Local Anesthetic-Induced Central Nervous System Toxicity during Interscalene Brachial Plexus Block: A Case Series Study of Three Patients
Abstract
:1. Introduction
2. Experimental Section
2.1. Patients
2.2. Definitions
2.3. Diagnosis and Treatment
3. Results
3.1. On-Scene Clinical Presentation
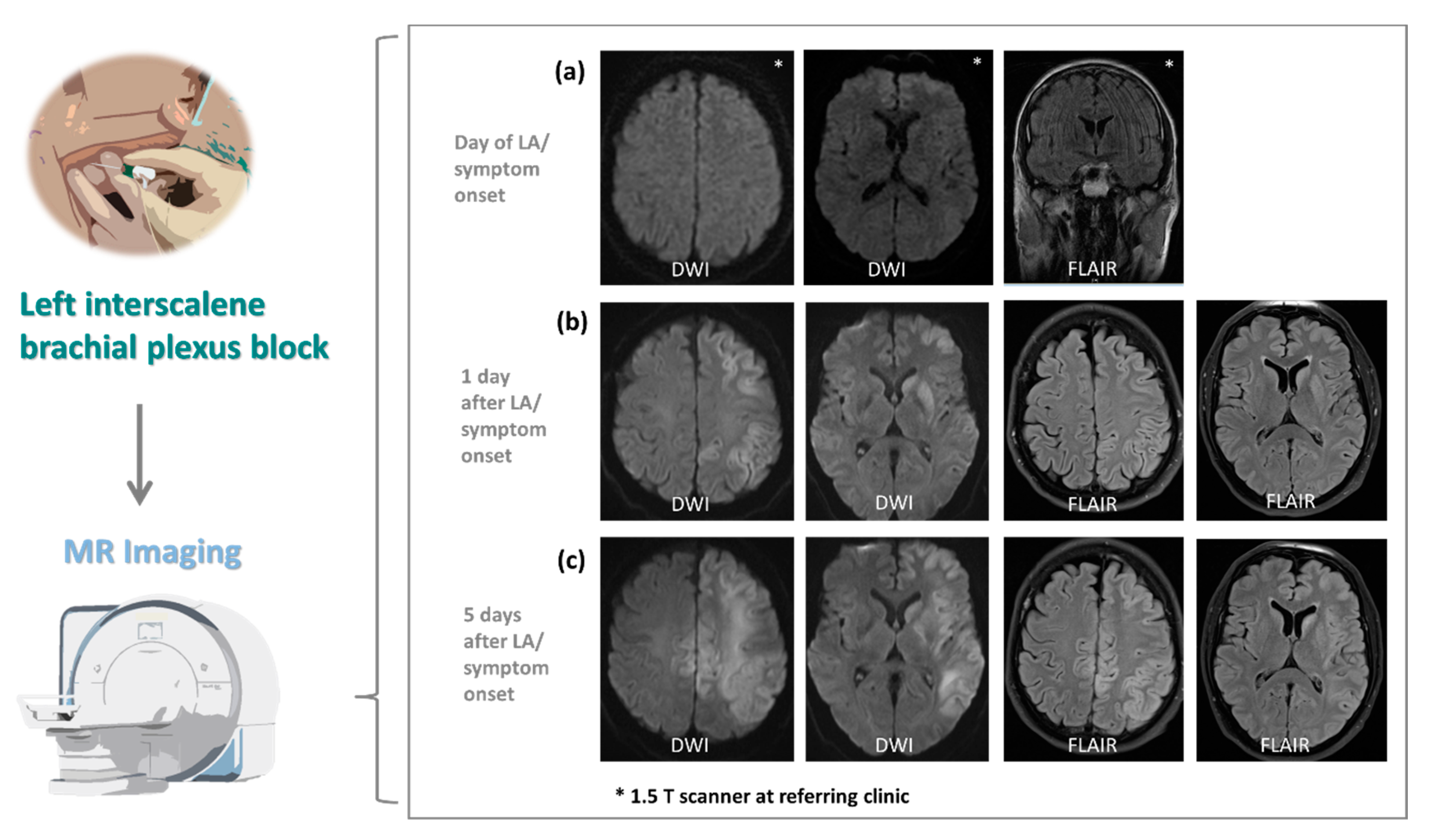
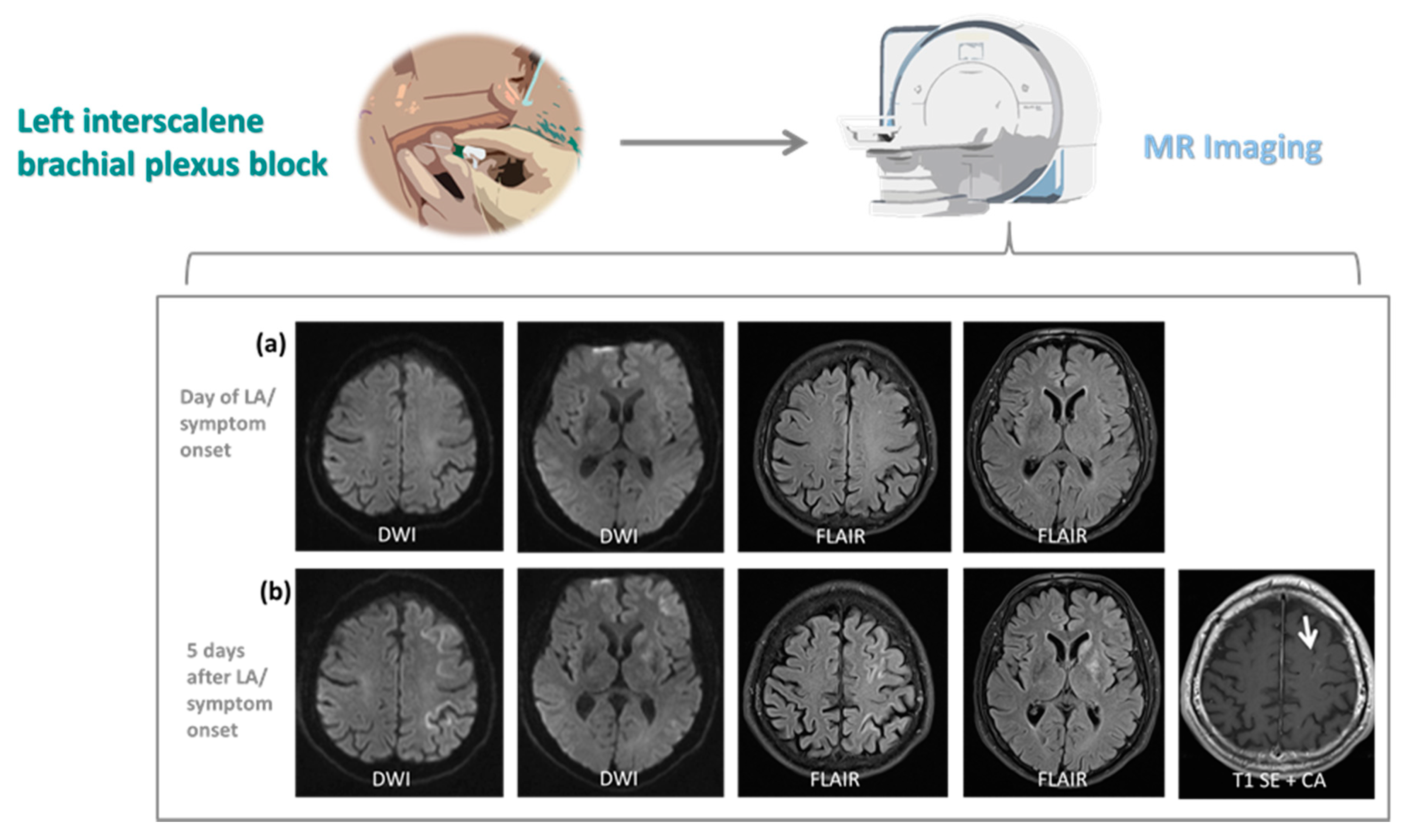
3.2. Neuroimaging
3.3. Clinical Management and Neurological Outcome at Discharge
3.4. Follow-Up and Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Watts, S.A.; Sharma, D.J. Long-term neurological complications associated with surgery and peripheral nerve blockade: Outcomes after 1065 consecutive blocks. Anaesth. Intensive Care 2007, 35, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Allen, H.W.; Liu, S.S.; Ware, P.D.; Nairn, C.S.; Owens, B.D. Peripheral nerve blocks improve analgesia after total knee replacement surgery. Anesth. Analg. 1998, 87, 93–97. [Google Scholar] [CrossRef]
- Iskandar, H.; Benard, A.; Ruel-Raymond, J.; Cochard, G.; Manaud, B. Femoral block provides superior analgesia compared with intra-articular ropivacaine after anterior cruciate ligament reconstruction. Reg. Anesth. Pain Med. 2003, 28, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Laurila, P.A.; Löppönen, A.; Kanga-Saarela, T.; Flinkkilä, T.; Salomäki, T.E. Interscalene brachial plexus block is superior to subacromial bursa block after arthroscopic shoulder surgery. Acta Anaesthesiol. Scand. 2002, 46, 1031–1036. [Google Scholar] [CrossRef]
- Singelyn, F.J.; Deyaert, M.; Joris, D.; Pendeville, E.; Gouverneur, J.M. Effects of intravenous patient-controlled analgesia with morphine, continuous epidural analgesia, and continuous three-in-one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesth. Analg. 1998, 87, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Brull, R.; McCartney, C.J.L.; Wong, P.; Kumar, N.; Essandoh, M.; Sawyer, T.; Sullivan, T.; Abdallah, F.W. Pectoralis-II Myofascial Block and Analgesia in Breast Cancer Surgery: A Systematic Review and Meta-analysis. Anesthesiology 2019, 131, 630–648. [Google Scholar] [CrossRef] [PubMed]
- Long, T.R.; Wass, C.T.; Burkle, C.M. Perioperative interscalene blockade: An overview of its history and current clinical use. J. Clin. Anesth. 2002, 14, 546–556. [Google Scholar] [CrossRef]
- Stav, A.; Reytman, L.; Stav, M.-Y.; Portnoy, I.; Kantarovsky, A.; Galili, O.; Luboshitz, S.; Sevi, R.; Sternberg, A. Comparison of the Supraclavicular, Infraclavicular and Axillary Approaches for Ultrasound-Guided Brachial Plexus Block for Surgical Anesthesia. Rambam Maimonides Med. J. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Ryu, T.; Kil, B.T.; Kim, J.H. Comparison between Ultrasound-Guided Supraclavicular and Interscalene Brachial Plexus Blocks in Patients Undergoing Arthroscopic Shoulder Surgery: A Prospective, Randomized, Parallel Study. Medicine 2015, 94, e1726. [Google Scholar] [CrossRef]
- Borgeat, A.; Ekatodramis, G.; Kalberer, F.; Benz, C. Acute and nonacute complications associated with interscalene block and shoulder surgery: A prospective study. Anesthesiology 2001, 95, 875–880. [Google Scholar] [CrossRef]
- Bollini, C.A.; Urmey, W.F.; Vascello, L.; Cacheiro, F. Relationship between evoked motor response and sensory paresthesia in interscalene brachial plexus block. Reg. Anesth. Pain Med. 2003, 28, 384–388. [Google Scholar] [CrossRef]
- Conroy, P.H.; Awad, I.T. Ultrasound-guided blocks for shoulder surgery. Curr. Opin. Anaesthesiol. 2011, 24, 638–643. [Google Scholar] [CrossRef]
- Salem, M.H.; Winckelmann, J.; Geiger, P.; Mehrkens, H.-H.; Salem, K.H. Electrostimulation with or without ultrasound-guidance in interscalene brachial plexus block for shoulder surgery. J. Anesth. 2012, 26, 610–613. [Google Scholar] [CrossRef]
- Liu, S.S.; Zayas, V.M.; Gordon, M.A.; Beathe, J.C.; Maalouf, D.B.; Paroli, L.; Liguori, G.A.; Ortiz, J.; Buschiazzo, V.; Ngeow, J.; et al. A prospective, randomized, controlled trial comparing ultrasound versus nerve stimulator guidance for interscalene block for ambulatory shoulder surgery for postoperative neurological symptoms. Anesth. Analg. 2009, 109, 265–271. [Google Scholar] [CrossRef]
- Crews, J.C.; Rothman, T.E. Seizure after levobupivacaine for interscalene brachial plexus block. Anesth. Analg. 2003, 96, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Sardesai, A.M.; Chakrabarti, A.J.; Denny, N.M. Lower lobe collapse during continuous interscalene brachial plexus local anesthesia at home. Reg. Anesth. Pain Med. 2004, 29, 65–68. [Google Scholar] [CrossRef]
- Rose, M.; Ness, T.J. Hypoxia following interscalene block. Reg. Anesth. Pain Med. 2002, 27, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Reinikainen, M.; Hedman, A.; Pelkonen, O.; Ruokonen, E. Cardiac arrest after interscalene brachial plexus block with ropivacaine and lidocaine. Acta Anaesthesiol. Scand. 2003, 47, 904–906. [Google Scholar] [CrossRef] [PubMed]
- Souron, V.; Reiland, Y.; Delaunay, L. Pleural effusion and chest pain after continuous interscalene brachial plexus block. Reg. Anesth. Pain Med. 2003, 28, 535–538. [Google Scholar] [CrossRef]
- Auroy, Y.; Benhamou, D.; Bargues, L.; Ecoffey, C.; Falissard, B.; Mercier, F.J.; Bouaziz, H.; Samii, K. Major complications of regional anesthesia in France: The SOS Regional Anesthesia Hotline Service. Anesthesiology 2002, 97, 1274–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaragoza-Lemus, G.; Limón-Muñoz, M.; García-Reyes, W. Ultrasonographic assessment of hemidiaphragm paralysis secondary to interscalene block. Cir. Cir. 2012, 80, 352–356. [Google Scholar]
- Vorobeichik, L.; Brull, R.; Bowry, R.; Laffey, J.G.; Abdallah, F.W. Should continuous rather than single-injection interscalene block be routinely offered for major shoulder surgery? A meta-analysis of the analgesic and side-effects profiles. Br. J. Anaesth. 2018, 120, 679–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stasiowski, M.; Zuber, M.; Marciniak, R.; Kolny, M.; Chabierska, E.; Jałowiecki, P.; Pluta, A.; Missir, A. Risk factors for the development of Horner’s syndrome following interscalene brachial plexus block using ropivacaine for shoulder arthroscopy: A randomised trial. Anaesthesiol. Intensive Ther. 2018, 50, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Borgeat, A.; Dullenkopf, A.; Ekatodramis, G.; Nagy, L. Evaluation of the lateral modified approach for continuous interscalene block after shoulder surgery. Anesthesiology 2003, 99, 436–442. [Google Scholar] [CrossRef]
- Neal, J.M.; Gerancher, J.C.; Hebl, J.R.; Ilfeld, B.M.; McCartney, C.J.L.; Franco, C.D.; Hogan, Q.H. Upper extremity regional anesthesia: Essentials of our current understanding, 2008. Reg. Anesth. Pain Med. 2009, 34, 134–170. [Google Scholar] [CrossRef] [PubMed]
- Turner, F.N.; Shih, R.D.; Fishman, I.; Calello, D.P.; Solano, J.J. Total Spinal Anesthesia Following an Interscalene Block Treated with Intravenous Lipid Emulsion. Cureus 2019, 11, e4491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanovski, B.; Gaitini, L.; Volodarski, D.; Ben-David, B. Catastrophic complication of an interscalene catheter for continuous peripheral nerve block analgesia. Anaesthesia 2012, 67, 1166–1169. [Google Scholar] [CrossRef] [PubMed]
- Korman, B.; Riley, R.H. Convulsions induced by ropivacaine during interscalene brachial plexus block. Anesth. Analg. 1997, 85, 1128–1129. [Google Scholar] [CrossRef]
- Klein, S.M.; Benveniste, H. Anxiety, vocalization, and agitation following peripheral nerve block with ropivacaine. Reg. Anesth. Pain Med. 1999, 24, 175–178. [Google Scholar] [CrossRef]
- Dhir, S.; Ganapathy, S.; Lindsay, P.; Athwal, G.S. Case report: Ropivacaine neurotoxicity at clinical doses in interscalene brachial plexus block. Can. J. Anaesth. 2007, 54, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, K.; Oda, Y.; Sato, H. Successful treatment of ropivacaine-induced central nervous system toxicity by use of lipid emulsion: Effect on total and unbound plasma fractions. J. Anesth. 2011, 25, 442–445. [Google Scholar] [CrossRef]
- Wippold, F.J. 2nd Focal neurologic deficit. AJNR. Am. J. Neuroradiol. 2008, 29, 1998–2000. [Google Scholar]
- Spilker, J.; Kongable, G.; Barch, C.; Braimah, J.; Brattina, P.; Daley, S.; Donnarumma, R.; Rapp, K.; Sailor, S. Using the NIH Stroke Scale to assess stroke patients. The NINDS rt-PA Stroke Study Group. J. Neurosci. Nurs. J. Am. Assoc. Neurosci. Nurses 1997, 29, 384–392. [Google Scholar] [CrossRef]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Bateman, D.E. Neurological assessment of coma. J. Neurol. Neurosurg. Psychiatry 2001, 71 (Suppl. 1), i13–i17. [Google Scholar] [CrossRef]
- Kaplan, P.W. The EEG of status epilepticus. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 2006, 23, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Leitinger, M.; Beniczky, S.; Rohracher, A.; Gardella, E.; Kalss, G.; Qerama, E.; Höfler, J.; Hess Lindberg-Larsen, A.; Kuchukhidze, G.; Dobesberger, J.; et al. Salzburg Consensus Criteria for Non-Convulsive Status Epilepticus--approach to clinical application. Epilepsy Behav. 2015, 49, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Brophy, G.M.; Bell, R.; Claassen, J.; Alldredge, B.; Bleck, T.P.; Glauser, T.; Laroche, S.M.; Riviello, J.J.J.; Shutter, L.; Sperling, M.R.; et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit. Care 2012, 17, 3–23. [Google Scholar] [CrossRef]
- Weber, S.C.; Jain, R. Scalene regional anesthesia for shoulder surgery in a community setting: An assessment of risk. J. Bone Joint Surg. Am. 2002, 84, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Walton, J.S.; Folk, J.W.; Friedman, R.J.; Dorman, B.H. Complete brachial plexus palsy after total shoulder arthroplasty done with interscalene block anesthesia. Reg. Anesth. Pain Med. 2000, 25, 318–321. [Google Scholar] [CrossRef]
- Tetzlaff, J.E.; Dilger, J.; Yap, E.; Brems, J. Idiopathic brachial plexitis after total shoulder replacement with interscalene brachial plexus block. Anesth. Analg. 1997, 85, 644–646. [Google Scholar] [CrossRef]
- Pohl, A.; Cullen, D.J. Cerebral ischemia during shoulder surgery in the upright position: A case series. J. Clin. Anesth. 2005, 17, 463–469. [Google Scholar] [CrossRef]
- Drummond, J.C.; Lee, R.R.; Howell, J.P.J. Focal cerebral ischemia after surgery in the “beach chair” position: The role of a congenital variation of circle of Willis anatomy. Anesth. Analg. 2012, 114, 1301–1303. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.; Bluwi, M.; Elshamaa, K. Postoperative brain stroke after shoulder arthroscopy in the lateral decubitus position. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2014, 23, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, C.; Stanescu, R.; Dormont, D.; Crozier, S.; Marro, B.; Samson, Y.; Rancurel, G.; Marsault, C. False-negative diffusion-weighted MR findings in acute ischemic stroke. AJNR Am. J. Neuroradiol. 2000, 21, 1434–1440. [Google Scholar] [PubMed]
- Watts, J.; Wood, B.; Kelly, A.; Alvaro, A. Stroke syndromes associated with DWI-negative MRI include ataxic hemiparesis and isolated internuclear ophthalmoplegia. Neurol. Clin. Pract. 2013, 3, 186–191. [Google Scholar] [CrossRef] [Green Version]
- Edlow, B.L.; Hurwitz, S.; Edlow, J.A. Diagnosis of DWI-negative acute ischemic stroke: A meta-analysis. Neurology 2017, 89, 256–262. [Google Scholar] [CrossRef]
- Zuo, L.; Zhang, Y.; Xu, X.; Li, Y.; Bao, H.; Hao, J.; Wang, X.; Li, G. A retrospective analysis of negative diffusion-weighted image results in patients with acute cerebral infarction. Sci. Rep. 2015, 5, 8910. [Google Scholar] [CrossRef] [Green Version]
- Zubkov, A.Y.; Uschmann, H.; Rabinstein, A.A. Rate of arterial occlusion in patients with acute ischemic stroke. Neurol. Res. 2008, 30, 835–838. [Google Scholar] [CrossRef]
- Beume, L.-A.; Hieber, M.; Kaller, C.P.; Nitschke, K.; Bardutzky, J.; Urbach, H.; Weiller, C.; Rijntjes, M. Large Vessel Occlusion in Acute Stroke. Stroke 2018, 49, 2323–2329. [Google Scholar] [CrossRef]
- Caplan, L.R.; Wong, K.S.; Gao, S.; Hennerici, M.G. Is hypoperfusion an important cause of strokes? If so, how? Cerebrovasc. Dis. 2006, 21, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.L.; Ransom, D.M.; Hall, J.A.; Leicht, C.H.; Schroeder, D.R.; Offord, K.P. Regional anesthesia and local anesthetic-induced systemic toxicity: Seizure frequency and accompanying cardiovascular changes. Anesth. Analg. 1995, 81, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.H.; Bogard, T.D.; Owen, M.D. Incidence and characteristics of failures in obstetric neuraxial analgesia and anesthesia: A retrospective analysis of 19,259 deliveries. Int. J. Obstet. Anesth. 2004, 13, 227–233. [Google Scholar] [CrossRef]
- Alsukhni, R.A.; Ghoubari, M.S.; Farfouti, M.T.; Aboras, Y.A. Status epilepticus following local anesthesia in a previously healthy adult. BMC Res. Notes 2016, 9, 300. [Google Scholar] [CrossRef] [Green Version]
- Friederich, P.; Schmitz, T.P. Lidocaine-induced cell death in a human model of neuronal apoptosis. Eur. J. Anaesthesiol. 2002, 19, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Di Gregorio, G.; Neal, J.M.; Rosenquist, R.W.; Weinberg, G.L. Clinical presentation of local anesthetic systemic toxicity: A review of published cases, 1979 to 2009. Reg. Anesth. Pain Med. 2010, 35, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.E.; Reed, K.L. Essentials of local anesthetic pharmacology. Anesth. Prog. 2006, 53, 10–98. [Google Scholar] [CrossRef]
- McClure, J.H. Ropivacaine. Br. J. Anaesth. 1996, 76, 300–307. [Google Scholar] [CrossRef]
- Johnson, M.E.; Uhl, C.B.; Spittler, K.-H.; Wang, H.; Gores, G.J. Mitochondrial injury and caspase activation by the local anesthetic lidocaine. Anesthesiology 2004, 101, 1184–1194. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, L.; Zhang, Y.; Yang, X. The role of DRP1 in ropivacaine-induced mitochondrial dysfunction and neurotoxicity. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1788–1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werdehausen, R.; Fazeli, S.; Braun, S.; Hermanns, H.; Essmann, F.; Hollmann, M.W.; Bauer, I.; Stevens, M.F. Apoptosis induction by different local anaesthetics in a neuroblastoma cell line. Br. J. Anaesth. 2009, 103, 711–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Castro, R.; Patel, S.; Garavito-Aguilar, Z.V.; Rosenberg, A.; Recio-Pinto, E.; Zhang, J.; Blanck, T.J.J.; Xu, F. Cytotoxicity of local anesthetics in human neuronal cells. Anesth. Analg. 2009, 108, 997–1007. [Google Scholar] [CrossRef]
- Ganapathy, S.; Sandhu, H.B.; Stockall, C.A.; Hurley, D. Transient neurologic symptom (TNS) following intrathecal ropivacaine. Anesthesiology 2000, 93, 1537–1539. [Google Scholar] [CrossRef]
- Müller, M.; Litz, R.J.; Hüler, M.; Albrecht, D.M. Grand mal convulsion and plasma concentrations after intravascular injection of ropivacaine for axillary brachial plexus blockade. Br. J. Anaesth. 2001, 87, 784–787. [Google Scholar] [CrossRef] [Green Version]
- Rodolà, F.; Anastasi, F.; Vergari, A. Ropivacaine induced acute neurotoxicity after epidural injection. Eur. Rev. Med. Pharmacol. Sci. 2007, 11, 133–135. [Google Scholar]
- Perkins, W.J.J.; Lanier, W.L.; Sharbrough, F.W. Cerebral and hemodynamic effects of lidocaine accidentally injected into the carotid arteries of patients having carotid endarterectomy. Anesthesiology 1988, 69, 787–790. [Google Scholar] [CrossRef]
- Tanaka, K.; Yamasaki, M. Blocking of cortical inhibitory synapses by intravenous lidocaine. Nature 1966, 209, 207–208. [Google Scholar] [CrossRef] [PubMed]
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Demographics | |||
| Age (y) | 52 | 52 | 71 |
| Sex | M | M | M |
| Pre-existing comorbidities | MS | HT | none |
| Surgical procedure | Arthroscopy | Arthroscopy | Arthroscopy |
| Underlying disease, affected side | RCI, left | RCI, left | RCI, left |
| Brachial plexus block | |||
| Approach, side | ISB, left | ISB, left | ISB, left |
| Local anesthetic | LDC, RPV | LDC, RPV | LDC, RPV |
| Neurological symptoms and signs on admission | |||
| Consciousness impairment | yes | yes | yes |
| Hemisphere syndrome, side | yes, left | yes, left | yes, left |
| Seizure activity | yes | yes | no |
| Slow wave activity in affected hemisphere | yes | yes | yes |
| Severity scores on admission | |||
| NIHSS | 24 | 25 | 16 |
| GCS | 8 | 8 | 10 |
| SAPS II | 54 | 41 | 29 |
| Complications during hospitalisation | |||
| Status epilepticus | no | yes | no |
| Ischemic and/or hemorrhagic complications | no | no | no |
| Aspiration pneumonia due to dysphagia | yes | yes | no |
| Treatment of toxic hemisphere syndrome | |||
| No. of anticonvulsants needed to control seizures | 2 | 2 | 0 |
| Need for mechanical ventilation | yes | no | no |
| Length (d) of hospital stay | 19 | 9 | 10 |
| Persisting neurological symptoms and signs at discharge | |||
| Consciousness impairment | no | no | no |
| Hemisphere syndrome | yes, partial | yes, partial | yes, partial |
| Seizure activity | no | no | no |
| Slow wave activity in affected hemisphere | yes, partial | yes, partial | no |
| Patient 1 | Patient 2 | Patient 3 | Median | |||
|---|---|---|---|---|---|---|
| Severity scores on admission | ||||||
| NIHSS | 24 | 25 | 16 | 24 | ||
| GCS | 8 | 8 | 10 | 8 | ||
| SAPS II | 54 | 41 | 29 | 41 | ||
| Severity scores at discharge | ||||||
| NIHSS | 10 | 17 | 7 | 10 | ||
| GCS | 14 | 14 | 15 | 14 | ||
| GOS | 3 | 3 | 4 | 3 | ||
| Severity scores after follow-up | ||||||
| NIHSS | 2 | 0 | 1 | |||
| GCS | 15 | 15 | 15 | |||
| GOS | 4 | 1 | 5 | 4 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spitzer, D.; Wenger, K.J.; Neef, V.; Divé, I.; Schaller-Paule, M.A.; Jahnke, K.; Kell, C.; Foerch, C.; Burger, M.C. Local Anesthetic-Induced Central Nervous System Toxicity during Interscalene Brachial Plexus Block: A Case Series Study of Three Patients. J. Clin. Med. 2021, 10, 1013. https://doi.org/10.3390/jcm10051013
Spitzer D, Wenger KJ, Neef V, Divé I, Schaller-Paule MA, Jahnke K, Kell C, Foerch C, Burger MC. Local Anesthetic-Induced Central Nervous System Toxicity during Interscalene Brachial Plexus Block: A Case Series Study of Three Patients. Journal of Clinical Medicine. 2021; 10(5):1013. https://doi.org/10.3390/jcm10051013
Chicago/Turabian StyleSpitzer, Daniel, Katharina J. Wenger, Vanessa Neef, Iris Divé, Martin A. Schaller-Paule, Kolja Jahnke, Christian Kell, Christian Foerch, and Michael C. Burger. 2021. "Local Anesthetic-Induced Central Nervous System Toxicity during Interscalene Brachial Plexus Block: A Case Series Study of Three Patients" Journal of Clinical Medicine 10, no. 5: 1013. https://doi.org/10.3390/jcm10051013






