Balloon Pulmonary Angioplasty in Technically Operable and Technically Inoperable Chronic Thromboembolic Pulmonary Hypertension
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Diagnosis of CTEPH
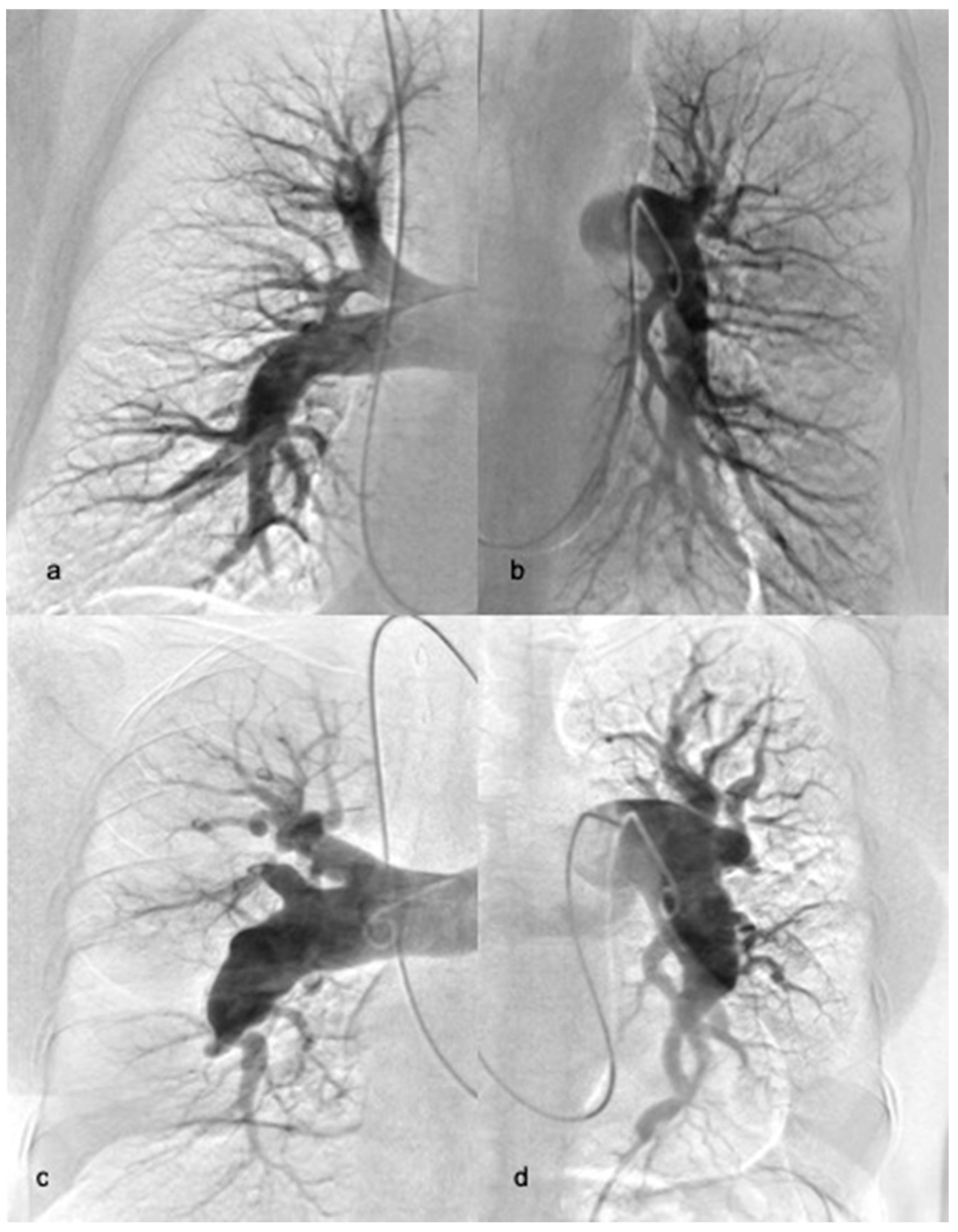
2.3. Balloon Pulmonary Angioplasty
2.4. Complications and Survival
2.5. Statistical Analysis
3. Results
3.1. Demographic and Procedural Parameters
3.2. Subpopulation of Proximal CTEPH
3.3. Treatment Response
3.4. Complications
3.5. Follow-Up
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.V.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Kardiol. Pol. 2015, 73, 1127–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, D.; Madani, M.; Fadel, E.; D’Armini, A.M.; Mayer, E. Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017, 26, 160111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madani, M.M.; Auger, W.R.; Pretorius, V.; Sakakibara, N.; Kerr, K.M.; Kim, N.H.; Fedullo, P.F.; Jamieson, S.W. Pulmonary endarterectomy: Recent changes in a single institution’s experience of more than 2,700 patients. Ann. Thorac. Surg. 2012, 94, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, J.A.; Goldhaber, S.Z.; Lock, J.E.; Ferndandes, S.M.; Landzberg, M.J. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation 2001, 103, 10–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condliffe, R.; Kiely, D.G.; Gibbs, J.S.; Corris, P.A.; Peacock, A.J.; Jenkins, D.P.; Goldsmith, K.; Coghlan, J.G.; Pepke-Zaba, J. Prognostic and aetiological factors in chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2009, 33, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, H.; Ogino, H.; Minatoya, K.; Sasaki, H.; Nakanishi, N.; Kyotani, S.; Kobayashi, J.; Yagihara, T.; Kitamura, S. Long-term recovery of exercise ability after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. Ann. Thorac. Surg. 2006, 82, 1338–1343. [Google Scholar] [CrossRef]
- Wieteska, M.; Biederman, A.; Kurzyna, M.; Dyk, W.; Burakowski, J.; Wawrzynska, L.; Szturmowicz, M.; Fijałkowska, A.; Szatkowski, P.; Torbicki, A. Outcome of Medically Versus Surgically Treated Patients with Chronic Thromboembolic Pulmonary Hypertension. Clin. Appl. Thromb. Hemost. 2016, 22, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Kurzyna, M.; Darocha, S.; Koteja, A.; Pietura, R.; Torbicki, A. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Postepy Kardiol. Interwencyjnej. 2015, 11, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.V.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Respir. J. 2015, 46, 903–975. [Google Scholar]
- Kurzyna, M.; Araszkiewicz, A.; Blaszczak, P.; Grabka, M.; Hawranek, M.; Kopec, G.; Mroczek, E.; Zembala, M.; Torbicki, A.; Ochała, A. Summary of recommendations for the haemodynamic and angiographic assessment of the pulmonary circulation. Joint statement of the Polish Cardiac Society’s Working Group on Pulmonary Circulation and Association of Cardiovascular Interventions. Kardiol. Pol. 2015, 73, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Kurzyna, M.; Darocha, S.; Pietura, R.; Pietrasik, A.; Norwa, J.; Manczak, R.; Wieteska, M.; Biederman, A.; Matsubara, H.; Torbicki, A. Changing the strategy of balloon pulmonary angioplasty resulted in a reduced complication rate in patients with chronic thromboembolic pulmonary hypertension. A single-centre European experience. Kardiol. Pol. 2017, 75, 645–654. [Google Scholar] [CrossRef]
- Araszkiewicz, A.; Darocha, S.; Pietrasik, A.; Pietura, R.; Jankiewicz, S.; Banaszkiewicz, M.; Sławek-Szmyt, S.; Biederman, A.; Mularek-Kubzdela, T.; Lesiak, M.; et al. Balloon pulmonary angioplasty for the treatment of residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int. J. Cardiol. 2019, 278, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, H.; Ogawa, A.; Munemasa, M.; Mikouchi, H.; Ito, H.; Matsubara, H. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ. Cardiovasc. Interv. 2012, 5, 748–755. [Google Scholar] [CrossRef] [Green Version]
- Araszkiewicz, A.; Jankiewicz, S.; Łanocha, M.; Janus, M.; Mularek-Kubzdela, T.; Lesiak, M. Optical coherence tomography improves the results of balloon pulmonary angioplasty in inoperable chronic thrombo-embolic pulmonary hypertension. Postepy Kardiol. Interwencyjnej. 2017, 13, 180–181. [Google Scholar] [CrossRef] [Green Version]
- Araszkiewicz, A.; Jankiewicz, S.; Mularek-Kubzdela, T.; Lesiak, M. Stepwise optimisation of balloon pulmonary angioplasty in a patient with severe non-operable chronic thromboembolic pulmonary hypertension. EuroIntervention 2018, 13, 1728–1729. [Google Scholar] [CrossRef]
- Inami, T.; Kataoka, M.; Shimura, N.; Ishiguro, H.; Yanagisawa, R.; Taguchi, H.; Fukuda, K.; Yoshino, H.; Satoh, T. Pulmonary edema predictive scoring index (PEPSI), a new index to predict risk of reperfusion pulmonary edema and improvement of hemodynamics in percutaneous transluminal pulmonary angioplasty. JACC Cardiovasc. Interv. 2013, 6, 725–736. [Google Scholar] [CrossRef] [Green Version]
- Madani, M.; Mayer, E.; Fadel, E.; Jenkins, D.P. Pulmonary Endarterectomy. Patient Selection, Technical Challenges, and Outcomes. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. 3), S240–S247. [Google Scholar] [CrossRef]
- Brenot, P.; Jais, X.; Taniguchi, Y.; Garcia Alonso, C.; Gerardin, B.; Mussot, S.; Mercier, O.; Fabre, D.; Parent, F.; Jevnikar, M.; et al. French experience of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2019, 53, 1802095. [Google Scholar] [CrossRef] [Green Version]
- Olsson, K.M.; Wiedenroth, C.B.; Kamp, J.C.; Breithecker, A.; Fuge, J.; Krombach, G.A.; Haas, M.; Hamm, C.; Kramm, T.; Guth, S.; et al. Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension: The initial German experience. Eur. Respir. J. 2017, 49, 1602409. [Google Scholar] [CrossRef] [PubMed]
- Inami, T.; Kataoka, M.; Yanagisawa, R.; Ishiguro, H.; Shimura, N.; Fukuda, K.; Yoshino, H.; Satoh, T. Long-Term Outcomes After Percutaneous Transluminal Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension. Circulation 2016, 134, 2030–2032. [Google Scholar] [CrossRef]
- Ogawa, A.; Satoh, T.; Fukuda, T.; Sugimura, K.; Fukumoto, Y.; Emoto, N.; Yamada, N.; Yao, A.; Ando, M.; Ogino, H.; et al. Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension: Results of a Multicenter Registry. Circ. Cardiovasc. Qua. Outcomes 2017, 10, e004029. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.M.; Matsubara, H. Balloon pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension: Is Europe behind? Eur. Respir. J. 2019, 53, 1900843. [Google Scholar] [CrossRef] [Green Version]
- Delcroix, M.; Lang, I.; Pepke-Zaba, J.; Jansa, P.; D’Armini, A.M.; Snijder, R.; Bresser, P.; Torbicki, A.; Mellemkjaer, S.; Lewczuk, J.; et al. Long-Term Outcome of Patients with Chronic Thromboembolic Pulmonary Hypertension: Results from an International Prospective Registry. Circulation 2016, 133, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Pepke-Zaba, J.; Delcroix, M.; Lang, I.; Mayer, E.; Jansa, P.; Ambroz, D.; Treacy, C.; D’Armini, A.M.; Morsolini, M.; Snijder, A.; et al. Chronic thromboembolic pulmonary hypertension (CTEPH): Results from an international prospective registry. Circulation 2011, 124, 1973–1981. [Google Scholar] [CrossRef] [Green Version]
- Mayer, E.; Jenkins, D.; Lindner, J.; D’Armini, A.; Kloek, J.; Meyns, B.; Ilkjaer, L.B.; Klepetko, W.; Delcroix, M.; Lang, I.; et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: Results from an international prospective registry. J. Thorac. Cardiovasc. Surg. 2011, 141, 702–710. [Google Scholar] [CrossRef] [Green Version]
- Bonderman, D.; Skoro-Sajer, N.; Jakowitsch, J.; Adlbrecht, C.; Dunkler, D.; Taghavi, S.; Klepetko, W.; Kneussl, M.; Lang, I.M. Predictors of outcome in chronic thromboembolic pulmonary hypertension. Circulation 2007, 115, 2153–2158. [Google Scholar] [CrossRef]
- Condliffe, R.; Kiely, D.G.; Gibbs, J.S.; Corris, P.A.; Peacock, A.J.; Jenkins, D.P.; Hodgkins, D.; Goldsmith, K.; Hughes, R.J.; Sheares, K.; et al. Improved outcomes in medically and surgically treated chronic thromboembolic pulmonary hypertension. Am. J. Respi.r Crit. Care Med. 2008, 177, 1122–1127. [Google Scholar] [CrossRef]
- Jamieson, S.W.; Kapelanski, D.P.; Sakakibara, N.; Manecke, G.R.; Thistlethwaite, P.A.; Kerr, K.M.; Channick, R.N.; Fedullo, P.F.; Auger, W.R. Pulmonary endarterectomy: Experience and lessons learned in 1,500 cases. Ann. Thorac. Surg. 2003, 76, 1457–1462. [Google Scholar] [CrossRef]
- Thistlethwaite, P.A.; Kaneko, K.; Madani, M.M.; Jamieson, S.W. Technique and outcomes of pulmonary endarterectomy surgery. Ann. Thorac. Cardiovasc. Surg. 2008, 14, 274–282. [Google Scholar]
- Amsallem, M.; Guihaire, J.; Arthur, A.J.; Lamrani, L.; Boulate, D.; Mussot, S.; Fabre, D.; Taniguchi, Y.; Haddad, F.; Sitbon, O.; et al. Impact of the initiation of balloon pulmonary angioplasty program on referral of patients with chronic thromboembolic pulmonary hypertension to surgery. J. Heart Lung Transplant. 2018, 37, 1102–1110. [Google Scholar] [CrossRef]
- Kataoka, M.; Inami, T.; Hayashida, K.; Shimura, N.; Ishiguro, H.; Abe, T.; Tamura, Y.; Ando, M.; Fukuda, K.; Yoshino, H.; et al. Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ. Cardiovasc. Interv. 2012, 5, 756–762. [Google Scholar] [CrossRef] [Green Version]
- Ishiguro, H.; Kataoka, M.; Inami, T.; Yanagisawa, R.; Shimura, N.; Taguchi, H.; Kohshoh, H.; Yoshino, H.; Satoh, T. Percutaneous transluminal pulmonary angioplasty for central-type chronic thromboembolic pulmonary hypertension. JACC Cardiovasc. Interv. 2013, 6, 1212–1213. [Google Scholar] [CrossRef] [Green Version]
- Minatsuki, S.; Kiyosue, A.; Kodera, S.; Hara, T.; Saito, A.; Maki, H.; Hatano, M.; Takimoto, E.; Ando, M.; Komuro, I. Effectiveness of balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension despite having lesion types suitable for surgical treatment. J. Cardiol. 2020, 75, 182–188. [Google Scholar] [CrossRef]
- Darocha, S.; Banaszkiewicz, M.; Pietrasik, A.; Siennicka, A.; Piorunek, M.; Grochowska, E.; Piłka, M.; Dobosiewicz, A.; Florczyk, M.; Pietura, R.; et al. Changes in Estimated Glomerular Filtration after Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension. Cardiorenal Med. 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Siennicka, A.; Darocha, S.; Banaszkiewicz, M.; Kedzierski, P.; Dobosiewicz, A.; Blaszczak, P.; Peregud-Pogorzelska, M.; Kasprzak, J.D.; Tomaszewski, M.; Mroczek, E.; et al. Treatment of chronic thromboembolic pulmonary hypertension in a multidisciplinary team. Ther. Adv. Respir. Dis. 2019, 13. [Google Scholar] [CrossRef] [Green Version]
- Shinkura, Y.; Nakayama, K.; Yanaka, K.; Kinutani, H.; Tamada, N.; Tsuboi, Y.; Satomi-Kobayashi, S.; Otake, H.; Shinke, T.; Emoto, N.; et al. Extensive revascularisation by balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension beyond haemodynamic normalisation. EuroIntervention 2018, 13, 2060–2068. [Google Scholar] [CrossRef] [PubMed]
- Lewczuk, J.; Piszko, P.; Jagas, J.; Porada, A.; Wojciak, S.; Sobkowicz, B.; Wrabec, K. Prognostic factors in medically treated patients with chronic pulmonary embolism. Chest 2001, 119, 818–823. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Jais, X.; Jevnikar, M.; Boucly, A.; Weatherald, J.; Brenot, P.; Planche, O.; Parent, F.; Savale, L.; Fadel, E.; et al. Predictors of survival in patients with not-operated chronic thromboembolic pulmonary hypertension. J. Heart Lung Transplant. 2019, 38, 833–842. [Google Scholar] [CrossRef] [Green Version]

| All Patients (n = 70) | d-CTEPH Group (n = 54) | p-CTEPH Group (n = 16) | p | |
|---|---|---|---|---|
| Age (years) | 64.5 (52–73) | 62.5 (48–70) | 73 (62–82) | 0.008 |
| Females (%) | 37 (53%) | 29 (54%) | 8 (50%) | 0.5 |
| BMI (kg/m2) | 25.8 (23.3–28.5) | 25.5 (23.4–28.1) | 26.4 (23.0–33.3) | 0.35 |
| History of PE | 52 (74%) | 41 (76%) | 11 (69%) | 0.39 |
| History of DVT | 24 (34%) | 17 (31%) | 7 (44%) | 0.27 |
| Comorbidities | ||||
| Coronary heart disease | 13 (19%) | 8 (15%) | 5 (31%) | 0.13 |
| Systemic hypertension | 33 (47%) | 24 (44%) | 9 (56%) | 0.29 |
| Type II diabetes | 11 (16%) | 9 (17%) | 2 (13%) | 0.51 |
| COPD | 6 (9%) | 4 (7%) | 2 (13%) | 0.42 |
| Chronic kidney disease (eGFR < 60 mL/min) | 12 (17%) | 6 (11%) | 6 (38%) | 0.02 |
| Atrial fibrillation/flutter | 12 (17%) | 9 (17%) | 3 (19%) | 0.55 |
| Pacemaker leads or ventriculoatrial shunts | 6 (9%) | 6 (11%) | 0 | 0.19 |
| Concomitant therapy | ||||
| Sildenafil | 20 (29%) | 15 (28%) | 5 (31%) | 0.51 |
| Riociguat | 42 (60%) | 31 (57%) | 11 (69%) | 0.30 |
| Prostanoids | 1 (1%) | 1 (2%) | 0 | - |
| PAH-like monotherapy | 61 (87%) | 45 (83%) | 16 (100%) | 0.08 |
| PAH-like combination therapy | 1 (1%) | 1(2%) | 0 | - |
| VKA | 22 (31%) | 17 (31%) | 5 (31%) | 0.9 |
| LMWH | 8 (11%) | 5 (9%) | 3 (19%) | 0.26 |
| DOAC | 40 (57%) | 32 (59%) | 8 (50%) | 0.35 |
| All Patients (n = 70) | |||
|---|---|---|---|
| Before | p | After | |
| HR (bpm) | 77 ± 15 | <0.001 | 68 ± 12 |
| BP systolic (mmHg) | 129 ± 26 | 0.18 | 126 ± 20 |
| BP diastolic (mmHg) | 76 ± 14 | 0.002 | 71 ± 10 |
| WHO class (I–II/III–IV) | 20%/80% | <0.001 | 77%/23% |
| 6mWT (m) | 365 ± 142 | <0.001 | 433 ± 120 |
| NT-pro-BNP (pg/mL) | |||
| (median) | 1307 | 206 | |
| (IQR)(IQR) | (510–3294) | <0.001 | (83–531) |
| mRAP (mmHg) | 9.1 ± 4.4 | <0.001 | 5.6 ± 3.2 |
| PAPs (mmHg) | 81.6 ± 18.3 | <0.001 | 53.8 ± 16.4 |
| PAPd (mmHg) | 28.5 ± 7.4 | <0.001 | 17.0 ± 5.8 |
| PAPm (mmHg) (median) (IQR) | 48.6 ± 10.0 48 (41–55) | <0.001 | 31.3 ± 8.6 30 (26–36) |
| PCWP (mmHg) | 9.9 ± 2.7 | 0.99 | 10.0 ± 3.6 |
| CO (L/min) | 4.96 ± 1.58 | 0.04 | 5.44 ± 1.45 |
| CI (L/min*m2) | 2.75 ± 0.78 | 0.03 | 2.95 ± 0.78 |
| SV (ml) | 65 ± 20 | <0.001 | 80 ± 20 |
| Art Sat. O2(%) | 93.4 ± 3.3 | 0.01 | 94.9 ± 3.8 |
| MV Sat.O2(%) | 65.2 ± 6.8 | <0.001 | 72.2 ± 6.3 |
| PVR (dynes*s*cm−5) (median) (IQR) | 694 ± 296 643 (465–892) | <0.001 | 333 ± 162 282 (229–396) |
| CPa (mL/mmHg) | 1.40 ± 0.76 | <0.001 | 2.39 ± 0.88 |
| Absolute change of mPAP(mmHg) | −16.8 ± 9.9 | ||
| % decrease of mPAP (%) | −34 ± 17 | ||
| % decrease of PVR (%) | −46 ± 22 | ||
| Anatomically Inoperable (d-CTEPH Group) (n = 54) | Inoperable Because of Comorbiditie (p-CTEPH Group) (n = 16) | |||||
|---|---|---|---|---|---|---|
| Before | p | After | Before | p | After | |
| HR (bpm) | 78 ± 15 | <0.001 | 69 ± 13 | 75 ± 16 | 0.04 | 67 ± 9 |
| BP systolic (mmHg) | 126 ± 24 | 0.24 | 121 ± 16 ** | 140 ± 30 | 0.55 | 142 ± 25 ** |
| BP diastolic (mmHg) | 76 ± 14 | <0.001 | 70 ± 10 * | 77 ± 14 | 0.88 | 77 ± 9 * |
| WHO class (I–II/III–IV) | 20%/80% | <0.001 | 77%/23% | 19%/81% | 0.02 | 79%/21% |
| 6mWT (m) | 384 ± 131 | <0.001 | 453 ± 115 * | 300 + 164 | 0.11 | 355 ± 112 * |
| NT-pro-BNP (pg/mL) (median) (IQR) | 1380 (330–3294) | <0.001 | 157 (77–448) | 1288 (754–2870) | <0.001 | 255 (203–897) |
| mRAP (mmHg) | 9.4 ± 4.7 | <0.001 | 5.6 ± 3.4 | 8.4 ± 3.3 | 0.05 | 5.6 ± 2.5 |
| PAPs (mmHg) | 82.1 ± 18.3 | <0.001 | 54.1 ± 17.7 | 80.0 ± 18.5 | 0.001 | 52.9 ± 10.8 |
| PAPd (mmHg) | 29.3±6.7 | <0.001 | 25.8 ± 9.1 * | 25.8 ± 9.1 | 0.02 | 16.9 ± 3.9 * |
| PAPm (mmHg) (median) (IQR) | 49.0 ± 10.0 49 (43–55) | <0.001 | 31.3 ± 9.3 31 (25–36) | 47.1 ± 10.1 46 (41–52) | 0.001 | 31.2 ± 5.6 29 (27–36) |
| PCWP (mmHg) | 9.7 ± 2.8 | 0.65 | 9.4 ± 3.3 * | 10.4 ± 2.3 | 0.44 | 12.1 ± 4.2 * |
| CO (L/min) | 4.94 ± 1.65 | 0.06 | 5.43 ± 1.53 | 5.03 ± 1.39 | 0.43 | 5.47 ± 1.17 |
| CI (L/min*m2) | 2.72 ± 0.78 | 0.04 | 2.95 ± 0.80 | 2.88 ± 0.78 | 0.50 | 2.96 ± 0.67 |
| SV (ml) | 65 ± 21 | <0.001 | 79 ± 20 | 68 ± 18 | 0.01 | 83 ± 18 |
| Art Sat. O2(%) | 93.8 ± 3.1 | 0.01 | 95.2 ± 3.8 | 93.7 ± 3.1 | 0.45 | 93.9 ± 3.6 |
| MV Sat.O2(%) | 65.7 ± 6.7 | <0.001 | 72.5 ± 6.4 | 63.6 ± 7.0 | 0.01 | 70.9 ± 6.1 |
| PVR (dynes*s*cm−5) (median) (IQR) | 713 ± 305 683 (479–935) | <0.001 | 345 ± 176 286 (223–420) | 628 ± 263 556 (429–862) | 0.001 | 288 ± 87 278 (245–303) |
| CPa (mL/mmHg) | 1.38 ± 0.72 | <0.001 | 2.39 ± 0.93 | 1.50 ± 0.89 | 0.01 | 2.39 ± 0.61 |
| Absolute change of mPAP(mmHg) | −17.1 ± 9.4 | −15.9 ± 12.0 | ||||
| % decrease of mPAP (%) | −35 ± 16 | −31 ± 19 | ||||
| % decrease of PVR (%) | −46 ± 23 | −46 ± 19 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darocha, S.; Araszkiewicz, A.; Kurzyna, M.; Banaszkiewicz, M.; Jankiewicz, S.; Dobosiewicz, A.; Sławek-Szmyt, S.; Janus, M.; Grymuza, M.; Pietrasik, A.; et al. Balloon Pulmonary Angioplasty in Technically Operable and Technically Inoperable Chronic Thromboembolic Pulmonary Hypertension. J. Clin. Med. 2021, 10, 1038. https://doi.org/10.3390/jcm10051038
Darocha S, Araszkiewicz A, Kurzyna M, Banaszkiewicz M, Jankiewicz S, Dobosiewicz A, Sławek-Szmyt S, Janus M, Grymuza M, Pietrasik A, et al. Balloon Pulmonary Angioplasty in Technically Operable and Technically Inoperable Chronic Thromboembolic Pulmonary Hypertension. Journal of Clinical Medicine. 2021; 10(5):1038. https://doi.org/10.3390/jcm10051038
Chicago/Turabian StyleDarocha, Szymon, Aleksander Araszkiewicz, Marcin Kurzyna, Marta Banaszkiewicz, Stanisław Jankiewicz, Anna Dobosiewicz, Sylwia Sławek-Szmyt, Magdalena Janus, Maciej Grymuza, Arkadiusz Pietrasik, and et al. 2021. "Balloon Pulmonary Angioplasty in Technically Operable and Technically Inoperable Chronic Thromboembolic Pulmonary Hypertension" Journal of Clinical Medicine 10, no. 5: 1038. https://doi.org/10.3390/jcm10051038







