Effects of β-Lactolin on Regional Cerebral Blood Flow within the Dorsolateral Prefrontal Cortex during Working Memory Task in Healthy Adults: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
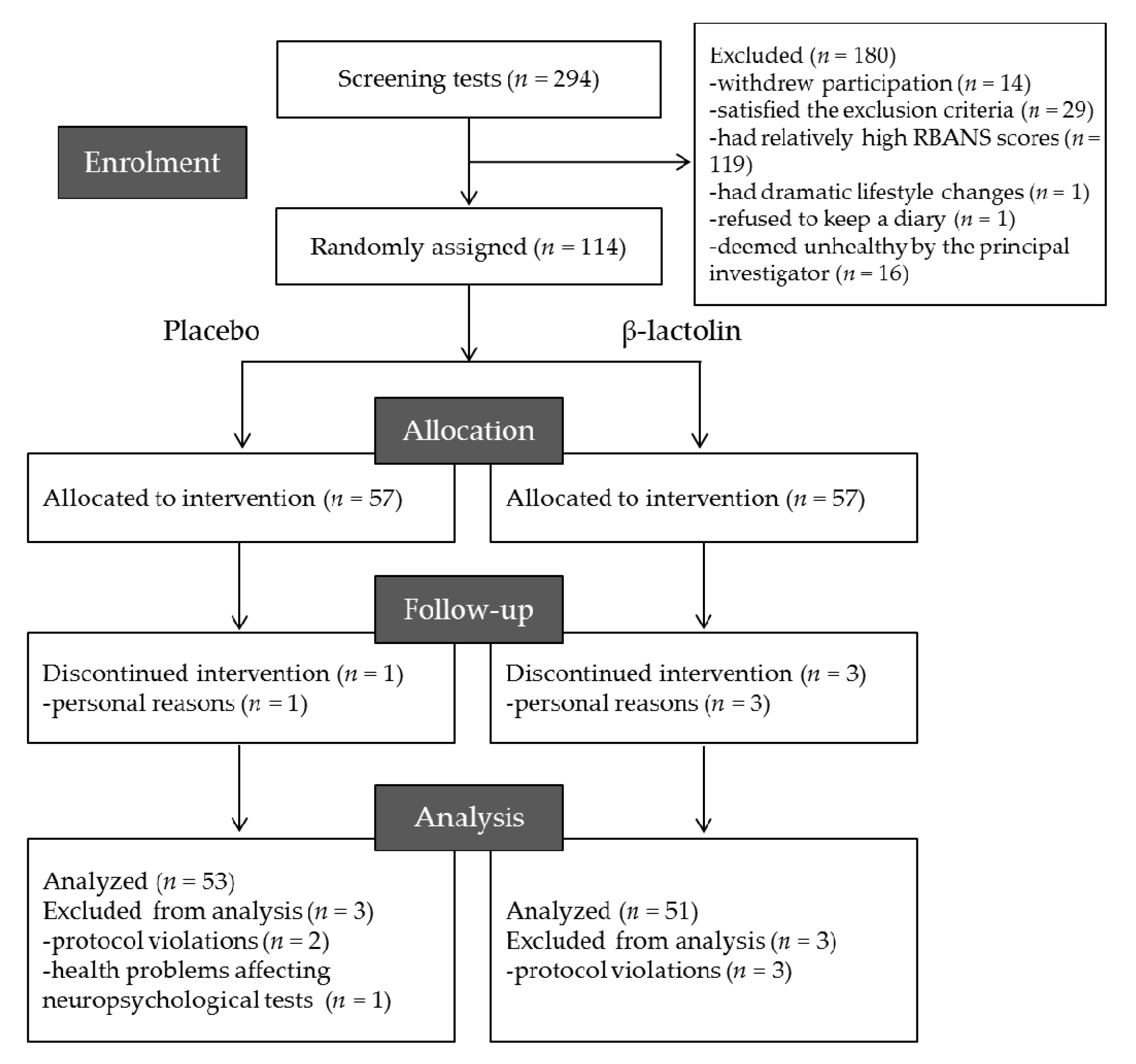
2.1. Participants
2.2. Ethics and Registration
2.3. Experimental Supplements
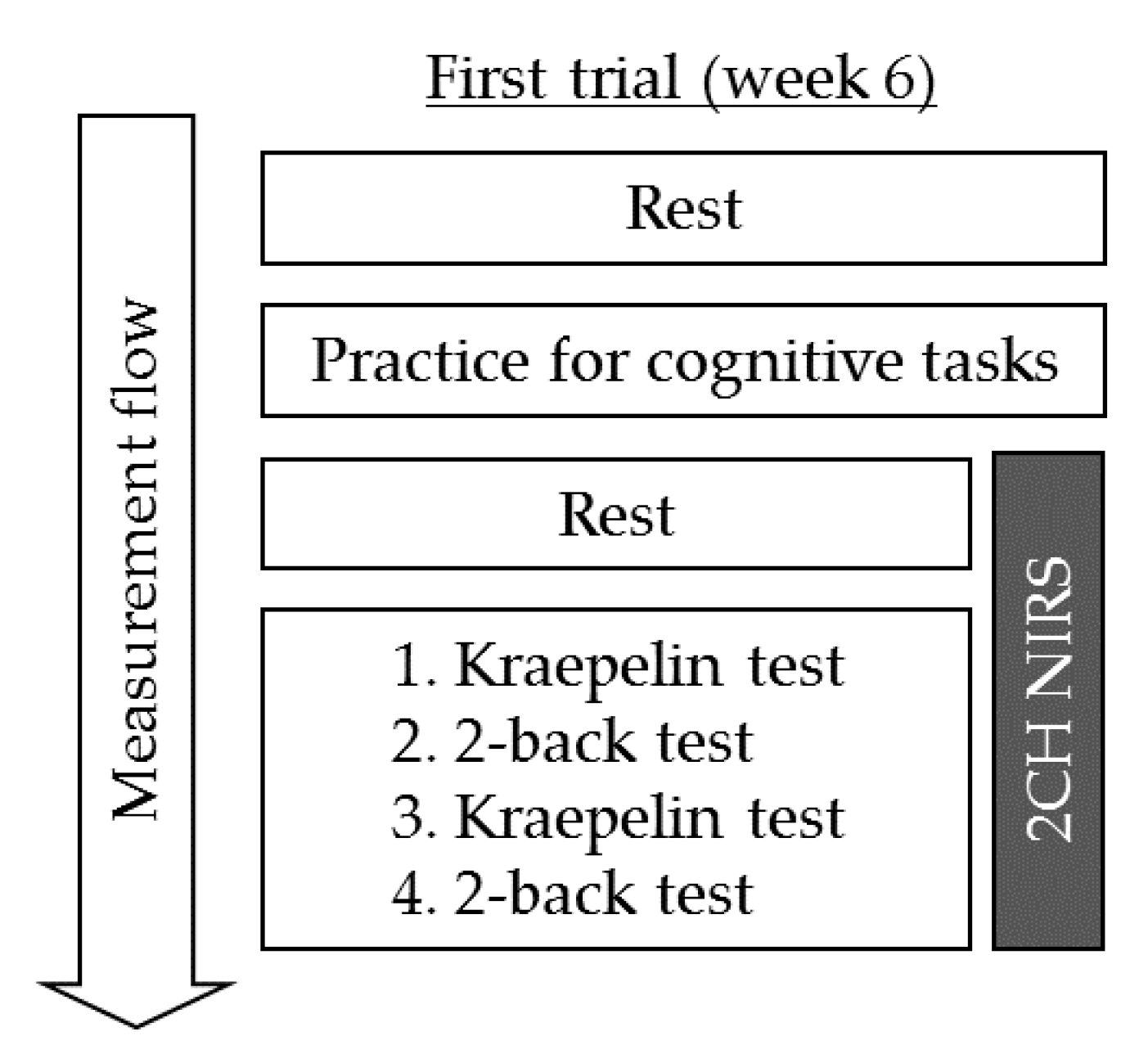
2.4. Procedures
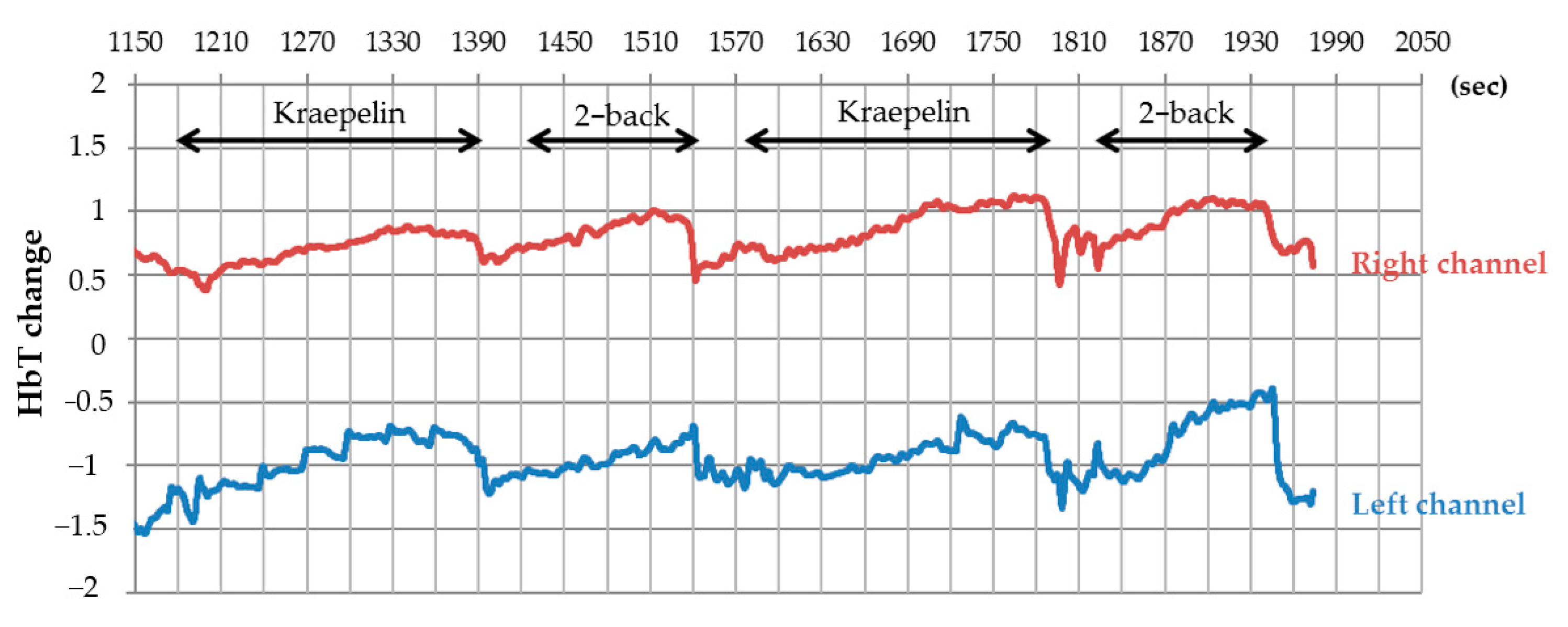
2.5. Cerebral Blood Flow Measurements
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Group
3.2. Cerebral Blood Flow Measurement
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.-C.; Wu, Y.-T.; Prina, A.M. World Alzheimer Report 2015 The Global Impact of Dementia. An analysis of prevalence, incidence, cost and trends. World Alzheimer Rep. 2015, 2015, 1–84. [Google Scholar]
- Crichton, G.E.; Bryan, J.; Murphy, K.J.; Buckley, J. Review of dairy consumption and cognitive performance in adults: Findings and methodological issues. Dement. Geriatr. Cogn. Disord. 2010, 30, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Ninomiya, T.; Ohara, T.; Doi, Y.; Uchida, K.; Shirota, T.; Yonemoto, K.; Kitazono, T.; Kiyohara, Y. Dietary patterns and risk of dementia in an elderly Japanese population: The Hisayama Study. Am. J. Clin. Nutr. 2013, 97, 1076–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozawa, M.; Ohara, T.; Ninomiya, T.; Hata, J.; Yoshida, D.; Mukai, N.; Nagata, M.; Uchida, K.; Shirota, T.; Kitazono, T.; et al. Milk and dairy consumption and risk of dementia in an elderly Japanese population: The Hisayama Study. J. Am. Geriatr. Soc. 2014, 62, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Ano, Y.; Nakayama, H. Preventive Effects of Dairy Products on Dementia and the Underlying Mechanisms. Int. J. Mol. Sci. 2018, 19, 1927. [Google Scholar] [CrossRef] [Green Version]
- Ano, Y.; Ayabe, T.; Kutsukake, T.; Ohya, R.; Takaichi, Y.; Uchida, S.; Yamada, K.; Uchida, K.; Takashima, A.; Hiroyuki, N. Novel lactopeptides in fermented dairy products improve memory function and cognitive decline. Neurobiol. Aging 2018, 72, 23–31. [Google Scholar] [CrossRef]
- Kita, M.; Kobayashi, K.; Obara, K.; Koikeda, T.; Umeda, S.; Ano, Y. Supplementation with Whey Peptide Rich in β-Lactolin Improves Cognitive Performance in Healthy Older Adults: A Randomized, Double-Blind, Placebo-Controlled Study. Front. Neurosci. 2019, 13, 399. [Google Scholar] [CrossRef]
- Ano, Y.; Kutsukake, T.; Hoshi, A.; Yoshida, A.; Nakayama, H. Identification of a novel dehydroergosterol enhancing microglial anti-inflammatory activity in a dairy product fermented with Penicillium candidum. PLoS ONE 2015, 10, e0116598. [Google Scholar] [CrossRef]
- Suzuki, T.; Kojima, N.; Osuka, Y.; Tokui, Y.; Takasugi, S.; Kawashima, A.; Yamaji, T.; Hosoi, E.; Won, C.W.; Kim, H. The Effects of Mold-Fermented Cheese on Brain-Derived Neurotrophic Factor in Community-Dwelling Older Japanese Women with Mild Cognitive Impairment: A Randomized, Controlled, Crossover Trial. J. Am. Med Dir. Assoc. 2019, 20, 1509–1514.e2. [Google Scholar] [CrossRef]
- Ano, Y.; Nakayama, H. Novel lacto-peptides improve cognitive decline. Aging 2019, 11, 1615–1616. [Google Scholar] [CrossRef]
- Ayabe, T.; Ohya, R.; Ano, Y. β-lactolin, a whey-derived glycine-threonine-tryptophan-tyrosine lactotetrapeptide, improves prefrontal cortex-associated reversal learning in mice. Biosci. Biotechnol. Biochem. 2020, 84, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Ayabe, T.; Ano, Y.; Ohya, R.; Kitaoka, S.; Furuyashiki, T. The Lacto-Tetrapeptide Gly-Thr-Trp-Tyr, β-Lactolin, Improves Spatial Memory Functions via Dopamine Release and D1 Receptor Activation in the Hippocampus. Nutrients 2019, 11, 2469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ano, Y.; Ohya, R.; Takaichi, Y.; Washinuma, T.; Uchida, K.; Takashima, A.; Nakayama, H. β-Lactolin, a Whey-Derived Lacto-Tetrapeptide, Prevents Alzheimer’s Disease Pathologies and Cognitive Decline. J. Alzheimer’s Dis. 2020, 73, 1331–1342. [Google Scholar] [CrossRef] [Green Version]
- Kita, M.; Obara, K.; Kondo, S.; Umeda, S.; Ano, Y. Effect of Supplementation of a Whey Peptide Rich in Tryptophan-Tyrosine-Related Peptides on Cognitive Performance in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2018, 10, 899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ano, Y.; Kobayashi, K.; Hanyuda, M.; Kawashima, R. β-lactolin increases cerebral blood flow in dorsolateral prefrontal cortex in healthy adults: A randomized controlled trial. Aging 2020, 12, 18660–18675. [Google Scholar] [CrossRef] [PubMed]
- Boushel, R.; Langberg, H.; Olesen, J.; Gonzales-Alonzo, J.; Bülow, J.; Kjaer, M. Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scand. J. Med. Sci. Sports 2001, 11, 213–222. [Google Scholar] [CrossRef]
- Tseng, Y.L.; Lu, C.F.; Wu, S.M.; Shimada, S.; Huang, T.; Lu, G.Y. A Functional Near-Infrared Spectroscopy Study of State Anxiety and Auditory Working Memory Load. Front. Hum. Neurosci. 2018, 12, 313. [Google Scholar] [CrossRef]
- Jahani, S.; Fantana, A.L.; Harper, D.; Ellison, J.M.; Boas, D.A.; Forester, B.P.; Yücel, M.A. fNIRS can robustly measure brain activity during memory encoding and retrieval in healthy subjects. Sci. Rep. 2017, 7, 9533. [Google Scholar] [CrossRef]
- Baker, J.M.; Bruno, J.L.; Gundran, A.; Hosseini, S.M.H.; Reiss, A.L. fNIRS measurement of cortical activation and functional connectivity during a visuospatial working memory task. PLoS ONE 2018, 13, e0201486. [Google Scholar]
- Santos, R.F.; Galduroz, J.C.; Barbieri, A.; Castiglioni, M.L.; Ytaya, L.Y.; Bueno, O.F. Cognitive performance, SPECT, and blood viscosity in elderly non-demented people using Ginkgo biloba. Pharmacopsychiatry 2003, 36, 127–133. [Google Scholar]
- Sakatani, K.; Tanida, M.; Hirao, N.; Takemura, N. Ginkobiloba extract improves working memory performance in middle-aged women: Role of asymmetry of prefrontal cortex activity during a working memory task. Adv. Exp. Med. Biol. 2014, 812, 295–301. [Google Scholar] [PubMed]
- Nouchi, R.; Kawata, N.Y.D.S.; Saito, T.; Himmelmeier, R.M.; Nakamura, R.; Nouchi, H.; Kawashima, R. Dorsolateral Prefrontal Cortex Activity during a Brain Training Game Predicts Cognitive Improvements after Four Weeks’ Brain Training Game Intervention: Evidence from a Randomized Controlled Trial. Brain Sci. 2020, 10, 560. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Murata, N.; Noma, T.; Itoh, H.; Kayano, M.; Nakamura, K.; Urashima, T. Relationship of a Special Acidified Milk Protein Drink with Cognitive Performance: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study in Healthy Young Adults. Nutrients 2018, 10, 574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, M.; Ikeda, T.; Tokuda, T.; Monden, Y.; Nagashima, M.; Mizushima, S.G.; Takeshi, I.; Keiichi, S.; Yuta, U.; Akari, A.; et al. Acute administration of methylphenidate differentially affects cortical processing of emotional facial expressions in attention-deficit hyperactivity disorder children as studied by functional near-infrared spectroscopy. Neurophotonics 2020, 7, 025003. [Google Scholar] [CrossRef]
- Monden, Y.; Dan, H.; Nagashima, M.; Dan, I.; Tsuzuki, D.; Kyutoku, Y.; Gunji, Y.; Yamagata, T.; Watanabe, E.; Momoi, M.Y. Right prefrontal activation as a neuro-functional biomarker for monitoring acute effects of methylphenidate in ADHD children: An fNIRS study. Neuroimage Clin. 2012, 1, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Elliott, R. Executive functions and their disorders. Br. Med Bull. 2003, 65, 49–59. [Google Scholar] [CrossRef]
- Monsell, S. Task switching. Trends Cogn. Sci. 2003, 7, 134–140. [Google Scholar] [CrossRef]
- Chan, R.C.; Shum, D.; Toulopoulou, T.; Chen, E.Y. Assessment of executive functions: Review of instruments and identification of critical issues. Arch. Clin. Neuropsychol. 2008, 23, 201–216. [Google Scholar] [CrossRef] [Green Version]
- Goldman-Rakic, P.S. Working memory dysfunction in schizophrenia. J. Neuropsychiatry Clin. Neurosci. 1994, 6, 348–357. [Google Scholar]
- Brosnan, M.B.; Wiegand, I. The Dorsolateral Prefrontal Cortex, a Dynamic Cortical Area to Enhance Top-Down Attentional Control. J. Neurosci. 2017, 37, 3445–3446. [Google Scholar] [CrossRef]
- Kaller, C.P.; Heinze, K.; Frenkel, A.; Lappchen, C.H.; Unterrainer, J.M.; Weiller, C.; Lange, R.; Rahm, B. Differential impact of continuous theta-burst stimulation over left and right DLPFC on planning. Hum. Brain Mapp. 2013, 34, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Cools, R.; Frobose, M.; Aarts, E.; Hofmans, L. Dopamine and the motivation of cognitive control. Handb. Clin. Neurol. 2019, 163, 123–143. [Google Scholar] [PubMed]
- Weinberger, D.R.; Berman, K.F.; Illowsky, B.P. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia III. A new cohort and evidence for a monoaminergic mechanism. Arch. Gen. Psychiatry 1988, 45, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Koenigs, M.; Grafman, J. The functional neuroanatomy of depression: Distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav. Brain Res. 2009, 201, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Arnsten, A.F.; Raskind, M.A.; Taylor, F.B.; Connor, D.F. The Effects of Stress Exposure on Prefrontal Cortex: Translating Basic Research into Successful Treatments for Post-Traumatic Stress Disorder. Neurobiol. Stress 2015, 1, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Zomorrodi, R.; Ghazala, Z.; Goodman, M.S.; Blumberger, D.M.; Cheam, A.; Fischer, C.; Daskalakis, Z.J.; Mulsant, B.H.; Pollock, B.G.; et al. Extent of Dorsolateral Prefrontal Cortex Plasticity and Its Association with Working Memory in Patients With Alzheimer Disease. JAMA Psychiatry 2017, 74, 1266–1274. [Google Scholar] [CrossRef]
- Nagahama, Y.; Fukuyama, H.; Yamauchi, H.; Katsumi, Y.; Magata, Y.; Shibasaki, H.; Kimura, J. Age-related changes in cerebral blood flow activation during a Card Sorting Test. Exp. Brain Res. 1997, 114, 571–577. [Google Scholar] [CrossRef]
- Kazumata, K.; Tokairin, K.; Sugiyama, T.; Ito, M.; Uchino, H.; Osanai, T.; Kawabori, M.; Nakayama, N.; Houkin, K. Association of cognitive function with cerebral blood flow in children with moyamoya disease. J. Neurosurg. Pediatrics 2019, 25, 1–17. [Google Scholar] [CrossRef]
- León-Domínguez, U.; Martín-Rodríguez, J.F.; León-Carrión, J. Executive n-back tasks for the neuropsychological assessment of working memory. Behav. Brain Res. 2015, 292, 167–173. [Google Scholar] [CrossRef]
- Woodcock, E.A.; Greenwald, M.K.; Khatib, D.; Diwadkar, V.A.; Stanley, J.A. Pharmacological stress impairs working memory performance and attenuates dorsolateral prefrontal cortex glutamate modulation. NeuroImage 2019, 186, 437–445. [Google Scholar] [CrossRef]
- Wang, H.; He, W.; Wu, J.; Zhang, J.; Jin, Z.; Li, L. A coordinate-based meta-analysis of the n-back working memory paradigm using activation likelihood estimation. Brain Cogn. 2019, 132, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Boggio, P.S.; Ferrucci, R.; Mameli, F.; Martins, D.; Martins, O.; Vergari, M.; Tadini, L.; Scarpini, E.; Fregni, F.; Priori, A. Prolonged visual memory enhancement after direct current stimulation in Alzheimer’s disease. Brain Stimul. 2012, 5, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Boggio, P.S.; Khoury, L.P.; Martins, D.C.; Martins, O.E.; de Macedo, E.C.; Fregni, F. Temporal cortex direct current stimulation enhances performance on a visual recognition memory task in Alzheimer disease. J. Neurol. Neurosurg. Psychiatry 2009, 80, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Lane, H.Y.; Lin, C.H. Brain Stimulation in Alzheimer’s Disease. Front. Psychiatry 2018, 9, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, L.; Liu, H.; Du, W.; Chao, F.; Zhang, L.; Wang, K.; Huang, C.; Gao, Y.; Tang, Y. Stimulated left DLPFC-nucleus accumbens functional connectivity predicts the anti-depression and anti-anxiety effects of rTMS for depression. Transl. Psychiatry 2018, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Taki, Y.; Nouchi, R.; Hashizume, H.; Sekiguchi, A.; Kotozaki, Y.; Nakagawa, S.; Miyauchi, C.M.; Sassa, Y.; Kawashima, R. Effects of working memory training on functional connectivity and cerebral blood flow during rest. Cortex J. Devoted Study Nerv. Syst. Behav. 2013, 49, 2106–2125. [Google Scholar] [CrossRef]
- Volkow, N.D.; Fowler, J.S.; Wang, G.J.; Goldstein, R.Z. Role of dopamine, the frontal cortex and memory circuits in drug addiction: Insight from imaging studies. Neurobiol. Learn. Mem. 2002, 78, 610–624. [Google Scholar] [CrossRef]
- Ott, T.; Nieder, A. Dopamine and Cognitive Control in Prefrontal Cortex. Trends Cogn. Sci. 2019, 23, 213–234. [Google Scholar] [CrossRef]
- Williams, G.V.; Castner, S.A. Under the curve: Critical issues for elucidating D1 receptor function in working memory. Neuroscience 2006, 139, 263–276. [Google Scholar] [CrossRef]
- Backman, L.; Karlsson, S.; Fischer, H.; Karlsson, P.; Brehmer, Y.; Rieckmann, A.; MacDonald, S.W.S.; Farde, L.; Nyberg, L. Dopamine D1 receptors and age differences in brain activation during working memory. Neurobiol. Aging 2011, 32, 1849–1856. [Google Scholar] [CrossRef]
- Cools, R.; Stefanova, E.; Barker, R.A.; Robbins, T.W.; Owen, A.M. Dopaminergic modulation of high-level cognition in Parkinson’s disease: The role of the prefrontal cortex revealed by PET. Brain J. Neurol. 2002, 125, 584–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ano, Y.; Ayabe, T.; Ohya, R.; Kondo, K.; Kitaoka, S.; Furuyashiki, T. Tryptophan-Tyrosine Dipeptide, the Core Sequence of beta-Lactolin, Improves Memory by Modulating the Dopamine System. Nutrients 2019, 11, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ano, Y.; Yoshino, Y.; Kutsukake, T.; Ohya, R.; Fukuda, T.; Uchida, K.; Takashima, A.; Nakayama, H. Tryptophan-related dipeptides in fermented dairy products suppress microglial activation and prevent cognitive decline. Aging 2019, 11, 2949–2967. [Google Scholar] [CrossRef]
- Jackson, P.A.; Forster, J.S.; Bell, J.G.; Dick, J.G.; Younger, I.; Kennedy, D.O. DHA Supplementation Alone or in Combination with Other Nutrients Does not Modulate Cerebral Hemodynamics or Cognitive Function in Healthy Older Adults. Nutrients 2016, 8, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wightman, E.L.; Jackson, P.A.; Khan, J.; Forster, J.; Heiner, F.; Feistel, B.; Suarez, C.G.; Pischel, I.; Kennedy, D.O. The Acute and Chronic Cognitive and Cerebral Blood Flow Effects of a Sideritis scardica (Greek Mountain Tea) Extract: A Double Blind, Randomized, Placebo Controlled, Parallel Groups Study in Healthy Humans. Nutrients 2018, 10, 955. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | Placebo | β-Lactolin | p |
|---|---|---|---|
| Age | 61.2 ± 5.6 | 60.7 ± 5.7 | 0.703 |
| Male/female | 20/33 | 17/34 | 0.639 |
| BMI | 22.1 ± 3.3 | 21.6 ± 2.8 | 0.414 |
| MMSE | 28.7 ± 1.4 | 28.5 ± 1.5 | 0.565 |
| RBANS | 54.5 ± 12.1 | 53.7 ± 14.1 | 0.772 |
| (A) | ||||
| Group | n | Week 6 | p | |
| Kraepelin (1st) | Placebo | 47 | 0.29 ± 0.29 | 0.51 |
| β-lactolin | 46 | 0.27 ± 0.32 | ||
| 2-back (1st) | Placebo | 45 | 0.073 ± 0.18 | 0.027 |
| β-lactolin | 47 | 0.15 ± 0.16 | ||
| Kraepelin (2nd) | Placebo | 47 | 0.23 ± 0.26 | 0.84 |
| β-lactolin | 46 | 0.25 ± 0.31 | ||
| 2-back (2nd) | Placebo | 46 | 0.096 ± 0.17 | 0.42 |
| β-lactolin | 47 | 0.14 ± 0.19 | ||
| Kraepelin (Average) | Placebo | 47 | 0.26 ± 0.26 | 0.80 |
| β-lactolin | 46 | 0.26 ± 0.30 | ||
| 2-back (Average) | Placebo | 46 | 0.086 ± 0.16 | 0.071 |
| β-lactolin | 47 | 0.14 ± 0.15 | ||
| (B) | ||||
| Group | n | Week 6 | p | |
| Kraepelin (1st) | Placebo | 49 | 0.26 ± 0.23 | 0.11 |
| β-lactolin | 44 | 0.18 ± 0.29 | ||
| 2-back | Placebo | 47 | 0.12 ± 0.19 | 0.73 |
| β-lactolin | 46 | 0.13 ± 0.19 | ||
| Kraepelin (2nd) | Placebo | 49 | 0.22 ± 0.30 | 0.24 |
| β-lactolin | 44 | 0.15 ± 0.24 | ||
| 2-back (2nd) | Placebo | 48 | 0.15 ± 0.25 | 0.52 |
| β-lactolin | 46 | 0.10 ± 0.15 | ||
| Kraepelin (Average) | Placebo | 49 | 0.24 ± 0.25 | 0.14 |
| β-lactolin | 44 | 0.16 ± 0.25 | ||
| 2-back (Average) | Placebo | 48 | 0.14 ± 0.20 | 0.62 |
| β-lactolin | 46 | 0.12 ± 0.16 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ano, Y.; Kita, M.; Kobayashi, K.; Koikeda, T.; Kawashima, R. Effects of β-Lactolin on Regional Cerebral Blood Flow within the Dorsolateral Prefrontal Cortex during Working Memory Task in Healthy Adults: A Randomized Controlled Trial. J. Clin. Med. 2021, 10, 480. https://doi.org/10.3390/jcm10030480
Ano Y, Kita M, Kobayashi K, Koikeda T, Kawashima R. Effects of β-Lactolin on Regional Cerebral Blood Flow within the Dorsolateral Prefrontal Cortex during Working Memory Task in Healthy Adults: A Randomized Controlled Trial. Journal of Clinical Medicine. 2021; 10(3):480. https://doi.org/10.3390/jcm10030480
Chicago/Turabian StyleAno, Yasuhisa, Masahiro Kita, Keiko Kobayashi, Takashi Koikeda, and Ryuta Kawashima. 2021. "Effects of β-Lactolin on Regional Cerebral Blood Flow within the Dorsolateral Prefrontal Cortex during Working Memory Task in Healthy Adults: A Randomized Controlled Trial" Journal of Clinical Medicine 10, no. 3: 480. https://doi.org/10.3390/jcm10030480






