Negligible Correlation between Radiographic Measurements and Clinical Outcomes in Patients Following Primary Reverse Total Shoulder Arthroplasty
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Outcome Measures
2.3. Surgical Procedure
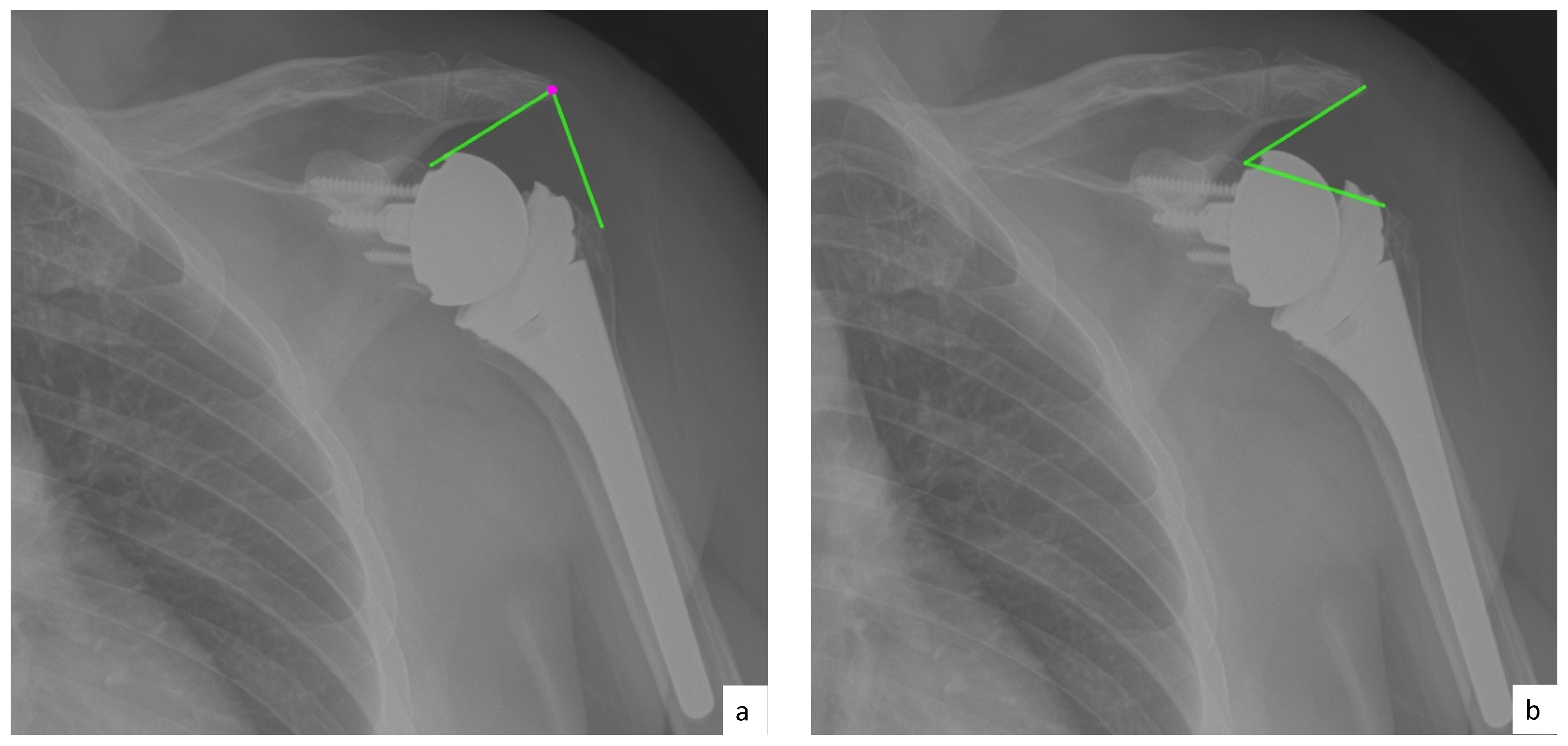
2.4. Radiographic Evaluation
2.5. Statistical Analysis
3. Results
3.1. Subjects
3.2. Clinical Outcome
3.3. Inter-Rater Reliability of Radiographic Analysis
3.4. Correlation between Preoperative Radiographic Measurements and Clinical Outcomes
3.5. Correlation between Lateralization and Clinical Outcomes
3.6. Correlation between Distalization and Clinical Outcomes
3.7. Prediction of Active ROM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| ASES | SANE | SST | VAS | FE | ABD | ER | ||
|---|---|---|---|---|---|---|---|---|
| COR | r | −0.079 | 0.080 | 0.006 | 0.154 | 0.170 | −0.176 | −0.011 |
| p−value | 0.547 | 0.545 | 0.966 | 0.235 | 0.194 | 0.190 | 0.937 | |
| Pre CSA | r | −0.035 | 0.056 | −0.056 | 0.051 | −0.211 | −0.212 | −0.187 |
| p−value | 0.794 | 0.672 | 0.677 | 0.697 | 0.105 | 0.113 | 0.156 | |
| Pre AHD | r | 0.085 | 0.142 | 0.124 | −0.059 | −0.051 | −0.015 | 0.314 |
| p−value | 0.518 | 0.284 | 0.349 | 0.652 | 0.697 | 0.910 | 0.016 | |
| Pre LHO | r | −0.277 | −0.243 | −0.251 | 0.177 | −0.048 | −0.037 | 0.035 |
| p−value | 0.032 | 0.064 | 0.055 | 0.173 | 0.716 | 0.783 | 0.790 | |
| Post−AHD | r | 0.150 | 0.179 | 0.293 | −0.135 | 0.398 | 0.111 | 0.233 |
| p−value | 0.253 | 0.174 | 0.025 | 0.299 | 0.002 | 0.411 | 0.075 | |
| Post LHO | r | −0.281 | −0.215 | −0.197 | 0.193 | 0.086 | 0.045 | −0.003 |
| p−value | 0.030 | 0.102 | 0.135 | 0.136 | 0.513 | 0.739 | 0.985 | |
| DSA | r | 0.169 | 0.099 | 0.234 | −0.145 | 0.299 | 0.145 | 0.133 |
| p−value | 0.198 | 0.456 | 0.075 | 0.266 | 0.020 | 0.283 | 0.317 | |
| LSA | r | −0.327 | −0.308 | −0.410 | 0.272 | −0.276 | 0.030 | −0.096 |
| p−value | 0.011 | 0.012 | 0.001 | 0.034 | 0.033 | 0.824 | 0.471 | |
| Inclination Glenoind. | r | −0.066 | −0.156 | −0.095 | 0.132 | 0.072 | 0.176 | 0.104 |
| p−value | 0.614 | 0.238 | 0.473 | 0.310 | 0.583 | 0.191 | 0.435 | |
| Inclination Baseplate | r | −0.121 | 0.038 | −0.102 | 0.123 | 0.122 | 0.106 | 0.050 |
| p−value | 0.356 | 0.776 | 0.442 | 0.347 | 0.353 | 0.433 | 0.710 | |
| Hamada | r | −0.009 | 0.067 | −0.031 | 0.013 | 0.156 | −0.141 | −0.289 |
| p−value | 0.947 | 0.613 | 0.817 | 0.919 | 0.233 | 0.297 | 0.026 | |
| Notching | r | −0.030 | −0.165 | 0.042 | 0.151 | −0.246 | −0.133 | −0.214 |
| p−value | 0.818 | 0.213 | 0.754 | 0.244 | 0.058 | 0.325 | 0.104 |
References
- Routman, H.D.; Flurin, P.H.; Wright, T.W.; Zuckerman, J.D.; Hamilton, M.A.; Roche, C.P. Reverse Shoulder Arthroplasty Prosthesis Design Classification System. Bull. NYU Hosp. Jt. Dis. 2015, 73, S5–S14. [Google Scholar]
- Grammont, P. Etude et réalisation d’une nouvelle prothèse d’épaule. Rheumatologie 1987, 39, 27–38. [Google Scholar]
- Grammont, P.M.; Baulot, E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics 1993, 16, 65–68. [Google Scholar] [CrossRef]
- Boileau, P.; Watkinson, D.J.; Hatzidakis, A.M.; Balg, F. Grammont reverse prosthesis: Design, rationale, and biomechanics. J. Shoulder Elbow Surg. 2005, 14, 147s–161s. [Google Scholar] [CrossRef] [PubMed]
- Boileau, P.; Moineau, G.; Roussanne, Y.; O’Shea, K. Bony increased-offset reversed shoulder arthroplasty: Minimizing scapular impingement while maximizing glenoid fixation. Clin. Orthop. Relat. Res. 2011, 469, 2558–2567. [Google Scholar] [CrossRef] [Green Version]
- Ladermann, A.; Denard, P.J.; Boileau, P. Effect of humeral stem design on humeral position and range of motion in reverse shoulder arthroplasty. Int. Orthop. 2015, 39, 2205–2213. [Google Scholar] [CrossRef] [Green Version]
- Bacle, G.; Nove-Josserand, L.; Garaud, P.; Walch, G. Long-Term Outcomes of Reverse Total Shoulder Arthroplasty: A Follow-up of a Previous Study. J. Bone Jt. Surg. Am. 2017, 99, 454–461. [Google Scholar] [CrossRef]
- Mizuno, N.; Denard, P.J.; Raiss, P.; Walch, G. The clinical and radiographical results of reverse total shoulder arthroplasty with eccentric glenosphere. Int. Orthop. 2012, 36, 1647–1653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutierrez, S.; Levy, J.C.; Frankle, M.A. Evaluation of abduction range of motion and avoidance of inferior scapular impingement in a reverse shoulder model. J. Shoulder Elbow Surg. 2008, 17, 608–615. [Google Scholar] [CrossRef]
- Berliner, J.L.; Regalado-Magdos, A.; Ma, C.B.; Feeley, B.T. Biomechanics of reverse total shoulder arthroplasty. J. Shoulder Elbow Surg. 2015, 24, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Frankle, M.; Levy, J.C.; Pupello, D. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. a minimum two-year follow-up study of sixty patients surgical technique. J. Bone Jt. Surg. Am. 2006, 88, 178–190. [Google Scholar] [CrossRef]
- Katz, D.; Valenti, P.; Kany, J.; Elkholti, K.; Werthel, J.D. Does lateralisation of the centre of rotation in reverse shoulder arthroplasty avoid scapular notching? Clinical and radiological review of one hundred and forty cases with forty five months of follow-up. Int. Orthop. 2016, 40, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Ernstbrunner, L.; Suter, A.; Catanzaro, S.; Rahm, S.; Gerber, C. Reverse Total Shoulder Arthroplasty for Massive, Irreparable Rotator Cuff Tears Before the Age of 60 Years: Long-Term Results. J. Bone Jt. Surg. Am. 2017, 99, 1721–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerber, C.; Canonica, S.; Catanzaro, S.; Ernstbrunner, L. Longitudinal observational study of reverse total shoulder arthroplasty for irreparable rotator cuff dysfunction: Results after 15 years. J. Shoulder Elbow Surg. 2018, 27, 838. [Google Scholar] [CrossRef]
- Mahendraraj, K.A.; Colliton, E.; Muniz, A.; Menendez, M.E.; Jawa, A. Assessing the validity of the distalization and lateralization shoulder angles following reverse total shoulder arthroplasty. Semin. Arthroplast. JSES 2020. [Google Scholar] [CrossRef]
- Li, X.; Knutson, Z.; Choi, D. Effects of glenosphere positioning on impingement-free internal and external rotation after reverse total shoulder arthroplasty. J. Shoulder Elbow Surg. 2013, 22, 807–813. [Google Scholar] [CrossRef]
- Berhouet, J.; Garaud, P.; Favard, L. Evaluation of the role of glenosphere design and humeral component retroversion in avoiding scapular notching during reverse shoulder arthroplasty. J. Shoulder Elbow Surg. 2014, 23, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Jobin, C.M.; Brown, G.D.; Bahu, M.J. Reverse total shoulder arthroplasty for cuff tear arthropathy: The clinical effect of deltoid lengthening and center of rotation medialization. J. Shoulder Elbow Surg. 2012, 21, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Marcoin, A.; Ferrier, A.; Blasco, L.; De Boissieu, P.; Nerot, C.; Ohl, X. Reproducibility of a new method for measuring lowering and medialisation of the humerus after reverse shoulder arthroplasty. Int. Orthop. 2018, 42, 141–147. [Google Scholar] [CrossRef]
- Boutsiadis, A.; Lenoir, H.; Denard, P.J. The lateralization and distalization shoulder angles are important determinants of clinical outcomes in reverse shoulder arthroplasty. J. Shoulder Elbow Surg. 2018, 27, 1226–1234. [Google Scholar] [CrossRef]
- Jeon, Y.S.; Rhee, Y.G. Factors associated with poor active anterior elevation after reverse total shoulder arthroplasty. J. Shoulder Elbow Surg. 2018, 27, 786–793. [Google Scholar] [CrossRef]
- Richards, R.R.; An, K.N.; Bigliani, L.U. A standardized method for the assessment of shoulder function. J. Shoulder Elbow Surg. 1994, 3, 347–352. [Google Scholar] [CrossRef]
- Lippitt, S. A practical tool for evaluating shoulder function. The Simple Shoulder Test. Shoulder A Balance Mobil. Stab. 1993, 501–518. [Google Scholar]
- Rhee, S.M.; Lee, J.D.; Park, Y.B.; Yoo, J.C.; Oh, J.H. Prognostic Radiological Factors Affecting Clinical Outcomes of Reverse Shoulder Arthroplasty in the Korean Population. Clin. Orthop. Surg. 2019, 11, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Maurer, A.; Fucentese, S.F.; Pfirrmann, C.W. Assessment of glenoid inclination on routine clinical radiographs and computed tomography examinations of the shoulder. J. Shoulder Elbow Surg. 2012, 21, 1096–1103. [Google Scholar] [CrossRef] [Green Version]
- Moor, B.K.; Bouaicha, S.; Rothenfluh, D.A.; Sukthankar, A.; Gerber, C. Is there an association between the individual anatomy of the scapula and the development of rotator cuff tears or osteoarthritis of the glenohumeral joint?: A radiological study of the critical shoulder angle. Bone Jt. J. 2013, 95, 935–941. [Google Scholar] [CrossRef]
- Sirveaux, F.; Favard, L.; Oudet, D.; Huquet, D.; Walch, G.; Mole, D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff: Results of a multicentre study of 80 shoulders. J. Bone Jt. Surg. Br. Vol. 2004, 86, 388–395. [Google Scholar] [CrossRef]
- Hamada, K.; Yamanaka, K.; Uchiyama, Y.; Mikasa, T.; Mikasa, M. A radiographic classification of massive rotator cuff tear arthritis. Clin. Orthop. Relat. Res. 2011, 469, 2452–2460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabesan, V.J.; Lombardo, D.; Josserand, D. The effect of deltoid lengthening on functional outcome for reverse shoulder arthroplasty. Musculoskelet. Surg. 2016, 100, 127–132. [Google Scholar] [CrossRef]
- Werner, B.S.; Chaoui, J.; Walch, G. The influence of humeral neck shaft angle and glenoid lateralization on range of motion in reverse shoulder arthroplasty. J. Shoulder Elbow Surg. 2017, 26, 1726–1731. [Google Scholar] [CrossRef]
- Greiner, S.; Schmidt, C.; Konig, C.; Perka, C.; Herrmann, S. Lateralized reverse shoulder arthroplasty maintains rotational function of the remaining rotator cuff. Clin. Orthop. Relat. Res. 2013, 471, 940–946. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, S.; Comiskey, C.A.; Luo, Z.P.; Pupello, D.R.; Frankle, M.A. Range of impingement-free abduction and adduction deficit after reverse shoulder arthroplasty. Hierarchy of surgical and implant-design-related factors. J. Bone Jt. Surg. Am. 2008, 90, 2606–2615. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.G.; Cottrell, B.J.; Teusink, M.J. Factors that predict postoperative motion in patients treated with reverse shoulder arthroplasty. J. Shoulder Elbow Surg. 2014, 23, 1289–1295. [Google Scholar] [CrossRef]
- Roberson, T.A.; Shanley, E.; Abildgaard, J.T. The influence of radiographic markers of biomechanical variables on outcomes in reverse shoulder arthroplasty. JSES Open Access 2019, 3, 59–64. [Google Scholar] [CrossRef] [Green Version]







| n | % | |
|---|---|---|
| Sex | ||
| Male | 27 | 44.3 |
| Female | 34 | 55.7 |
| Mean age ± SD (years) | 69.2 ± 8.2 | |
| Mean follow-up ± SD (years) | 3.1 ± 0.7 | |
| Dominant Arm Involved | 32 | 52.0 |
| Right Shoulders | 33 | 54.0 |
| BMI | 29.9 ± 7.0 |
| Mean ± SD | ICC | ICC 95% CI | Reliability | |
|---|---|---|---|---|
| COR | 20.9 ± 3.9 mm | 0.68 | [0.51, 0.8] | Moderate-Good |
| Pre CSA | 35.2 ± 4.5 deg | 0.9 | [0.9, 0.94] | Good |
| Pre AHD | 5.1 ± 3.2 mm | 0.37 | [0.18, 0.55] | Poor |
| Post AHD | 26.3 ± 9.5 mm | 0.88 | [0.82, 0.93] | Good |
| DSA | 38.6 ± 9.6 deg | 0.66 | [0.32, 0.82] | Moderate-Good |
| Pre LHO | 9.9 ± 5.7 mm | 0.86 | [0.79, 0.91] | Good |
| Post LHO | 9.5 ± 6 mm | 0.84 | [0.75, 0.89] | Good |
| LSA | 89.2 ± 11.9 deg | 0.84 | [0.73, 0.9] | Good |
| Glenoid inclination | 81.2 ± 6.8 deg | 0.66 | [0.47, 0.79] | Moderate-Good |
| Baseplate inclination | 83.2 ± 6.4 deg | 0.79 | [0.69, 0.86] | Good |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berthold, D.P.; Morikawa, D.; Muench, L.N.; Baldino, J.B.; Cote, M.P.; Creighton, R.A.; Denard, P.J.; Gobezie, R.; Lederman, E.; Romeo, A.A.; et al. Negligible Correlation between Radiographic Measurements and Clinical Outcomes in Patients Following Primary Reverse Total Shoulder Arthroplasty. J. Clin. Med. 2021, 10, 809. https://doi.org/10.3390/jcm10040809
Berthold DP, Morikawa D, Muench LN, Baldino JB, Cote MP, Creighton RA, Denard PJ, Gobezie R, Lederman E, Romeo AA, et al. Negligible Correlation between Radiographic Measurements and Clinical Outcomes in Patients Following Primary Reverse Total Shoulder Arthroplasty. Journal of Clinical Medicine. 2021; 10(4):809. https://doi.org/10.3390/jcm10040809
Chicago/Turabian StyleBerthold, Daniel P., Daichi Morikawa, Lukas N. Muench, Joshua B. Baldino, Mark P. Cote, R. Alexander Creighton, Patrick J. Denard, Reuben Gobezie, Evan Lederman, Anthony A. Romeo, and et al. 2021. "Negligible Correlation between Radiographic Measurements and Clinical Outcomes in Patients Following Primary Reverse Total Shoulder Arthroplasty" Journal of Clinical Medicine 10, no. 4: 809. https://doi.org/10.3390/jcm10040809





