Learning the Ropes of Platelet Count Regulation: Inherited Thrombocytopenias
Abstract
:1. Introduction
2. Megakaryocytopoiesis and Platelet Production
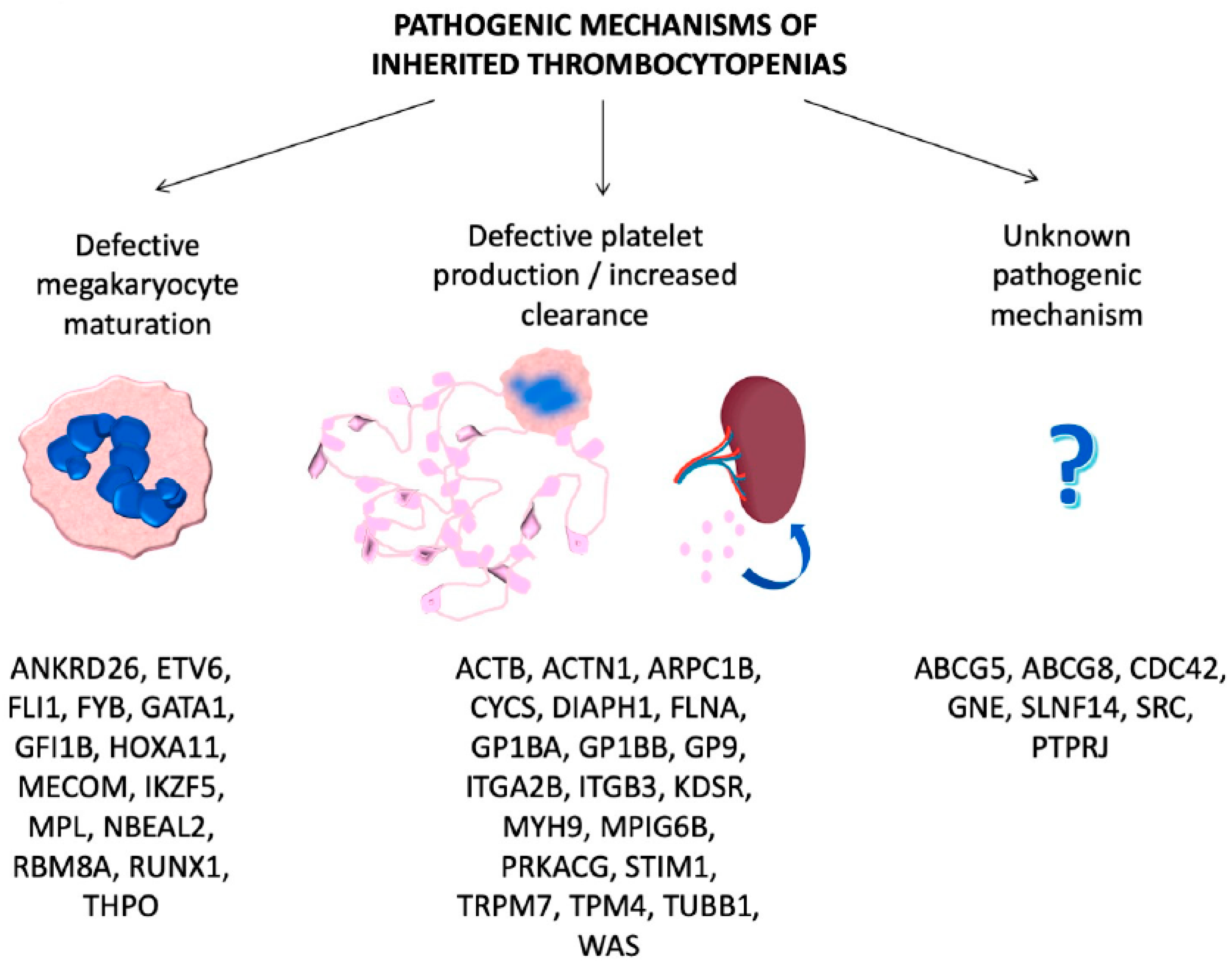
3. Hereditary Disorders of Platelet Number
3.1. ITs Caused by Defective Megakaryocyte Maturation and Differentiation
3.2. ITs Caused by Defective Platelet Production/Clearance
3.3. ITs Caused by Unknown Pathogenic Mechanisms
4. Diagnostic Approach
4.1. Introduction
4.2. Clinical Examination
4.3. Laboratory Tests
4.4. Genetic Analysis
4.5. Undefined Aspects and Possible Future Research Lines
5. Bleeding and Other Manifestations
6. Prophylaxis and Treatment Options
6.1. General Prophylactic Measures
6.2. Female Hormones
6.3. Local Hemostatic Measures
6.4. Platelet Transfusions
6.5. Antifibrinolytic Agents
6.6. Desmopressin
6.7. VWF-Rich Concentrates
6.8. Activated Recombinant Factor VIIa (rFVIIa)
6.9. Eltrombopag
6.10. Hematopoietic Stem Cell Transplantation (HSCT) and Gene Therapy
6.11. Splenectomy
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Balduini, C.L.; Pecci, A.; Noris, P. Inherited thrombocytopenias: The evolving spectrum. Hamostaseologie 2012, 32, 259–270. [Google Scholar] [PubMed]
- Oved, J.H.; Lambert, M.P.; Kowalska, M.A.; Poncz, M.; Karczewski, K.J. Population based frequency of naturally occurring loss-of-function variants in genes associated with platelet disorders. J. Thromb. Haemost. 2021, 19, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Bury, L.; Falcinelli, E.; Gresele, P. Qualitative Disorders of Platelet Function. In Wintrobe’s Clinical Hematology, 14th ed.; Greer, J.P., Appelbaum, F., Arber, D.A., Dispenzieri, A., Fehniger, T., Glader, B., List, A.F., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2018; pp. 3482–3527. [Google Scholar]
- Pecci, A.; Balduini, C.L. Inherited thrombocytopenias: An updated guide for clinicians. Blood Rev. 2020, 100784. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.; Doak, R.; Allsup, D.; Astwood, E.; Evans, G.; Grimley, C.; James, B.; Myers, B.; Stokley, S.; Thachil, J.; et al. A comprehensive targeted next-generation sequencing panel for genetic diagnosis of patients with suspected inherited thrombocytopenia. Res. Pract. Thromb. Haemost. 2018, 2, 640–652. [Google Scholar] [CrossRef] [Green Version]
- Machlus, K.R.; Italiano, J.E. The incredible journey: From megakaryocyte development to platelet formation. J. Cell Biol. 2013, 201, 785–796. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, S.; Nagasaki, M.; Kunishima, S.; Sawaguchi, A.; Sakata, A.; Sakaguchi, H.; Ohmori, T.; Manabe, I.; Italiano, J.E.; Ryu, T.; et al. IL-1α induces thrombopoiesis through megakaryocyte rupture in response to acute platelet needs. J. Cell Biol. 2015, 209, 453–466. [Google Scholar] [CrossRef] [Green Version]
- Potts, K.S.; Farley, A.; Dawson, C.A.; Rimes, J.S.; Biben, C.; De Graaf, C.A.; Potts, M.A.; Stonehouse, O.J.; Carmagnac, A.; Gangatirkar, P.; et al. Membrane budding is a major mechanism of in vivo platelet biogenesis. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef]
- Looney, M.R. The incomparable platelet: Holy alveoli. Blood 2018, 132, 1088–1089. [Google Scholar] [CrossRef]
- AlMazni, I.; Stapley, R.; Morgan, N.V. Inherited Thrombocytopenia: Update on Genes and Genetic Variants Which may be Associated with Bleeding. Front. Cardiovasc. Med. 2019, 6, 80. [Google Scholar] [CrossRef] [Green Version]
- Nurden, A.T.; Nurden, P. Inherited thrombocytopenias: History, advances and perspectives. Haematologica 2020, 105, 2004–2019. [Google Scholar] [CrossRef]
- Balduini, C.L.; Melazzini, F.; Pecci, A. Inherited thrombocytopenias—recent advances in clinical and molecular aspects. Platelets 2017, 28, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Bluteau, D.; Balduini, A.; Balayn, N.; Currao, M.; Nurden, P.; Deswarte, C.; Leverger, G.; Noris, P.; Perrotta, S.; Solary, E.; et al. Thrombocytopenia associated mutations in the ANKRD26 regulatory region induce MAPK hyperactivation. J. Clin. Investig. 2014, 124, 580–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noetzli, L.; Lo, R.W.; Lee-Sherick, A.B.; Callaghan, M.; Noris, P.; Savoia, A.; Rajpurkar, M.; Jones, K.; Gowan, K.; Balduini, C.L.; et al. Germline mutations in ETV6 are associated with thrombocytopenia, red cell macrocytosis and predisposition to lymphoblastic leukemia. Nat. Genet. 2015, 47, 535–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurden, P.; Debili, N.; Coupry, I.; Bryckaert, M.; Youlyouz-Marfak, I.; Sole´, G.; Pons, A.C.; Berrou, E.; Adam, F.; Kauskot, A.; et al. Thrombocytopenia resulting from mutations in filamin A can be expressed as an isolated syndrome. Blood 2011, 118, 5928–5937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, C.; Koren, A.; Pretorius, E.; Rosenberg, N.; Shenkman, B.; Hauschner, H.; Zalman, L.; Khayat, M.; Salama, I.; Elpeleg, O.; et al. Deleterious mutation in the FYB gene is associated with congenital autosomal recessive small-platelet thrombocytopenia. J. Thromb. Haemost. 2015, 13, 1285–1292. [Google Scholar] [CrossRef]
- Songdej, N.; Rao, A.K. Hematopoietic transcription factor mutations and inherited platelet dysfunction. F1000Prime Rep. 2015, 7, 66. [Google Scholar] [CrossRef]
- Gresele, P. Subcommittee on Platelet Physiology of the International Society on Thrombosis and Hemostasis. Diagnosis of inherited platelet function disorders: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2015, 13, 314–322. [Google Scholar] [CrossRef]
- Horvat-Switzer, R.D.; Thompson, A.A. HOXA11 mutation in amegakaryocytic thrombocytopenia with radio-ulnar synostosis syndrome inhibits megakaryocytic differentiation in vitro. Blood Cells Mol. Dis. 2006, 37, 55–63. [Google Scholar] [CrossRef]
- Germeshausen, M.; Ancliff, P.; Estrada, J.; Metzler, M.; Ponstingl, E.; Rütschle, H.; Schwabe, D.; Scott, R.H.; Unal, S.; Wawer, A.; et al. MECOM-associated syndrome: A heterogeneous inherited bone marrow failure syndrome with amegakaryocytic thrombocytopenia. Blood Adv. 2018, 2, 586–596. [Google Scholar] [CrossRef] [Green Version]
- Lentaigne, C.; Greene, D.; Sivapalaratnam, S.; Favier, R.; Seyres, D.; Thys, C.; Grassi, L.; Mangles, S.; Sibson, K.; Stubbs, M.J.; et al. Germline mutations in the transcription factor IKZF5 cause thrombocytopenia. Blood 2019, 134, 2070–2081. [Google Scholar] [CrossRef]
- Hirata, S.; Takayama, N.; Jono-Ohnishi, R.; Endo, H.; Nakamura, S.; Dohda, T.; Nishi, M.; Hamazaki, Y.; Ishii, E.; Kaneko, S.; et al. Congenital amegakaryocytic thrombocytopenia iPS cells exhibit defective MPL-mediated signaling. J. Clin. Investig. 2013, 123, 3802–3814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluthero, F.G.; Di Paola, J.; Carcao, M.D.; Kahr, W.H.A. NBEAL2 mutations and bleeding in patients with gray platelet syndrome. Platelets 2018, 29, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Albers, C.A.; Newbury-Ecob, R.; Ouwehand, W.H.; Ghevaert, C. New insights into the genetic basis of TAR (thrombocytopenia-absent radii) syndrome. Curr. Opin. Genet. Dev. 2013, 23, 316–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakurai, M.; Kunimoto, H.; Watanabe, N.; Fukuchi, Y.; Yuasa, S.; Yamazaki, S.; Nishimura, T.; Sadahira, K.; Fukuda, K.; Okano, H.; et al. Impaired hematopoietic differentiation of RUNX1-mutated induced pluripotent stem cells derived from FPD/AML patients. Leukemia 2014, 28, 2344–2354. [Google Scholar] [CrossRef]
- Dasouki, M.J.; Rafi, S.K.; Olm-Shipman, A.J.; Wilson, N.R.; Abhyankar, S.; Ganter, B.; Furness, L.M.; Fang, J.; Calado, R.T.; Saadi, I. Exome sequencing reveals a thrombopoietin ligand mutation in a Micronesian family with autosomal recessive aplastic anemia. Blood 2013, 122, 3440–3449. [Google Scholar] [CrossRef] [Green Version]
- Latham, S.L.; Ehmke, N.; Reinke, P.Y.A.; Taft, M.H.; Eicke, D.; Reindl, T.; Stenzel, W.; Lyons, M.J.; Friez, M.J.; Lee, J.A.; et al. Variants in exons 5 and 6 of ACTB cause syndromic thrombocytopenia. Nat. Commun. 2018, 9, 4250. [Google Scholar] [CrossRef]
- Kunishima, S.; Okuno, Y.; Yoshida, K.; Shiraishi, Y.; Sanada, M.; Muramatsu, H.; Chiba, K.; Tanaka, H.; Miyazaki, K.; Sakai, M.; et al. ACTN1 mutations cause congenital macrothrombocytopenia. Am. J. Hum. Genet. 2013, 92, 431–438. [Google Scholar] [CrossRef] [Green Version]
- Kahr, W.H.; Pluthero, F.G.; Elkadri, A.; Warner, N.; Drobac, M.; Chen, C.H.; Lo, R.W.; Li, L.; Li, R.; Li, Q.; et al. Loss of the Arp2/3 complex component ARPC1B causes platelet abnormalities and predisposes to inflammatory disease. Nat. Commun. 2017, 8, 14816. [Google Scholar] [CrossRef] [Green Version]
- Morison, I.M.; Cramer Borde, E.M.; Cheesman, E.J.; Cheong, P.L.; Holyoake, A.J.; Fichelson, S.; Weeks, R.J.; Lo, A.; Davies, S.M.; Wilbanks, S.M.; et al. A mutation of human cytochrome c enhances the intrinsic apoptotic pathway but causes only thrombocytopenia. Nat. Genet. 2008, 40, 387–389. [Google Scholar] [CrossRef]
- Stritt, S.; Nurden, P.; Turro, E.; Greene, D.; Jansen, S.B.; Westbury, S.K.; Petersen, R.; Astle, W.J.; Marlin, S.; Bariana, T.K.; et al. A gain-of-function variant in DIAPH1 causes dominant macrothrombocytopenia and hearing loss. Blood 2016, 127, 2903–2914. [Google Scholar] [CrossRef] [Green Version]
- Necchi, V.; Balduini, A.; Noris, P.; Barozzi, S.; Sommi, P.; di Buduo, C.; Balduini, C.L.; Solcia, E.; Pecci, A. Ubiqui-tin/proteasome-rich particulate cytoplasmic structures (PaCSs) in the platelets and megakaryocytes of ANKRD26-related thrombo-cytopenia. Thromb. Haemost. 2013, 109, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Balduini, A.; Malara, A.; Balduini, C.L.; Noris, P. Megakaryocytes derived from patients with the classical form of Bernard-Soulier syndrome show no ability to extend proplatelets in vitro. Platelets 2011, 22, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Bury, L.; Malara, A.; Momi, S.; Petito, E.; Balduini, A.; Gresele, P. Mechanisms of thrombocytopenia in platelet-type von Willebrand disease. Haematologica 2019, 104, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Bury, L.; Falcinelli, E.; Chiasserini, D.; Springer, T.A.; Italiano, J.E., Jr.; Gresele, P. Cytoskeletal perturbation leads to platelet dysfunction and thrombocytopenia in Glanzmann variants. Haematologica 2016, 101, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Bury, L.; Malara, A.; Gresele, P.; Balduini, A. Outside-in signalling generated by a constitutively activated integrin αIIbβ3 impairs proplatelet formation in human megakaryocytes. PLoS ONE 2012, 7, e34449. [Google Scholar] [CrossRef] [Green Version]
- Bariana, T.K.; Labarque, V.; Heremans, J.; Thys, C.; De Reys, M.; Greene, D.; Jenkins, B.; Grassi, L.; Seyres, D.; Burden, F.; et al. Sphingolipid dysregulation due to lack of functional KDSR impairs proplatelet formation causing thrombocytopenia. Haematologica 2019, 104, 1036–1045. [Google Scholar] [CrossRef] [Green Version]
- Pecci, A.; Malara, A.; Badalucco, S.; Bozzi, V.; Torti, M.; Balduini, C.L.; Balduini, A. Megakaryocytes of patients with MYH9-related thrombocytopenia present an altered proplatelet formation. Thromb. Haemost. 2009, 102, 90–96. [Google Scholar]
- Hofmann, I.; Geer, M.J.; Vögtle, T.; Crispin, A.; Campagna, D.R.; Barr, A.; Calicchio, M.L.; Heising, S.; van Geffen, J.P.; Kuijpers, M.J.E.; et al. Congenital macrothrombocytopenia with focal myelofibrosis due to mutations in human G6b-B is rescued in humanized mice. Blood 2018, 132, 1399–1412. [Google Scholar] [CrossRef] [Green Version]
- Manchev, V.T.; Hilpert, M.; Berrou, E.; Elaib, Z.; Aouba, A.; Boukour, S.; Souquere, S.; Pierron, G.; Rameau, P.; Andrews, R.; et al. A new form of macro-thrombocytopenia induced by germ-line mutation in the PRKACG gene. Blood 2014, 124, 2554–2563. [Google Scholar] [CrossRef] [Green Version]
- Morin, G.; Bruechle, N.O.; Singh, A.R.; Knopp, C.; Jedraszak, G.; Elbracht, M.; Bre´mond-Gignac, D.; Hartmann, K.; Sevestre, H.; Deutz, P.; et al. Gain-of-function mutation in STIM1 (P.R304W) is associated with Stormorken syndrome. Hum. Mutat. 2014, 35, 1221–1232. [Google Scholar] [CrossRef]
- Stritt, S.; Nurden, P.; Favier, R.; Favier, M.; Ferioli, S.; Gotru, S.K.; van Eeuwijk, J.M.M.; Schulze, H.; Nurden, A.T.; Lambert, M.P.; et al. Defects in TRPM7 channel function deregulate thrombopoiesis through altered cellular Mg(2+) homeostasis and cytoskeletal architecture. Nat. Commun. 2016, 7, 11097. [Google Scholar] [CrossRef] [PubMed]
- Pleines, I.; Woods, J.; Chappaz, S.; Kew, V.; Foad, N.; Ballester-Beltrán, J.; Aurbach, K.; Lincetto, C.; Lane, R.M.; Schevzov, G.; et al. Mutations in tropomyosin 4 underlie a rare form of human macrothrombocytopenia. J. Clin. Investig. 2017, 127, 814–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunishima, S.; Kobayashi, R.; Itoh, T.J.; Hamaguchi, M.; Saito, H. Mutation of the beta1-tubulin gene associated with congenital macrothrombocytopenia affecting microtubule assembly. Blood 2009, 113, 458–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massaad, M.J.; Ramesh, N.; Geha, R.S. Wiskott-Aldrich syndrome: A comprehensive review. Ann. N. Y. Acad. Sci. 2013, 1285, 26–43. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.C.; Iolascon, A.; Carella, M.; O’marcaigh, A.S.; Kendra, J.R.; Jowitt, S.N.; Wales, J.K.; Vora, A.; Makris, M.; Manning, N.; et al. Stomatocytic haemolysis and macrothrombocytopenia (Mediterranean stomatocytosis/macrothrombocytopenia) is the haematological presentation of phytosterolaemia. Br. J. Haematol. 2005, 130, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Takenouchi, T.; Okamoto, N.; Ida, S.; Uehara, T.; Kosaki, K. Further evidence of a mutation in CDC42 as a cause of a recognizable syndromic form of thrombocytopenia. Am. J. Med. Genet. A 2016, 170, 852–855. [Google Scholar] [CrossRef]
- Futterer, J.; Dalby, A.; Lowe, G.C.; Johnson, B.; Simpson, M.A.; Motwani, J.; Williams, M.; Watson, S.P.; Morgan, N.V. Mutation in GNE is associated with severe congenital thrombocytopenia. Blood 2018, 132, 1855–1858. [Google Scholar] [CrossRef]
- Fletcher, S.J.; Johnson, B.; Lowe, G.C.; Bem, D.; Drake, S.; Lordkipanidzé, M. SLFN14 mutations underlie thrombocytopenia with excessive bleeding and platelet secretion defects. J. Clin. Investig. 2015, 125, 3600–3605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turro, E.; Greene, D.; Wijgaerts, A.; Thys, C.; Lentaigne, C.; Bariana, T.K.; Westbury, S.K.; Kelly, A.M.; Selleslag, D.; Stephens, J.C.; et al. A dominant gain-of-function mutation in universal tyrosine kinase SRC causes thrombocytopenia, myelofibrosis, bleeding, and bone pathologies. Sci. Transl. Med. 2016, 8, 328ra30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marconi, C.; Di Buduo, C.A.; LeVine, K.; Barozzi, S.; Faleschini, M.; Bozzi, V.; Palombo, F.; McKinstry, S.; Lassandro, G.; Giordano, P.; et al. Loss-of-function mutations in PTPRJ cause a new form of inherited thrombocytopenia. Blood 2019, 133, 1346–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raslova, H.; Komura, E.; Le Couédic, J.P.; Larbret, F.; Debili, N.; Feunteun, J.; Danos, O.; Albagli, O.; Vainchenker, W.; Favier, R. FLI1 monoallelic expression combined with its hemizygous loss underlies Paris-Trousseau/Jacobsen thrombopenia. J. Clin. Investig. 2004, 114, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.A.; Woodruff, K.; Feig, S.A.; Nguyen, L.T.; Schanen, N.C. Congenital thrombocytopenia and radio-ulnar synostosis: A new familial syndrome. Br. J. Haematol. 2001, 113, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Gresele, P.; Falcinelli, E.; Giannini, S.; D’Adamo, P.; D’Eustacchio, A.; Corazzi, T.; Mezzasoma, A.M.; Di Bari, F.; Guglielmini, G.; Cecchetti, L.; et al. Dominant inheritance of a novel integrin beta3 mutation associated with a hereditary macrothrombocytopenia and platelet dysfunction in two Italian families. Haematologica 2009, 94, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Bury, L.; Zetterberg, E.; Leinøe, E.B.; Falcinelli, E.; Marturano, A.; Manni, G.; Nurden, A.T.; Gresele, P. A novel variant Glanzmann thrombasthenia due to co-inheritance of a loss- and a gain-of-function mutation of ITGB3: Evidence of a dominant effect of gain-of-function mutations. Haematologica. 2018, 103, e259–e263. [Google Scholar] [CrossRef] [Green Version]
- Sabri, S.; Foudi, A.; Boukour, S.; Franc, B.; Charrier, S.; Jandrot-Perrus, M.; Farndale, R.W.; Jalil, A.; Blundell, M.P.; Cramer, E.M.; et al. Deficiency in the Wiskott-Aldrich protein induces premature proplatelet formation and platelet production in the bone marrow compartment. Blood 2006, 108, 134–140. [Google Scholar] [CrossRef]
- Nesin, V.; Wiley, G.; Kousi, M.; Ong, E.-C.; Lehmann, T.; Nicholl, D.J.; Suri, M.; Shahrizaila, N.; Katsanis, N.; Gaffney, P.M.; et al. Activating mutations in STIM1 and ORAI1 cause overlapping syndromes of tubular myopathy and congenital miosis. Proc. Natl. Acad. Sci. USA 2014, 111, 4197–4202. [Google Scholar] [CrossRef] [Green Version]
- Grosse, J.; Braun, A.; Varga-Szabo, D.; Beyersdorf, N.; Schneider, B.; Zeitlmann, L.; Hanke, P.; Schropp, P.; Mühlstedt, S.; Zorn, C.; et al. An EF hand mu-tation in Stim1 causes premature platelet activation and bleeding in mice. J. Clin. Investig. 2007, 117, 3540–3550. [Google Scholar] [CrossRef] [Green Version]
- Berge, K.E.; Tian, H.; Graf, G.A.; Yu, L.; Grishin, N.V.; Schultz, J.; Kwiterovich, P.; Shan, B.; Barnes, R.; Hobbs, H.H. Accu-mulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science 2000, 290, 1771–1775. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Wang, Z.; Yang, H.; Cao, L.; Liu, F.; Bai, X.; Ruan, C. Clinical and molecular genetic analysis of a family with sitosterolemia and co-existing erythrocyte and platelet abnormalities. Haematologica 2006, 91, 1392–1395. [Google Scholar]
- Pisareva, V.P.; Muslimov, I.A.; Tcherepanov, A.; Pisarev, A.V. Characterization of novel ribosome-associated endoribonuclease SLFN14 from rabbit reticulocytes. Biochemistry 2015, 54, 3286–3301. [Google Scholar] [CrossRef] [Green Version]
- Marconi, C.; Di Buduo, C.A.; Barozzi, S.; Palombo, F.; Pardini, S.; Zaninetti, C.; Pippucci, T.; Noris, P.; Balduini, A.; Marconi, C.; et al. SLFN14-related thrombocytopenia: Identification within a large series of patients with inherited thrombocytopenia. Thromb. Haemost. 2016, 115, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, I.; Avidan, N.; Potikha, T.; Hochner, H.; Chen, M.; Olender, T.; Barash, M.; Shemesh, M.; Sadeh, M.; Grabov-Nardini, G.; et al. The UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene is mutated in recessive hereditary inclusion body myopathy. Nat. Genet. 2001, 29, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Rodeghiero, F.; Pabinger, I.; Ragni, M.; Abdul-Kadir, R.; Berntorp, E.; Blanchette, V.; Bodó, I.; Casini, A.; Gresele, P.; Lassila, R.; et al. Fundamentals for a systematic approach to mild and moderate inherited bleeding disorders: A EHA consensus report. Hemasphere 2019, 3, e286. [Google Scholar] [CrossRef] [PubMed]
- Gresele, P.; Orsini, S.; Noris, P.; Falcinelli, E.; Alessi, M.C.; Bury, L.; Borhany, M.; Santoro, C.; Glembotsky, A.C.; Cid, A.R.; et al. BAT-VAL study investigators. Validation of the ISTH/SSC bleeding assessment tool for inherited platelet disorders: A communication from the Platelet Physiology SSC. J. Thromb. Haemost. 2020, 18, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Balduini, C.L.; Cattaneo, M.; Fabris, F.; Gresele, P.; Iolascon, A.; Pulcinelli, F.M.; Savoia, A. Inherited thrombocytopenias: A proposed diagnostic algorithm from the Italian Gruppo di Studio delle Piastrine. Haematologica 2003, 88, 582–592. [Google Scholar] [PubMed]
- Noris, P.; Pecci, A.; Di Bari, F.; Di Stazio, M.T.; Di Pumpo, M.; Ceresa, I.F.; Arezzi, N.; Ambaglio, C.; Savoia, A.; Balduini, C.L. Application of a diagnostic algorithm for inherited thrombocytopenias to 46 consecutive patients. Haematologica 2004, 89, 1219–1225. [Google Scholar] [PubMed]
- Zaninetti, C.; Greinacher, A. Diagnosis of Inherited Platelet Disorders on a Blood Smear. J. Clin. Med. 2020, 9, 539. [Google Scholar] [CrossRef] [Green Version]
- Bury, L.; Falcinelli, E.; Gresele, P. Inherited Platelet Function Disorders: Algorithms for Phenotypic and Genetic Investigation. Semin. Thromb. Hemost. 2016, 42, 292–305. [Google Scholar] [CrossRef]
- Podda, G.M.; Pugliano, M.; Femia, E.A.; Mezzasoma, A.M.; Gresele, P.; Carpani, G.; Cattaneo, M. The platelet count in EDTA-anticoagulated blood from patients with thrombocytopenia may be underestimated when measured in routine laboratories. Am. J. Hematol. 2012, 87, 727–728. [Google Scholar] [CrossRef]
- Noris, P.; Biino, G.; Pecci, A.; Civaschi, E.; Savoia, A.; Seri, M.; Melazzini, F.; Loffredo, G.; Russo, G.; Bozzi, V.; et al. Platelet diameters in inherited thrombocytopenias: Analysis of 376 patients with all known disorders. Blood 2014, 124, e4–e10. [Google Scholar] [CrossRef]
- Monteferrario, D.; Bolar, N.A.; Marneth, A.E.; Hebeda, K.M.; Bergevoet, S.M.; Veenstra, H.; Laros-van Gorkom, B.A.; MacKenzie, M.A.; Khandanpour, C.; Botezatu, L.; et al. A dominant-negative GFI1B mutation in the gray platelet syndrome. N. Engl. J. Med. 2014, 370, 245–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gresele, P.; Bury, L.; Mezzasoma, A.M.; Falcinelli, E. Platelet function assays in diagnosis: An update. Expert Rev. Hematol. 2019, 12, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Gresele, P.; Falcinelli, E.; Bury, L. Laboratory diagnosis of clinically relevant platelet function disorders. Int. J. Lab. Hematol. 2018, 40, 34–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mumford, A.D.; Frelinger, A.L., 3rd; Gachet, C.; Gresele, P.; Noris, P.; Harrison, P.; Mezzano, D. A review of platelet secretion assays for the diagnosis of inherited platelet secretion disorders. Thromb. Haemost. 2015, 114, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.; Gresele, P. Guidance on the diagnosis and management of platelet-type von Willebrand disease: A communication from the Platelet Physiology Subcommittee of the ISTH. J. Thromb. Haemost. 2020, 18, 1855–1858. [Google Scholar] [CrossRef] [PubMed]
- Giannini, S.; Cecchetti, L.; Mezzasoma, A.M.; Gresele, P. Diagnosis of platelet-type von Willebrand disease by flow cytometry. Haematologica 2010, 95, 1021–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sims, M.C.; Mayer, L.; Collins, J.H.; Bariana, T.K.; Megy, K.; Lavenu-Bombled, C.; Seyres, D.; Kollipara, L.; Burden, F.S.; Greene, D.; et al. Novel manifestations of immune dysregulation and granule defects in gray platelet syndrome. Blood 2020, 136, 1956–1967. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, W.S.; Rabbolini, D.J.; Beutler, L.; Chen, Q.; Gabrielli, S.; Mackay, J.P.; Brighton, T.A.; Ward, C.M.; Morel-Kopp, M.C. Paris-Trousseau thrombocytopenia is phenocopied by the autosomal recessive inheritance of a DNA-binding domain mutation in FLI1. Blood 2015, 126, 2027–2030. [Google Scholar] [CrossRef] [Green Version]
- Pecci, A.; Biino, G.; Fierro, T.; Bozzi, V.; Mezzasoma, A.; Noris, P.; Ramenghi, U.; Loffredo, G.; Fabris, F.; Momi, S.; et al. Alteration of Liver Enzymes Is a Feature of the Myh9-Related Disease Syndrome. PLoS ONE 2012, 7, e35986. [Google Scholar] [CrossRef] [Green Version]
- Noris, P.; Pecci, A. Hereditary thrombocytopenias: A growing list of disorders. Hematology 2017, 2017, 385–399. [Google Scholar] [CrossRef] [Green Version]
- Simeoni, I.; Stephens, J.C.; Hu, F.; Deevi, S.V.V.; Megy, K.; Bariana, T.K.; Lentaigne, C.; Schulman, S.; Sivapalaratnam, S.; Vries, M.J.A.; et al. A high-throughput sequencing test for diagnosing inherited bleeding, thrombotic, and platelet disorders. Blood 2016, 127, 2791–2803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downes, K.; Megy, K.; Duarte, D.; Vries, M.; Gebhart, J.; Hofer, S.; Shamardina, O.; Deevi, S.V.V.; Stephens, J.; Mapeta, R.; et al. Diagnostic high-throughput sequencing of 2396 patients with bleeding, thrombotic, and platelet disorders. Blood 2019, 134, 2082–2091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Megy, K.; Downes, K.; Simeoni, I.; Bury, L.; Morales, J.; Mapeta, R.; Bellissimo, D.B.; Bray, P.F.; Goodeve, A.C.; Gresele, P.; et al. Subcommittee on Genomics in Thrombosis and Hemostasis. Cu-rated disease-causing genes for bleeding, thrombotic, and platelet disorders: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2019, 17, 1253–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downes, K.; Borry, P.; Ericson, K.; Gomez, K.; Greinacher, A.; Lambert, M.; Leinoe, E.; Noris, P.; Van Geet, C.; Freson, K. Subcommittee on Genomics in Thrombosis, Hemostasis. Clinical management, ethics and informed consent related to multi-gene panel-based high throughput sequencing testing for platelet disorders: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2020, 18, 2751–2758. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Drachman, J.G. Inherited thrombocytopenia: When a low platelet count does not mean ITP. Blood 2004, 103, 390–398. [Google Scholar] [CrossRef]
- Johnson, B.; Lowe, G.C.; Futterer, J.; Lordkipanidzé, M.; Macdonald, D.; Simpson, M.A.; Guiú, I.S.; Drake, S.; Bem, D.; Leo, V.; et al. Whole exome sequencing identifies genetic variants in inherited thrombocytopenia with secondary qualitative function defects. Haematologica 2016, 101, 1170–1179. [Google Scholar] [CrossRef] [Green Version]
- Gresele, P.; Falcinelli, E.; Bury, L. Inherited platelet disorders in women. Thromb. Res. 2019, 181, S54–S59. [Google Scholar] [CrossRef]
- Balduini, C.L.; Melazzini, F.; Pecci, A. Inherited Thrombocytopenias. In Platelets in Thrombotic and Non Thrombotic Disorders; Gresele, P., Kleiman, N.S., Lopez, J.A., Page, C.P., Eds.; Springer Gmbh: Cambridge, UK, 2017; pp. 727–747. [Google Scholar]
- Kaur, H.; Ozelo, M.; Scovil, S.; James, P.D.; Othman, M. Systematic analysis of bleeding phenotype in PT-VWD compared to type 2B VWD using an electronic bleeding questionnaire. Clin. Appl. Thromb. Hemost. 2014, 20, 765–771. [Google Scholar] [CrossRef] [Green Version]
- Nurden, P.; Nurden, A.; Favier, R.; Gleyze, M. Management of pregnancy for a patient with the new syndromic macrothrombocytopenia, DIAPH1-related disease. Platelets 2018, 29, 737–738. [Google Scholar] [CrossRef]
- Orsini, S.; Noris, P.; Bury, L.; Heller, P.G.; Santoro, C.; Kadir, R.A.; Butta, N.C.; Falcinelli, E.; Cid, A.R.; Fabris, F.; et al. European Hematology Association—Scientific Working Group (EHA-SWG) on thrombocytopenias and platelet function disorders. Bleeding risk of surgery and its pre-vention in patients with inherited platelet disorders. Haematologica 2017, 102, 1192–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, N.V.; Daly, M.E. Gene of the issue: RUNX1 mutations and inherited bleeding. Platelets 2017, 28, 208–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melazzini, F.; Zaninetti, C.; Balduini, C.L. Bleeding is not the main clinical issue in many patients with inherited thrombocytopaenias. Haemophilia 2017, 23, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Pecci, A.; Klersy, C.; Gresele, P.; Lee, K.J.; De Rocco, D.; Bozzi, V.; Russo, G.; Heller, P.G.; Loffredo, G.; Ballmaier, M.; et al. MYH9-related disease: A novel prognostic model to predict the clinical evolution of the disease based on genotype-phenotype correlations. Hum. Mutat. 2014, 35, 236–247. [Google Scholar] [CrossRef] [Green Version]
- Bury, L.; Megy, K.; Stephens, J.C.; Grassi, L.; Greene, D.; Gleadall, N.; Althaus, K.; Allsup, D.; Bariana, T.K.; Bonduel, M.; et al. Next-generation sequencing for the diagnosis of MYH9 -RD: Predicting pathogenic variants. Hum. Mutat. 2019, 41, 277–290. [Google Scholar] [CrossRef] [Green Version]
- Falcinelli, E.; Bury, L.; Gresele, P. Inherited platelet function disorders. Hämostaseologie 2016, 36, 265–278. [Google Scholar] [CrossRef]
- Makris, M.; Conlon, C.P.; Watson, H.G. Immunization of patients with bleeding disorders. Haemophilia 2003, 9, 541–546. [Google Scholar] [CrossRef]
- Bolton-Maggs, P.; Chalmers, E.A.; Collins, P.W.; Harrison, P.; Kitchen, S.; Liesner, R.J.; Minford, A.; Mumford, A.D.; Parapia, L.A.; Perry, D.J.; et al. A review of inherited platelet disorders with guidelines for their management on behalf of the UKHCDO. Br. J. Haematol. 2006, 135, 603–633. [Google Scholar] [CrossRef]
- Buijs, A.; Poddighe, P.; van Wijk, R.; van Solinge, W.; Borst, E.; Verdonck, L.; Hagenbeek, A.; Pearson, P.; Lokhorst, H. A novel CBFA2 single-nucleotide mutation in familial platelet disorder with propensity to develop myeloid malig-nancies. Blood 2001, 98, 2856–2858. [Google Scholar] [CrossRef] [Green Version]
- Demers, C.; Derzko, C.; David, M.; Douglas, J. Gynaecological and obstetric management of women with inherited bleeding disorders. Int. J. Gynaecol. Obstet. 2006, 95, 75–87. [Google Scholar] [CrossRef]
- Sogut, O.; Erdogan, M.O.; Kose, R.; Boleken, M.E.; Kaya, H.; Gokdemir, M.T.; Ozgonul, A.; Iynen, I.; Albayrak, L.; Dokuzoglu, M.A. Hemostatic Efficacy of a Traditional Medicinal Plant Extract (Ankaferd Blood Stopper) in Bleeding Control. Clin. Appl. Thromb. 2015, 21, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P. Subcommittee on Platelet Physiology. The use of platelets in regenerative medicine and proposal for a new classification system: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1895–1900. [Google Scholar] [CrossRef] [PubMed]
- Nurden, P.; Youlouz-Marfak, I.; Siberchicot, F.; Kostrzewa, E.; Andia, I.; Anitua, E.; Nurden, A.T. Use of autologous platelet-rich clots for the prevention of local injury bleeding in patients with severe inherited mucocutaneous bleeding disorders. Haemophilia 2011, 17, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Randhawa, M.S.; Ganesamoni, R.; Singh, S.K. Tranexamic Acid Reduces Blood Loss During Percutaneous Nephrolithotomy: A Prospective Randomized Controlled Study. J. Urol. 2013, 189, 1757–1761. [Google Scholar] [CrossRef] [PubMed]
- Vujkovac, B.; Sabovic, M. A successful treatment of life-threatening bleeding from polycystic kidneys with anti-fibrinolytic agent tranexamic acid. Blood Coagul. Fibrinolysis 2006, 17, 589–591. [Google Scholar] [CrossRef]
- Dunn, C.J.; Goa, K.L. Tranexamic acid: A review of its use in surgery and other indications. Drugs 1999, 57, 1005–1032. [Google Scholar] [CrossRef]
- Colucci, G.; Stutz, M.; Rochat, S.; Conte, T.; Pavicic, M.; Reusser, M.; Giabbani, E.; Huynh, A.; Thürlemann, C.; Keller, P.; et al. The effect of desmopressin on platelet function: A selective enhancement of procoagulant COAT platelets in patients with primary platelet function defects. Blood 2014, 123, 1905–1916. [Google Scholar] [CrossRef] [Green Version]
- Tosetto, A.; Balduini, C.L.; Cattaneo, M.; De Candia, E.; Mariani, G.; Molinari, A.C.; Rossi, E.; Siragusa, S. Italian Society for Haemostasis and Thrombosis. Management of bleeding and of invasive procedures in patients with platelet disorders and/or thrombocytopenia: Guidelines of the Italian Society for Haemostasis and Thrombosis (SISET). Thromb. Res. 2009, 124, 13–18. [Google Scholar] [CrossRef]
- Sehbai, A.S.; Abraham, J.; Brown, V.K. Perioperative management of a patient with May-Hegglin anomaly requiring craniotomy. Am. J. Hematol. 2005, 79, 303–308. [Google Scholar] [CrossRef]
- Poon, M.C. The evidence for the use of recombinant human activated factor VII in the treatment of bleeding patients with quantitative and qualitative platelet disorders. Transfus. Med. Rev. 2007, 21, 223–236. [Google Scholar] [CrossRef]
- Franchini, M. The use of recombinant activated factor VII in the treatment of bleeding patients with quantitative and qualitative platelet disorders. Blood Transfus. 2009, 7, 24–28. [Google Scholar] [PubMed]
- Pecci, A.; Gresele, P.; Klersy, C.; Savoia, A.; Noris, P.; Fierro, T.; Bozzi, V.; Mezzasoma, A.M.; Melazzini, F.; Balduini, C.L. Eltrombopag for the treatment of the inherited thrombocytopenia deriving from MYH9 mutations. Blood 2010, 116, 5832–5837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaninetti, C.; Gresele, P.; Bertomoro, A.; Klersy, C.; De Candia, E.; Veneri, D.; Barozzi, S.; Fierro, T.; Alberelli, M.A.; Musella, V.; et al. Eltrombopag for the treatment of inherited thrombocytopenias: A phase 2 clinical trial. Haematologica 2020, 105, 820–828. [Google Scholar] [CrossRef] [Green Version]
- Gerrits, A.J.; Leven, E.A.; Frelinger, A.L.; Brigstocke, S.L.; Berny-Lang, M.A.; Mitchell, W.B.; Revel-Vilk, S.; Tamary, H.; Carmichael, S.L.; Barnard, M.R.; et al. Effects of eltrombopag on platelet count and platelet activation in Wiskott-Aldrich syndrome/X-linked thrombocytopenia. Blood 2015, 126, 1367–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pecci, A.; Verver, E.J.; Schlegel, N.; Canzi, P.; Boccio, C.M.; Platokouki, H.; Krause, E.; Benazzo, M.; Topsakal, V.; Greinacher, A. Cochlear implantation is safe and effective in patients with MYH9-related disease. Orphanet. J. Rare Dis. 2014, 9, 100. [Google Scholar] [CrossRef] [Green Version]
- Favier, R.; Feriel, J.; Favier, M.; Denoyelle, F.; Martignetti, J.A. First Successful Use of Eltrombopag Before Surgery in a Child With MYH9-Related Thrombocytopenia. Pediatrics 2013, 132, e793–e795. [Google Scholar] [CrossRef] [Green Version]
- Zaninetti, C.; Barozzi, S.; Bozzi, V.; Gresele, P.; Balduini, C.L.; Pecci, A. Eltrombopag in preparation for surgery in patients with severe MYH9-related thrombocytopenia. Am. J. Hematol. 2019, 94, e199–e201. [Google Scholar] [CrossRef] [Green Version]
- Paciullo, F.; Bury, L.; Gresele, P. Eltrombopag to allow chemotherapy in a patient with MYH9-related inherited thrombocytopenia and pancreatic cancer. Int. J. Hematol. 2020, 112, 1–3. [Google Scholar] [CrossRef]
- Moratto, D.; Giliani, S.; Bonfim, C.; Mazzolari, E.; Fischer, A.; Ochs, H.D.; Cant, A.J.; Thrasher, A.J.; Cowan, M.J.; Albert, M.H.; et al. Long-term outcome and lineage-specific chimerism in 194 patients with Wiskott-Aldrich syndrome treated by hematopoietic cell transplantation in the period 1980–2009: An international collaborative study. Blood 2011, 118, 1675–1684. [Google Scholar] [CrossRef] [Green Version]
- Ballmaier, M.; Germeshausen, M. Congenital Amegakaryocytic Thrombocytopenia: Clinical Presentation, Diagnosis, and Treatment. Semin. Thromb. Hemost. 2011, 37, 673–681. [Google Scholar] [CrossRef]
- Bosticardo, M.; Marangoni, F.; Aiuti, A.; Villa, A.; Grazia Roncarolo, M. Recent advances in understanding the pathophysiology of Wiskott-Aldrich syndrome. Blood 2009, 113, 6288–6295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locatelli, F.; Rossi, G.; Balduini, C. Hematopoietic stem-cell transplantation for the Bernard-Soulier syndrome. Ann. Intern. Med. 2003, 138, 79. [Google Scholar] [CrossRef] [PubMed]
- Oshima, K.; Imai, K.; Albert, M.H.; Bittner, T.C.; Strauss, G.; Filipovich, A.H.; Morio, T.; Kapoor, N.; Dalal, J.; Schultz, K.R.; et al. Hematopoietic stem cell transplantation for X-linked thrombo-cytopenia with mutations in the WAS gene. J. Clin. Immunol. 2015, 35, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Aiuti, A.; Biasco, L.; Scaramuzza, S.; Ferrua, F.; Cicalese, M.P.; Baricordi, C.; Dionisio, F.; Calabria, A.; Giannelli, S.; Castiello, M.C.; et al. Lentiviral Hematopoietic Stem Cell Gene Therapy in Patients with Wiskott-Aldrich Syndrome. Science 2013, 341, 1233151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hacein-Bey Abina, S.; Gaspar, H.B.; Blondeau, J.; Caccavelli, L.; Charrier, S.; Buckland, K.; Picard, C.; Six, E.; Himoudi, N.; Gilmour, K.; et al. Outcomes following gene therapy in patients with severe Wiskott-Aldrich syndrome. JAMA 2015, 313, 1550–1563. [Google Scholar] [CrossRef]
- Pala, F.; Morbach, H.; Castiello, M.C.; Schickel, J.N.; Scaramuzza, S.; Chamberlain, N.; Cassani, B.; Glauzy, S.; Romberg, N.; Candotti, F.; et al. Lentiviral mediated gene therapy restores B cell tolerance in Wiskott-Aldrich syndrome patients. J. Clin. Investig. 2015, 125, 3941–3951. [Google Scholar] [CrossRef] [Green Version]
- Kanaji, S.; Kuether, E.L.; Fahs, S.A.; Schroeder, J.A.; Ware, J.; Montgomery, R.R.; Shi, Q. Correction of murine Bernard-Soulier syndrome by lentivirus-mediated gene therapy. Mol. Ther. 2012, 20, 625–632. [Google Scholar] [CrossRef] [Green Version]
- Albert, M.H.; Bittner, T.C.; Nonoyama, S.; Notarangelo, L.D.; Burns, S.; Imai, K.; Espanol, T.; Fasth, A.; Pellier, I.; Strauss, G.; et al. X-linked thrombocytopenia (XLT) due to WAS mutations: Clinical characteristics, long-term outcome, and treatment options. Blood 2010, 115, 3231–3238. [Google Scholar] [CrossRef] [Green Version]
- Mullen, C.A.; Anderson, K.D.; Blaese, R.M. Splenectomy and/or bone marrow transplantation in the management of the Wiskott-Aldrich syndrome: Long-term follow-up of 62 cases. Blood 1993, 82, 2961–2966. [Google Scholar] [CrossRef] [Green Version]

| Defective Step of Thrombopoiesis | Affected Gene | Disorder | Pathogenic Mechanism (Reference) | Additional Features (e.g., Syndromic Manifestations, Predisposition) |
|---|---|---|---|---|
| Defective megakaryocyte maturation | ANKRD26 | ANKRD26-related thrombocytopenia | Loss of ANKRD26 silencing during the last phases of megakaryocytopoiesis causes ERK1/2 phosphorylation that interferes with megakaryocyte maturation [13] | Predisposition to hematological malignancies |
| ETV6 | ETV6-related thrombocytopenia | ETV6 is a transcriptional repressor that promotes the late phases of megakaryopoiesis. Mutations in ETV6 cause defective megakaryocyte maturation and impaired proplatelet formation [14] | Predisposition to hematological malignancies | |
| FLI1 | FLI1-related thrombocytopenia | FLI1 is a transcription factor regulating many genes associated with megakaryocyte development. Therefore, FLI1 mutations promote defective megakaryocyte maturation [15] | Not reported | |
| FLI1 deletion | Paris-Trousseau syndrome/Jacobsen syndrome | Abnormalities of heart and face, intellectual disabilities | ||
| FYB | FYB-related thrombocytopenia | ADAP is a protein involved in the remodeling of cytoskeleton. Mutations in ADAP cause defective maturation of megakaryocytes and clearance of platelets [16] | Mild iron deficiency anemia | |
| GATA1 | GATA1-relate disease | GATA1 is a transcription factor regulating many genes associated with megakaryocyte development therefore GATA1 defects cause alterations of megakaryocyte maturation [17] | Dyserythropoietic anemia, beta-thalassemia, congenital erythropoietic porphyria, splenomegaly | |
| GFI1B | GFI1B-related thrombocytopenia | GFI1B is a transcription factor involved in homeostasis of hematopoietic stem cells and development of megakaryocytes therefore GFI1B defects cause alterations of megakaryocyte maturation [18] | Mild myelofibrosis | |
| HOXA11 | Amegakaryocytic thrombocytopenia with radio-ulnar synostosis | HOXA11 is a transcription factor involved in the regulation of early hematopoiesis, its defect causes reduced number of megakaryocytes [19] | Bilateral radioulnar synostosis, severe bone marrow failure culminating in aplastic anemia in majority of cases, cardiac and renal malformations, hearing loss, clinodactyly, skeletal abnormalities, pancytopenia | |
| MECOM | MECOM is a transcription factor involved in the regulation of early hematopoiesis, its defect causes reduced number of megakaryocytes [20] | |||
| IKZF5 | IKZF5-related thrombocytopenia | IKZF5 is a previously unknown transcriptional regulator of megakaryopoiesis [21] | Not reported | |
| MPL | Congenital amegakaryocytic thrombocytopenia | MPL is the receptor for thrombopoietin. MPL defects cause impaired thrombopoietin binding and thus impaired megakaryocyte maturation [22] | Acquired bone marrow aplasia | |
| NBEAL2 | Gray platelet syndrome | Mutations in NBEAL2 cause impaired megakaryocyte maturation however its role in megakaryocytopoiesis is not clear [23] | Myelofibrosis, immune dysregulation (autoimmune diseases, positive autoantibodies, reduced leukocyte counts), proinflammatory profile | |
| RBM8A | Thrombocytopenia-absent radius | RBM8A is a protein of the exon-junction complex involved in RNA processing. It has been hypothesized that RBM8A defects cause wrong mRNA processing of unknown components of the TPO-MPL pathway impairing megakaryocyte maturation [24] | Bilateral radial aplasia, anemia, skeletal, urogenital, kidney, heart defects | |
| RUNX1 | Familial platelet disorder with predisposition to hematological malignancies | RUNX1 is a transcription factor regulating many genes associated with megakaryocyte development therefore RUNX1 mutations promote defective megakaryocyte maturation [25] | Predisposition to hematological malignancies | |
| THPO | THPO-related disease | THPO is the gene for thrombopoietin, essential for hematopoietic stem cell survival and megakaryocyte maturation [26] | Bone marrow aplasia | |
| Defective platelet production/increased clearance | ACTB | Baraitser–Winter syndrome 1 with macrothrombocytopenia | Mutations in β-cytoplasmic actin inhibit the final stages of platelet maturation by compromising microtubule organization [27] | Microcephaly, facial anomalies, mild intellectual disability, developmental delay |
| ACTN1 | ACTN1-related thrombocytopenia | ACTN-1 is involved in cytoskeletal remodeling, defects in ACTN-1 cause defective proplatelet formation [28] | Not reported | |
| ARPC1B | Platelet abnormalities with eosinophilia and immune-mediated inflammatory disease | The actin-related protein 2/3 complex (Arp2/3) is a regulator of the actin cytoskeleton and its mutation causes impaired proplatelet formation [29] | Immunodeficiency, systemic inflammation, vasculitis, inflammatory colitis, eosinophilia, eczema, lymphadenomegaly, hepato-splenomegaly, growth failure | |
| CYCS | CYCS-related thrombocytopenia | CYCS is a mitochondrial protein with a role in respiration and apoptosis. Mutations in CYCS cause ectopic premature proplatelet formation with an unknown mechanism [30] | Not reported | |
| DIAPH1 | DIAPH1-related thrombocytopenia | DIAPH1 is involved in cytoskeletal remodeling, defects in DIAPH1 cause defective proplatelet formation [31] | Hearing loss | |
| FLNA | FLNA-related thrombocytopenia | Filamin A is involved in cytoskeletal remodeling, defects in FLNA cause defective proplatelet formation [32] | Periventricular nodular heterotopia and otopalatodigital syndrome spectrum of disorders | |
| GP1BA, GP1BB, GP9 (loss of function) | Bernard–Soulier syndrome monoallelic | The intracellular portion of the GPIb/IX/V complex links the receptor to the cytoskeleton. Disruption of this link causes impaired proplatelet formation [33] | Not reported | |
| Bernard–Soulier syndrome biallelic | ||||
| GP1BA (gain of function) | Platelet-type von Willebrand disease | The extracellular portion of the GPIb/IX/V complex binds VWF. Constitutive binding of VWF to its receptor triggers the Src kinases pathway causing impaired proplatelet formation, ectopic platelet production and increased platelet clearance [34] | Not reported | |
| ITGA2B, ITGB3 | ITGA2B/ITGB3-related thrombocytopenia | Constitutive activation of αIIbβ3 causes cytoskeletal perturbation leading to impaired proplatelet formation [35,36] | Not reported | |
| KDSR | Thrombocytopenia and erythrokeraderma | KDSR is an essential enzyme for de novo sphingolipid synthesis, this suggests an important role for sphingolipids as regulators of cytoskeletal organization during megakaryopoiesis and proplatelet formation [37] | Dermatologic involvement ranging from hyperkeratosis/ erythema to ichthyosis. One family with no or very mild skin lesions but associated anemia has been reported | |
| MYH9 | MYH9-related disorder | MYH9 regulates cytoskeleton remodeling and mediates signal transduction pathways involved in proplatelet formation. Abnormalities of MYH9 cause hyperactivation of the Rho/ROCK pathway causing ectopic platelet formation [38] | Kidney disease, cataract, deafness, elevated liver enzymes | |
| MPIG6B | Thrombocytopenia, anemia and myelofibrosis | G6b-B is a transmembrane receptor with an ITIM motif with a not well defined role in proplatelet formation [39] | Microcitic anemia, myelofibrosis, leukocytosis may be present | |
| PRKACG | PRKACG-related thrombocytopenia | PKA activates many proteins involved in megakaryocyte and platelet function, among them FLNa and GPIbβ therefore its dysfunction causes impaired proplatelet formation [40] | Not reported | |
| STIM1 | Stormorken syndrome | STIM1 mutations cause a constitutively active store operated Ca2+ release-activated Ca2+ (CRAC) channel which triggers Ca2+ entry with consequent increased clearance of activated platelets [41] | Tubular myopathy and congenital myosis. Severe immune dysfunction | |
| TRPM7 | TRPM7-related thrombocytopenia | Defects of the Mg2+ channel TRPM7, a regulator of embryonic development and cell survival, cause cytoskeletal alterations resulting in impaired proplatelet formation [42] | Atrial fibrillation | |
| TPM4 | TPM4-related thrombocytopenia | Tropomyosin 4 is an actin cytoskeletal regulator. Insufficient TPM4 expression in human and mouse megakaryocytes resulted in a defect in the terminal stages of platelet production [43] | Not reported | |
| TUBB1 | TUBB1-related thrombocytopenia | Tubulin beta1 is a major component of microtubules therefore defects in TUBB1 cause impaired proplatelet formation [44] | Not reported | |
| WAS | Wiskott–Aldrich syndrome | The WASP protein is a regulator of the actin cytoskeleton and its defect causes ectopic platelet formation and increased platelet clearance [45] | Immunodeficiency, hematopoietic malignancies, eczema, autoimmune hemolytic anemia. | |
| X-linked thrombocytopenia | Not reported | |||
| Other/unknown pathogenic mechanism | ABCG5, ABCG8 | Thrombocytopenia associated with sitosterolemia | ABCG5 and ABCG8 regulate plant sterol and cholesterol absorption. It is supposed that sterol-enriched platelets are more rapidly cleared [46] | Xanthomas and pre-mature coronary atherosclerosis due to hypercholesterolemia |
| CDC42 | Takenouchi-Kosaki syndrome with macrothrombocytopenia | CDC42 is a critical molecule in various biological processes including the cell cycle, cell division, and the formation of the actin cytoskeleton [47] | Defective growth and psychomotor development, intellectual disability, facial abnormalities, brain malformation, muscle tone abnormalities, immunodeficiency, eczema, hearing/visual disability, lymphedema, cardiac, genitourinary, and/or skeletal malformations | |
| GNE | GNE-related thrombocytopenia | GNE encodes an enzyme involved in the sialic acid biosynthesis pathway and it is known that thrombocytopenia is associated with increased platelet desialylation [48] | Some patients presented myopathy with rimmed vacuoles with onset in early adulthood | |
| SLNF14 | SLNF14-related thrombocytopenia | SLNF14 is an endoribonuclease and its role in the generation of thrombocytopenia is unknown [49] | Not reported | |
| SRC | SRC-related thrombocytopenia | Src-family kinase regulates multiple signaling pathways, its role in the generation of thrombocytopenia is unknown [50] | Myelofibrosis, bone pathologies, bone marrow dysplasia, splenomegaly, congenital facial dysmorphism | |
| PTPRJ | PTPRJ-related thrombocytopenia | PTPRJ is a protein tyrosine phosphatase expressed abundantly in platelets and megakaryocytes, its role in the generation of thrombocytopenia is unknown [51] | None |
| Disease | Inheritance | Gene | Bleeding Diathesis |
|---|---|---|---|
| Arthrogryposis, renal dysfunction and cholestasis | AR | VPS33B VIPAS39 | Severe |
| CalDAG-GEFI related platelet disorder | AR | RASGRP2 | Moderate-severe |
| Cediak-Higashi Syndrome | AR | CHS1 | Moderate-severe |
| Combined alpha-delta granule deficiency | AR/AD | Unknown | Mild-moderate |
| COX-1 deficiency | AR/AD | PTGSA | Moderate-severe |
| Delta granule deficiency | AR/AD | Unknown | Mild-moderate |
| Glanzmann thrombasthenia | AR | ITGA2B, ITGB3 | Moderate-severe |
| Glycoprotein IV (GPIV) deficiency | AR | GP4 | Mild |
| Glycoprotein VI (GPVI) deficiency | AR | GP6 | Mild |
| Gs platelet defect | AD (if paternally inherited) | GNAS | Mild |
| Hermansky–Pudlak syndrome | AR | HPS1, ADTB3A, HPS3, HPS4, HPS5, HPS6, DTNBP1, BLOC1S3, AP3D1, BLOC1S6 | Moderate-severe |
| Leukocyte adhesion deficiency, type III | AR | FERMT3 | Moderate-severe |
| P2Y12 deficiency | AR | P2RY12 | Moderate-severe |
| Phospholipase A2 (cPLA2) deficiency | not determined | PLA2G4A | Moderate-severe |
| PKCδ deficiency | AR | PRKCD | Absent |
| Primary secretion defect | AR/AD | Unknown | Mild-moderate |
| Quebec platelet disorder | AD | PLAU | Moderate-severe |
| Scott syndrome | AR | TMEM16F | Mild-moderate |
| Thromboxane A2 receptor defect | AD | TBXA2R | Mild |
| Tx synthase deficiency | AD/AR | TBXAS1 | Moderate |
| Form | Disease | Inheritance | Degree of Thrombocytopenia | Key Laboratory Features | References |
|---|---|---|---|---|---|
| Syndromic | Amegakaryocytic thrombocytopenia with radio-ulnar synostosis (ATRUS) | AD | severe | Normal platelet size and morphology | [19,20] |
| Baraitser–Winter syndrome 1 with macrothrombocytopenia | AD | absent | Macrothrombocytopenia; leukocytosis with eosinophilia, leukopenia | [27] | |
| FLNA-related thrombocytopenia | XL | moderate | Macrothrombocytopenia; impaired platelet aggregation GPVI-triggered; heterogeneous α-granules, occasionally giant; abnormal distribution of FLNa | [32] | |
| GATA-1-related disease | XL | severe | Macrothrombocytopenia; reduced platelet aggregation by collagen and ristocetin; reduced α-granule content and release | [17] | |
| GNE-related thrombocytopenia | AR | from mild to severe | Macrothrombocytopenia | [48] | |
| Gray platelet syndrome | AR | moderate/severe | Macrothrombocytopenia; grey or pale platelets; dyserytropoiesis; absence of α-granules; defective TRAP-induced platelet aggregation | [23] | |
| Paris-Trousseau thrombocytopenia, Jacobsen syndrome | AD | severe | Macrothrombocytopenia; defective platelet aggregation by thrombin; giant α-granules | [15] | |
| Platelet abnormalities with eosinophilia and immune-mediated inflammatory disease | AR | moderate | Small platelets; eosinophilia; reduced platelet spreading; decreased platelet dense granules | [29] | |
| PTPRJ-related thrombocytopenia | AR | moderate/severe | Microthrombocytopenia; impaired activation by the GPVI-specific agonist convulxin and the thrombin receptor-activating peptide but normal response to ADP | [51] | |
| SRC-related thrombocytopenia | AD | moderate/severe | Platelets deficient in granules and rich in vacuoles | [50] | |
| Stormorken syndrome | AD | moderate/severe | Howell-Jolly bodies in red blood cells; enhanced annexin V binding, defective GPIIb/IIIa activation (PAC-1) | [41] | |
| Takenouchi-Kosaki syndrome with macrothrombocytopenia | AD | absent | Macrothrombocytopenia, abnormal platelet spreading and filopodia formation | [47] | |
| Thrombocytopenia-absent radius syndrome (TAR) | AR | severe | Normal platelet size and morphology, thrombocytopenia | [24] | |
| Thrombocytopenia and erythrokeraderma | AR | moderate | Thrombocytopenia and presence of 3-keto-dihydrosphingosine in plasma | [37] | |
| Thrombocytopenia, anemia and myelofibrosis | AR | mild/moderate | Macrothrombocytopenia, anemia | [39] | |
| Wiskott–Aldrich syndrome | XL | severe | Microthrombocytopenia; Reduced α/δ granules release | [45] | |
| X-linked thrombocytopenia | XL | mild/moderate | Microthrombocytopenia; Reduced α/δ granules release | [45] | |
| Non-syndromic | ACTN1-related thrombocytopenia | AD | mild | Macrothrombocytopenia | [28] |
| Bernard Soulier syndrome monoallelic biallelic | AD AR | mild moderate/severe | Macrothrombocytopenia; lack of platelet agglutination to ristocetin with normal aggregation to other agonists; severe reduction or complete lack of GPIb/IX/V | [33] | |
| CYCS-related thrombocytopenia | AD | mild | Normal platelet size and morphology | [30] | |
| FLI1-related thrombocytopenia | AD/AR | moderate | Reduced platelet aggregation in response to collagen and PAR-1 agonists; δ-granule deficiency | [15] | |
| FYB-related thrombocytopenia | AR | moderate/severe | Microthrombocytopenia; increased expression of P-selectin and PAC-1 by resting platelets but impaired upon stimulation with ADP | [16] | |
| GFI1b-related thrombocytopenia | AD/AR | mild/moderate | Macrothrombocytopenia; dyserytropoiesis; reduced α-granule content and release; diminished expression of GPIbα, red cell anisocytosis | [18] | |
| IKZF5-related thrombocytopenia | AD | absent | Thrombocytopenia; deficiency of platelet alpha granules. | [21] | |
| ITGA2B/ITGB3-related thrombocytopenia | AD | mild/moderate | Macrothrombocytopenia; reduced GPIIb/IIIa; defective GPIIb/IIIa activation (PAC-1) | [35,36,54] | |
| PT-VWD | AD | mild/moderate | Macrothrombocytopenia; increased response to ristocetin and decreased VWF-ristocetin cofactor activity (VWF:RCo) Mixing tests discriminate the plasmatic (VWD type2B) from platelet (PT-VWD) origin of hyperreactivity to ristocetin | [36,76,77] | |
| PRKACG-related thrombocytopenia | AR | severe | Macrothrombocytopenia; defective platelet αIIbβ3 activation and P-selectin exposure in response to TRAP6; defective Ca2+ mobilization in response to thrombin | [40] | |
| THPO-related thrombocytopenia | AD | mild | Normal or slightly increased platelet size | [26] | |
| TRPM7-related thrombocytopenia | AD | mild/moderate | Macrothrombocytopenia; aberrant distribution of granules | [42] | |
| Tropomyosin 4 (TPM)-related thrombocytopenia | AD | mild | Macrothrombocytopenia | [43] | |
| TUBB-1-related thrombocytopenia | AD | mild | Macrothrombocytopenia; platelet anisocytosis | [44] | |
| SLFN14-related thrombocytopenia | AD | mild/moderate | Macrothrombocytopenia; δ-granule deficiency with decreased ATP secretion in response to ADP, collagen and TRAP-6 | [49] | |
| Forms predisposing to additional diseases | ANKRD26-related thrombocytopenia | AD | mild/moderate | Reduced α-granules in some patients | [13] |
| Congenital amegakaryocytic thrombocytopenia (CAMT) | AR | severe | Elevated serum levels of TPO | [22] | |
| DIAPH1-related thrombocytopenia | AD | mild/severe | Macrothrombocytopenia | [31] | |
| ETV6-related thrombocytopenia | AD | mild/moderate | Decreased ability of platelets to spread on fibrinogen covered surfaces; abnormal clot retraction | [14] | |
| Familial platelet disorder with predisposition to hematological malignancies (FPD/AML) | AD | moderate | Abnormal aggregation in response to multiple agonists; δ (occasionally α)-granule deficiency | [25] | |
| MYH9-related disease | AD | mild/severe | Macrothrombocytopenia; Döhl-like body cytoplasmic leukocyte inclusions | [38] | |
| Thrombocytopenia associated with sitosterolemia | moderate/severe | Macrothrombocytopenia; hyperactivatable platelets with constitutive binding of fibrinogen to αIIbβ3 integrin; shedding of GPIbα; impaired platelet adhesion to von Willebrand factor | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bury, L.; Falcinelli, E.; Gresele, P. Learning the Ropes of Platelet Count Regulation: Inherited Thrombocytopenias. J. Clin. Med. 2021, 10, 533. https://doi.org/10.3390/jcm10030533
Bury L, Falcinelli E, Gresele P. Learning the Ropes of Platelet Count Regulation: Inherited Thrombocytopenias. Journal of Clinical Medicine. 2021; 10(3):533. https://doi.org/10.3390/jcm10030533
Chicago/Turabian StyleBury, Loredana, Emanuela Falcinelli, and Paolo Gresele. 2021. "Learning the Ropes of Platelet Count Regulation: Inherited Thrombocytopenias" Journal of Clinical Medicine 10, no. 3: 533. https://doi.org/10.3390/jcm10030533
APA StyleBury, L., Falcinelli, E., & Gresele, P. (2021). Learning the Ropes of Platelet Count Regulation: Inherited Thrombocytopenias. Journal of Clinical Medicine, 10(3), 533. https://doi.org/10.3390/jcm10030533






