Otosurgery with the High-Definition Three-Dimensional (3D) Exoscope: Advantages and Disadvantages
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Groups’ Characteristics
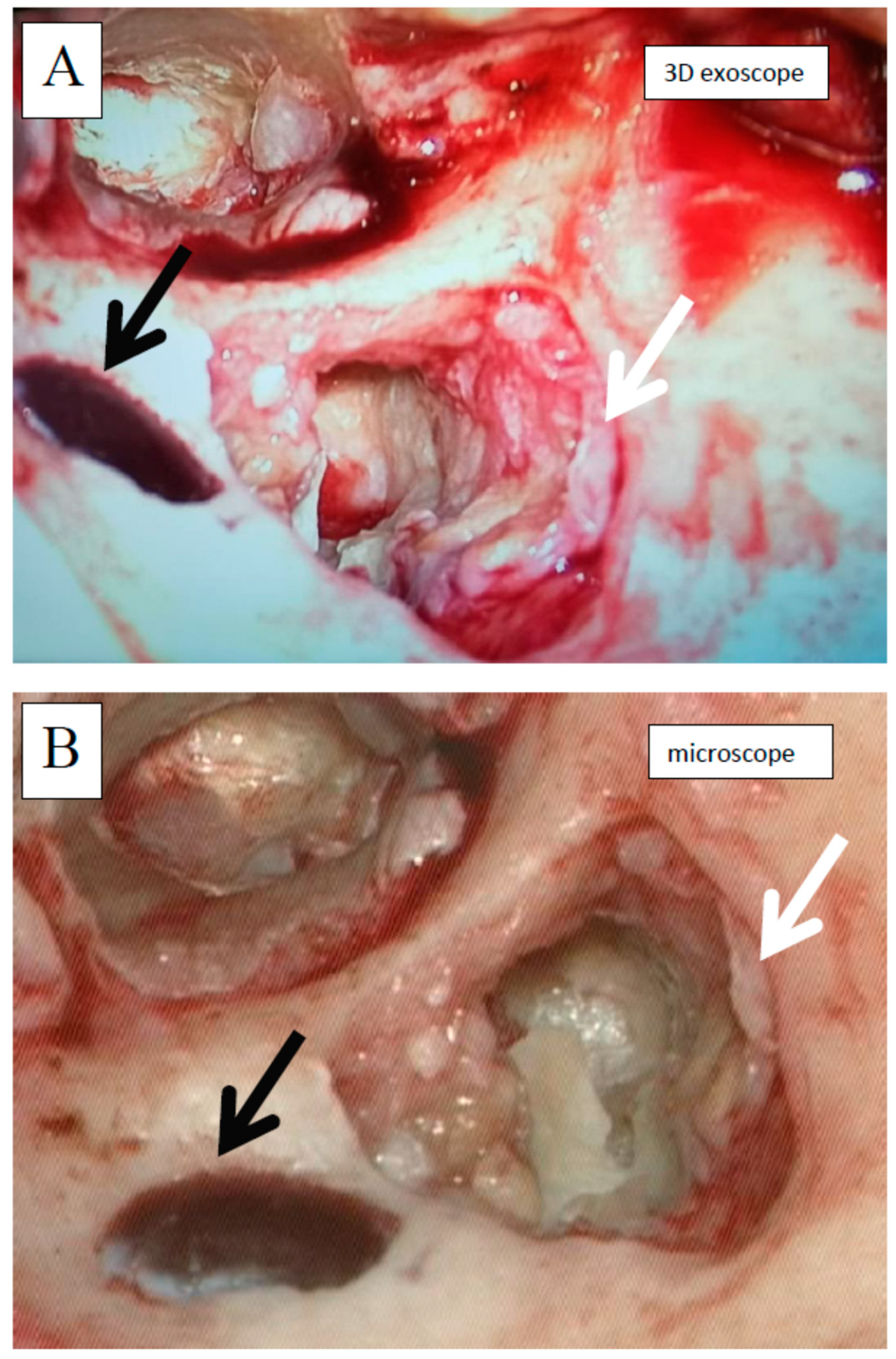
2.4. Equipment Description
2.5. Assessment Tools
2.6. Outcome Measures
2.7. Statistical Analysis
3. Results
3.1. Stapedotomy
3.2. Tympanoplasty
4. Discussion
Study Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langer, D.J.; White, T.G.; Schulder, M.; A Boockvar, J.; Labib, M.; Lawton, M.T. Advances in Intraoperative Optics: A Brief Review of Current Exoscope Platforms. Oper. Neurosurg. 2020, 19, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y.; Tamura, R.; Takahashi, S.; Morimoto, Y.; Sato, M.; Horikoshi, T.; Hassaan, S.; Yoshida, K.; Toda, M. A Comparative Study Between Traditional Microscopic Surgeries and Endoscopic Endonasal Surgery for Skull Base Chordomas. World Neurosurg. 2020, 134, e1099–e1107. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, G.M.; Certo, F. Three-Dimensional, High-Definition Exoscopic Anterior Cervical Discectomy and Fusion: A Valid Alternative to Microscope-Assisted Surgery. World Neurosurg. 2019, 130, e244–e250. [Google Scholar] [CrossRef] [PubMed]
- Garneau, J.C.; Laitman, B.M.; Cosetti, M.K.; Hadjipanayis, C.; Wanna, G. The Use of the Exoscope in Lateral Skull Base Surgery. Otol. Neurotol. 2019, 40, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Harput, M.V.; Türe, U. Commentary: First-in-Man Clinical Experience Using a High-Definition 3-Dimensional Exoscope System for Microneurosurgery. Oper. Neurosurg. 2019, 17, E85–E87. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Kozin, E.D.; Kanumuri, V.V.; Barber, S.R.; Backous, D.; Nogueira, J.F.; Lee, D.J. Initial Experience with 3-Dimensional Exoscope-Assisted Transmastoid and Lateral Skull Base Surgery. Otolaryngol. Neck Surg. 2019, 160, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, K. From Exoscope into the Next Generation. J. Korean Neurosurg. Soc. 2017, 60, 289–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricciardi, L.; Chaichana, K.L.; Cardia, A.; Stifano, V.; Rossini, Z.; Olivi, A.; Sturiale, C.L. The Exoscope in Neurosurgery: An Innovative “Point of View”. A Systematic Review of the Technical, Surgical, and Educational Aspects. World Neurosurg. 2019, 124, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Minoda, R.; Miwa, T. Non-microscopic Middle Ear Cholesteatoma Surgery. Otol. Neurotol. 2019, 40, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Ferreli, F.; Di Bari, M.; Cugini, G.; Miceli, S.; De Virgilio, A.; Spriano, G.; Poletti, A. Introducing the High-definition 3D exoscope in ear surgery: Preliminary analysis of advantages and limits compared with operative microscope. Eur. Arch. Oto-Rhino-Laryngology 2021, 1–7. [Google Scholar] [CrossRef]
- Yung, M.; Tono, T.; Olszewska, E.; Yamamoto, Y.; Sudhoff, H.; Sakagami, M.; Mulder, J.; Kojima, H.; Incesulu, A.; Trabalzini, F.; et al. EAONO/JOS Joint Consensus Statements on the Definitions, Classification and Staging of Middle Ear Cholesteatoma. J. Int. Adv. Otol. 2017, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.G.; Schöller, K.; Uhl, E. Application of a Compact High-Definition Exoscope for Illumination and Magnification in High-Precision Surgical Procedures. World Neurosurg. 2017, 97, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Pinto, V.; Giorgini, F.A.; Miralles, M.E.L.; Tarsitano, A.; Panella, M.M.; Cipriani, R.; Pignatti, M. 3D Exoscope-Assisted Microvascular Anastomosis: An Evaluation on Latex Vessel Models. J. Clin. Med. 2020, 9, 3373. [Google Scholar] [CrossRef] [PubMed]
- Preul, M.C.; Belykh, E.; George, L.; Zhao, X.; Carotenuto, A.; Moreira, L.B.; Yağmurlu, K.; Bozkurt, B.; Byvaltsev, V.A.; Nakaji, P. Microvascular anastomosis under 3D exoscope or endoscope magnification: A proof-of-concept study. Surg. Neurol. Int. 2018, 9, 115. [Google Scholar] [CrossRef] [PubMed]






| Visualization Grade: 3D Exoscope Relative to Microscope | ||||||
|---|---|---|---|---|---|---|
| Stapedotomy—Stages of the Procedure | WORSE (−1) | COMPARABLE (0) | SUPERIOR (1) | SCORE (Sum of Points) | p-Value (Test of Proportion) | |
| S1 | Initial view of the operating field | 0 (0.0%) | 4 (13.3%) | 26 (86.7%) | 26 | 0.0001 |
| S2 | Tympanomeatal flap formation | 4 (13.3%) | 0 (0.0%) | 26 (86.7%) | 22 | 0.0001 |
| S3 | Tympanic cavity opening | 4 (13.3%) | 4 (13.3%) | 22 (73.4%) | 18 | 0.0104 |
| S4 | External auditory canal widening | 4 (13.3%) | 3 (10.0%) | 23 (76.7%) | 19 | 0.0034 |
| S5 | Incudostapedial joint visualization | 7 (23.3%) | 4 (13.3%) | 19 (63.4%) | 12 | 0.1421 |
| S6 | Oval window area assessment | 7 (23.3%) | 6 (20.0%) | 17 (56.7%) | 10 | 0.4630 |
| S7 | Stapes superstructure removal | 18 (60.0%) | 8 (26.7%) | 4 (13.3%) | −14 | 0.0001 |
| S8 | Facial nerve assessment | 16 (53.3%) | 11 (36.7%) | 3 (10.0%) | −13 | 0.0001 |
| S9 | Footplate assessment and perforation | 20 (66.7%) | 8 (26.7%) | 2 (6.6%) | −18 | 0.0001 |
| S10 | Prosthesis placement maneuver | 17 (56.7%) | 11 (36.7%) | 2 (6.6%) | −15 | 0.0001 |
| Stapedotomy—the whole procedure (sum of S1–S10) | 97 (32.3%) | 59 (19.7%) | 144 (48.0%) | 47 | 0.4884 | |
| Visualization Grade: 3D Exoscope Relative to Microscope | ||||||
|---|---|---|---|---|---|---|
| Tympanoplasty—Stages of the Procedure | WORSE (−1) | COMPARABLE (0) | SUPERIOR (1) | SCORE (Sum of Points) | p-Value (Test of Proportion) | |
| T1 | Initial view of the operating field—planum mastoideum | 0 (0.0%) | 6 (20.0%) | 24 (80.0%) | 24 | 0.0010 |
| T2 | Posterior meatal wall skin incision + tympanic membrane assessment | 3 (10.0%) | 6 (20.0%) | 21 (70.0%) | 18 | 0.0285 |
| T3 | Antromastoidectomy | 1 (3.3%) | 7 (23.3%) | 22 (73.4%) | 21 | 0.0104 |
| T4 | Cholesteatoma or granulation removal from mastoid process | 5 (16.7%) | 5 (16.7%) | 20 (66.7%) | 15 | 0.0673 |
| T5 | Lateral semicircular canal visualization | 2 (6.7%) | 8 (26.7%) | 20 (66.7%) | 18 | 0.0673 |
| T6 | Cholesteatoma or granulation removal from tympanic cavity | 18 (60.0%) | 9 (30.0%) | 3 (10.0%) | −15 | 0.0001 |
| T7 | Cholesteatoma or granulation removal from hidden areas (protympanum and/or sinus tympani) | 19 (63.3%) | 9 (30.0%) | 2 (6.7%) | −17 | 0.0001 |
| T8 | Temporal fascia harvesting | 0 (0.0%) | 7 (23.3%) | 23 (76.7%) | 23 | 0.0034 |
| T9 | Ossiculoplasty | 2 (6.7%) | 12 (40.0%) | 16 (53.3%) | 14 | 0.7172 |
| T10 | Myringoplasty | 3 (10.0%) | 9 (30.0%) | 18 (60.0%) | 15 | 0.2733 |
| Tympanoplasty—the whole procedure (sum of T1–T10) | 53 (17.7%) | 78 (26.0%) | 169 (56.3%) | 116 | 0.0283 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wierzbicka, M.; Szyfter, W.; Greczka, G.; Gawęcki, W. Otosurgery with the High-Definition Three-Dimensional (3D) Exoscope: Advantages and Disadvantages. J. Clin. Med. 2021, 10, 777. https://doi.org/10.3390/jcm10040777
Wierzbicka M, Szyfter W, Greczka G, Gawęcki W. Otosurgery with the High-Definition Three-Dimensional (3D) Exoscope: Advantages and Disadvantages. Journal of Clinical Medicine. 2021; 10(4):777. https://doi.org/10.3390/jcm10040777
Chicago/Turabian StyleWierzbicka, Małgorzata, Witold Szyfter, Grażyna Greczka, and Wojciech Gawęcki. 2021. "Otosurgery with the High-Definition Three-Dimensional (3D) Exoscope: Advantages and Disadvantages" Journal of Clinical Medicine 10, no. 4: 777. https://doi.org/10.3390/jcm10040777






