Globotrioasylsphingosine Levels and Optical Coherence Tomography Angiography in Fabry Disease Patients
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sodi, A.; Guarducci, M.; Vauthier, L.; Ioannidis, A.S.; Pitz, S.; Abbruzzese, G.; Sofi, F.; Mecocci, A.; Miele, A.; Menchini, U. Computer assisted evaluation of retinal vessels tortuosity in Fabry disease. Acta Ophthalmol. 2012, 91, e113–e119. [Google Scholar] [CrossRef]
- Desnick, R.; Ioannou, Y.; Eng, C. α-galactosidase A deficiency: Fabry disease. In The Metabolic and Molecular Bases of Inherited Disease; Scriver, C.R., Beaudet, A.L., Sly, W.S., Valle, D., Eds.; The McGraw-Hill Companies Inc.: New York, NY, USA, 2001; pp. 3733–3774. [Google Scholar]
- Kramer, J.; Weidemann, F. Biomarkers for Diagnosing and Staging of Fabry Disease. Curr. Med. Chem. 2018, 25, 1530–1537. [Google Scholar] [CrossRef]
- San Roman, I.; Rodriguez, M.E.; Caporossi, O.; Zoppetti, C.; Sodi, A.; Mecocci, A.; López, D.; Rodríguez, B.; Gimeno, J.R. Computer Assisted Retinal Vessel Tortuosity Evaluation in Novel Mutation Fabry Disease: Towards New Prognostic Markers. Retina 2017, 37, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Siegenthaler, M.; Huynh-Do, U.; Krayenbuehl, P.; Pollock, E.; Widmer, U.; Debaix, H.; Olinger, E.; Frank, M.; Namdar, M.; Ruschitzka, F.; et al. Impact of cardio-renal syndrome on adverse outcomes in patients with Fabry disease in a long-term follow-up. Int. J. Cardiol. 2017, 249, 261–267. [Google Scholar] [CrossRef]
- Desnick, R.J.; Wasserstein, M.P.; Banikazemi, M. Fabry Disease (α-Galactosidase A Deficiency): Renal Involvement and Enzyme Replacement Therapy. Proteom. Nephrol. 2001, 136, 174–192. [Google Scholar] [CrossRef]
- Meikle, P.J.; Hopwood, J.J.; Clague, A.E.; Carey, W.F. Prevalence of Lysosomal Storage Disorders. JAMA 1999, 281, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Desnick, R.J.; Brady, R.; Barranger, J.; Collins, A.J.; Germain, D.P.; Goldman, M.; Grabowski, G.; Packman, S.; Wilcox, W.R. Fabry Disease, an Under-Recognized Multisystemic Disorder: Expert Recommendations for Diagnosis, Management, and Enzyme Replacement Therapy. Ann. Intern. Med. 2003, 138, 338–346. [Google Scholar] [CrossRef]
- Desnick, R.J.; Ioannou, Y.A.; Eng, C.M. α-Galactosidase A Deficiency: Fabry Disease. In The Online Metabolic and Molecular Bases of Inherited Disease; Beaudet, A.L., Vogelstein, B., Kinzler, K.W., Eds.; The McGraw-Hill Companies Inc.: New York, NY, USA, 2014. [Google Scholar]
- Echevarria, L.; Benistan, K.; Toussaint, A.; Dubourg, O.; Hagege, A.A.; Eladari, D.; Jabbour, F.; Beldjord, C.; De Mazancourt, P.; Germain, D.P. X-chromosome inactivation in female patients with Fabry disease. Clin. Genet. 2016, 89, 44–54. [Google Scholar] [CrossRef]
- Germain, D.P.; Charrow, J.; Desnick, R.J.; Guffon, N.; Kempf, J.; Lachmann, R.H.; Lemay, R.; Linthorst, G.E.; Packman, S.; Scott, C.R.; et al. Ten-year outcome of enzyme replacement therapy with agalsidase beta in patients with Fabry disease. J. Med Genet. 2015, 52, 353–358. [Google Scholar] [CrossRef]
- Francois, J.; Hanssens, M.; Teuchy, H. Corneal Ultrastructural Changes in Fabry’s Disease. Int. J. Ophthalmol. 1978, 176, 313–330. [Google Scholar] [CrossRef]
- Tuppurainen, K.; Collan, Y.; Rantanen, T.; Hollmen, A. Fabry’s disease and cornea verticillata. A report of 3 cases. Acta Ophthalmol. 1981, 59, 674–682. [Google Scholar] [CrossRef]
- Macrae, W.; Ghosh, M.; McCulloch, C. Corneal changes in Fabry’s disease: A clinico-pathologic case report of a heterozygote. Ophthalmic Paediatr. Genet. 1985, 5, 185–190. [Google Scholar] [CrossRef]
- Sodi, A.; Ioannidis, A.S.; Mehta, A.; Davey, C.; Beck, M.; Pitz, S. Ocular manifestations of Fabry’s disease: Data from the Fabry Outcome Survey. Br. J. Ophthalmol. 2007, 91, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Samiy, N. Ocular Features of Fabry Disease: Diagnosis of a Treatable Life-threatening Disorder. Surv. Ophthalmol. 2008, 53, 416–423. [Google Scholar] [CrossRef]
- Desnick, R.J. Enzyme replacement therapy for Fabry disease: Lessons from two α-galactosidase A orphan products and one FDA approval. Expert Opin. Biol. Ther. 2004, 4, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Desnick, R.; Schuchman, E. Enzyme Replacement Therapy for Lysosomal Diseases: Lessons from 20 Years of Experience and Remaining Challenges. Annu. Rev. Genom. Hum. Genet. 2012, 13, 307–335. [Google Scholar] [CrossRef] [PubMed]
- Schiffmann, R.; Kopp, J.B.; Austin, H.A., III; Sabnis, S.; Moore, D.F.; Weibel, T.; Balow, J.E.; Brady, R.O. Enzyme replacement therapy in Fabry disease: A randomized controlled trial. JAMA 2001, 285, 2743–2749. [Google Scholar] [CrossRef] [PubMed]
- Germain, D.P.; Hughes, D.A.; Nicholls, K.; Bichet, D.G.; Giugliani, R.; Wilcox, W.R.; Feliciani, C.; Shankar, S.P.; Ezgu, F.; Amartino, H.; et al. Treatment of Fabry’s Disease with the Pharmacologic Chaperone Migalastat. N. Engl. J. Med. 2016, 375, 545–555. [Google Scholar] [CrossRef]
- Germain, D.P.; Elliott, P.M.; Falissard, B.; Fomin, V.V.; Hilz, M.J.; Jovanovic, A.; Kantola, I.; Linhart, A.; Mignani, R.; Namdar, M.; et al. The effect of enzyme replacement therapy on clinical outcomes in male patients with Fabry disease: A systematic literature review by a European panel of experts. Mol. Genet. Metab. Rep. 2019, 19, 100454. [Google Scholar] [CrossRef]
- Lenders, M.; Brand, E. Effects of Enzyme Replacement Therapy and Antidrug Antibodies in Patients with Fabry Disease. J. Am. Soc. Nephrol. 2018, 29, 2265–2278. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]
- Spaide, R.F.; Klancnik, J.M.; Cooney, M.J. Retinal Vascular Layers Imaged by Fluorescein Angiography and Optical Coherence Tomography Angiography. JAMA Ophthalmol. 2015, 133, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Bonfiglio, V.; Ortisi, E.; Scollo, D.; Reibaldi, M.; Russo, A.; Pizzo, A.; Faro, G.; Macchi, I.; Fallico, M.; Toro, M.D.; et al. Vascular changes after vitrectomy for rhegmatogenous retinal detachment: Optical coherence tomography angiography study. Acta Ophthalmol. 2019, 98, e563–e569. [Google Scholar] [CrossRef]
- Wrzesińska, D.; Nowomiejska, K.; Nowakowska, D.; Toro, M.D.; Bonfiglio, V.; Reibaldi, M.; Avitabile, T.; Rejdak, R. Secondary Vitrectomy with Internal Limiting Membrane Plug due to Persistent Full-Thickness Macular Hole OCT-Angiography and Microperimetry Features: Case Series. J. Ophthalmol. 2020, 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Carnevali, A.; Mastropasqua, R.; Gatti, V.; Vaccaro, S.; Mancini, A.; D’Aloisio, R.; Lupidi, M.; Cerquaglia, A.; Sacconi, R.; Borrelli, E.; et al. Optical Coherence Tomography Angiography in Intermediate and Late Age-Related Macular Degeneration: Review of Current Technical Aspects and Applications. Appl. Sci. 2020, 10, 8865. [Google Scholar] [CrossRef]
- Cennamo, G.; Di Maio, L.G.; Montorio, D.; Tranfa, F.; Russo, C.; Pontillo, G.; Cocozza, S.; Esposito, R.; Di Risi, T.; Imbriaco, M.; et al. Optical Coherence Tomography Angiography Findings in Fabry Disease. J. Clin. Med. 2019, 8, 528. [Google Scholar] [CrossRef]
- Minnella, A.M.; Barbano, L.; Verrecchia, E.; Martelli, F.; Pagliei, V.; Gambini, G.; Placidi, G.; Falsini, B.; Caporossi, A.; Manna, R. Macular Impairment in Fabry Disease: A Morpho-functional Assessment by Swept-Source OCT Angiography and Focal Electroretinography. Investig. Opthalmol. Vis. Sci. 2019, 60, 2667–2675. [Google Scholar] [CrossRef]
- Cakmak, A.I.; Atalay, E.; Cankurtaran, V.; Yaşar, E.; Turgut, F.H. Optical coherence tomography angiography analysis of fabry disease. Int. Ophthalmol. 2020, 40, 3023–3032. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, G.; Montorio, D.; Santoro, C.; Cocozza, S.; Spinelli, L.; Di Risi, T.; Riccio, E.; Russo, C.; Pontillo, G.; Esposito, R.; et al. The Retinal Vessel Density as a New Vascular Biomarker in Multisystem Involvement in Fabry Disease: An Optical Coherence Tomography Angiography Study. J. Clin. Med. 2020, 9, 4087. [Google Scholar] [CrossRef]
- Donati, S.; Maresca, A.M.; Cattaneo, J.; Grossi, A.; Mazzola, M.; Caprani, S.M.; Premoli, L.; Docchio, F.; Rizzoni, D.; Guasti, L.; et al. Optical coherence tomography angiography and arterial hypertension: A role in identifying subclinical microvascular damage? Eur. J. Ophthalmol. 2021, 31, 158–165. [Google Scholar] [CrossRef]
- Gold, H.; Mirzaian, M.; Dekker, N.; Ferraz, M.J.; Lugtenburg, J.; Codée, J.D.C.; Van Der Marel, G.A.; Overkleeft, H.S.; Linthorst, G.E.; Groener, J.E.M.; et al. Quantification of Globotriaosylsphingosine in Plasma and Urine of Fabry Patients by Stable Isotope Ultraperformance Liquid Chromatography–Tandem Mass Spectrometry. Clin. Chem. 2013, 59, 547–556. [Google Scholar] [CrossRef]
- Nowak, A.; Mechtler, T.; Kasper, D.C.; Desnick, R.J. Correlation of Lyso-Gb3 levels in dried blood spots and sera from patients with classic and Later-Onset Fabry disease. Mol. Genet. Metab. 2017, 121, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Early Treatment Diabetic Retinopathy Study Design and Baseline Patient Characteristics; ETDRS report number 7. Ophthalmology 1991, 98, 741–756. [CrossRef]
- Al-Sheikh, M.; Falavarjani, K.G.; Akil, H.; Sadda, S.R. Impact of image quality on OCT angiography based quantitative measurements. Int. J. Retin. Vitr. 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Carnevali, A.; Sacconi, R.; Corbelli, E.; Tomasso, L.; Querques, L.; Zerbini, G.; Scorcia, V.; Bandello, F.; Querques, G. Optical coherence tomography angiography analysis of retinal vascular plexuses and choriocapillaris in patients with type 1 diabetes without diabetic retinopathy. Acta Diabetol. 2017, 54, 695–702. [Google Scholar] [CrossRef]
- Cicinelli, M.V.; Rabiolo, A.; Marchese, A.; De Vitis, L.; Carnevali, A.; Querques, L.; Bandello, F.; Querques, G. Choroid morphometric analysis in non-neovascular age-related macular degeneration by means of optical coherence tomography angiography. Br. J. Ophthalmol. 2017, 101, 1193–1200. [Google Scholar] [CrossRef]
- Wanner, C.; Feldt-Rasmussen, U.; Jovanovic, A.; Linhart, A.; Yang, M.; Ponce, E.; Brand, E.; Germain, D.P.; Hughes, D.A.; Jefferies, J.L.; et al. Cardiomyopathy and kidney function in agalsidase beta-treated female Fabry patients: A pre-treatment vs. post-treatment analysis. ESC Hear. Fail. 2020, 7, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Franzen, D.; Haile, S.R.; Kasper, D.C.; Mechtler, T.P.; Flammer, A.J.; Krayenbühl, P.A.; Nowak, A. Pulmonary involvement in Fabry disease: Effect of plasma globotriaosylsphingosine and time to initiation of enzyme replacement therapy. BMJ Open Respir. Res. 2018, 5, e000277. [Google Scholar] [CrossRef]
- Sims, K.; Politei, J.; Banikazemi, M.; Lee, P. Stroke in Fabry disease frequently occurs before diagnosis and in the absence of other clinical events: Natural history data from the Fabry Registry. Stroke 2009, 40, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Mechtler, T.P.; Hornemann, T.; Gawinecka, J.; Theswet, E.; Hilz, M.J.; Kasper, D.C. Genotype, phenotype and disease severity reflected by serum LysoGb3 levels in patients with Fabry disease. Mol. Genet. Metab. 2018, 123, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Aerts, J.M.; Groener, J.E.; Kuiper, S.; Donker-Koopman, W.E.; Strijland, A.; Ottenhoff, R.; Van Roomen, C.; Mirzaian, M.; Wijburg, F.A.; Linthorst, G.E.; et al. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc. Natl. Acad. Sci. USA 2008, 105, 2812–2817. [Google Scholar] [CrossRef]
- Jung, S.C.; Park, E.S.; Choi, J.O.; Park, J.W.; Lee, M.H.; Park, H.Y. Expression of genes and their responses to enzyme replacement therapy in a Fabry disease mouse model. Int. J. Mol. Med. 2009, 24, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Rozenfeld, P.; Agriello, E.; De Francesco, N.; Martinez, P.; Fossati, C. Leukocyte perturbation associated with Fabry disease. J. Inherit. Metab. Dis. 2009, 32, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Biancini, G.B.; Vanzin, C.S.; Rodrigues, D.B.; Deon, M.; Ribas, G.S.; Barschak, A.G.; Manfredini, V.; Netto, C.B.; Jardim, L.B.; Giugliani, R.; et al. Globotriaosylceramide is correlated with oxidative stress and inflammation in Fabry patients treated with enzyme replacement therapy. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 226–232. [Google Scholar] [CrossRef]
- Biancini, G.B.; Moura, D.J.; Manini, P.R.; Faverzani, J.L.; Netto, C.B.O.; Deon, M.; Giugliani, R.; Saffi, J.; Vargas, C.R. DNA damage in Fabry patients: An investigation of oxidative damage and repair. Mutat. Res. Toxicol. Environ. Mutagen. 2015, 784–785, 31–36. [Google Scholar] [CrossRef]
- Matafora, V.; Cuccurullo, M.; Beneduci, A.; Petrazzuolo, O.; Simeone, A.; Anastasio, P.; Mignani, R.; Feriozzi, S.; Pisani, A.; Comotti, C.; et al. Early markers of Fabry disease revealed by proteomics. Mol. Biosyst. 2015, 11, 1543–1551. [Google Scholar] [CrossRef]
- Ko, Y.; Lee, C.; Moon, M.H.; Hong, G.R.; Cheon, C.K.; Lee, J.S. Unravelling the mechanism of action of enzyme replacement therapy in Fabry disease. J. Hum. Genet. 2015, 61, 143–149. [Google Scholar] [CrossRef]
- Weidemann, F.; Sanchez-Niño, M.D.; Politei, J.; Oliveira, J.P.; Wanner, C.; Warnock, D.G.; Ortiz, A. Fibrosis: A key feature of Fabry disease with potential therapeutic implications. Orphanet J. Rare Dis. 2013, 8, 116. [Google Scholar] [CrossRef]
- Nowak, A.; Mechtler, T.P.; Desnick, R.J.; Kasper, D.C. Plasma LysoGb3: A useful biomarker for the diagnosis and treatment of Fabry disease heterozygotes. Mol. Genet. Metab. 2017, 120, 57–61. [Google Scholar] [CrossRef]
- Nowak, A.; Huynh-Do, U.; Krayenbuehl, P.; Beuschlein, F.; Schiffmann, R.; Barbey, F. Fabry disease genotype, phenotype, and migalastat amenability: Insights from a national cohort. J. Inherit. Metab. Dis. 2020, 43, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Cordon, B.; Vilades, E.; Orduna, E.; Satue, M.; Perez-Velilla, J.; Sebastian, B.; Polo, V.; Larrosa, J.M.; Pablo, L.E.; Garcia-Martin, E. Angiography with optical coherence tomography as a biomarker in multiple sclerosis. PLoS ONE 2020, 15, e0243236. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Vagge, A.; Desideri, L.F.F.; Bernabei, F.; Triolo, G.; Mastropasqua, R.; Del Del Noce, C.; Borrelli, E.; Sacconi, R.; Iovino, C.; et al. Optical Coherence Tomography Angiography in Neurodegenerative Disorders. J. Clin. Med. 2020, 9, 1706. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Pan, X.; Mao, K.; Jiao, Q.; Chen, Y.; Zhong, Y.; Cheng, Y. Quantitative evaluation of retinal and choroidal changes in Fabry disease using optical coherence tomography angiography. Lasers Med. Sci. 2021, 1–9. [Google Scholar] [CrossRef]
| FD Patients n = 57 | ||
|---|---|---|
| gender | male | 23 |
| female | 34 | |
| mean age (years) ± SD | 43.4 ± 15.3 | |
| FD phenotype | classic | 41 |
| later-onset | 16 | |
| mean lysoGb3 (ng/mL) ± SD | overall | 16.7 ± 21.03 |
| male | 31.8 ± 27.03 | |
| female | 6.9 ± 3.52 | |
| enzyme replacement therapy * | yes | 40 |
| no | 17 | |
| Mutation | Frequency | ||||
|---|---|---|---|---|---|
| Pooled FD | Male * | Female * | Classic * | Later-Onset * | |
| c.1033T > C | 6 | 1 | 5 | 6 | 0 |
| c.1055-1057dupCTA | 2 | 1 | 1 | 2 | 0 |
| c.114delCTT | 1 | 0 | 1 | 1 | 0 |
| c.1167dupT | 2 | 0 | 2 | 2 | 0 |
| c.1168insT | 2 | 0 | 2 | 2 | 0 |
| c.1196G > C | 1 | 1 | 0 | 0 | 1 |
| c.125T > C | 3 | 1 | 2 | 3 | 0 |
| c.337T > C | 8 | 3 | 5 | 1 | 7 |
| c.370-2A > G | 1 | 1 | 0 | 1 | 0 |
| c.514T > C | 1 | 0 | 1 | 1 | 0 |
| c.518C > T | 1 | 1 | 0 | 1 | 0 |
| c.559-560delAT | 1 | 1 | 0 | 1 | 0 |
| c.581C > T | 7 | 2 | 5 | 7 | 0 |
| c.613C > T | 1 | 1 | 0 | 0 | 1 |
| c.640-3C > G | 1 | 0 | 1 | 1 | 0 |
| c.644A > G | 1 | 1 | 0 | 0 | 1 |
| c.680G > A | 1 | 0 | 1 | 1 | 0 |
| c.704C > G | 2 | 0 | 2 | 2 | 0 |
| c.743-744delTA | 2 | 1 | 1 | 2 | 0 |
| c.744-745delTA | 3 | 2 | 1 | 3 | 0 |
| c.796G > T | 1 | 1 | 0 | 1 | 0 |
| c.827G > A | 1 | 1 | 0 | 1 | 0 |
| c.870G > C | 1 | 0 | 1 | 0 | 1 |
| c.899T > A | 1 | 1 | 0 | 1 | 0 |
| c.901C > T | 1 | 2 | 1 | 1 | 0 |
| c.902G > A | 5 | 3 | 2 | 0 | 5 |
| Eyes n = 109 | ||
|---|---|---|
| laterality | right | 57 |
| left | 52 | |
| cornea verticillata | yes | 74 |
| no | 35 | |
| retinal vessel tortuosity | yes | 52 |
| no | 57 | |
| mean BCVA(EDTRS letters) | 85.32 ± 4.92 | |
| mean IOP(mmHg) | 14.8 ± 2.9 | |
| OCTA Parameter | Mean | ±SD | |
|---|---|---|---|
| pooled FD group | |||
| SCP | VD | 0.382 | 0.023 |
| VLD | 17.183 | 1.261 | |
| DCP | VD | 0.275 | 0.049 |
| VLD | 12.747 | 2.248 | |
| male FD subgroup | |||
| SCP | VD | 0.379 | 0.020 |
| VLD | 16.955 | 1.113 | |
| DCP | VD | 0.262 | 0.049 |
| VLD | 12.137 | 2.207 | |
| female FD subgroup | |||
| SCP | VD | 0.384 | 0.026 |
| VLD | 17.338 | 1.340 | |
| DCP | VD | 0.283 | 0.047 |
| VLD | 13.167 | 2.196 | |
| classic phenotype FD subgroup | |||
| SCP | VD | 0.379 | 0.025 |
| VLD | 16.983 | 1.276 | |
| DCP | VD | 0.269 | 0.051 |
| VLD | 12.486 | 2.320 | |
| later-onset phenotype FD subgroup | |||
| SCP | VD | 0.391 | 0.017 |
| VLD | 17.712 | 1.073 | |
| DCP | VD | 0.289 | 0.043 |
| VLD | 13.427 | 1.922 | |
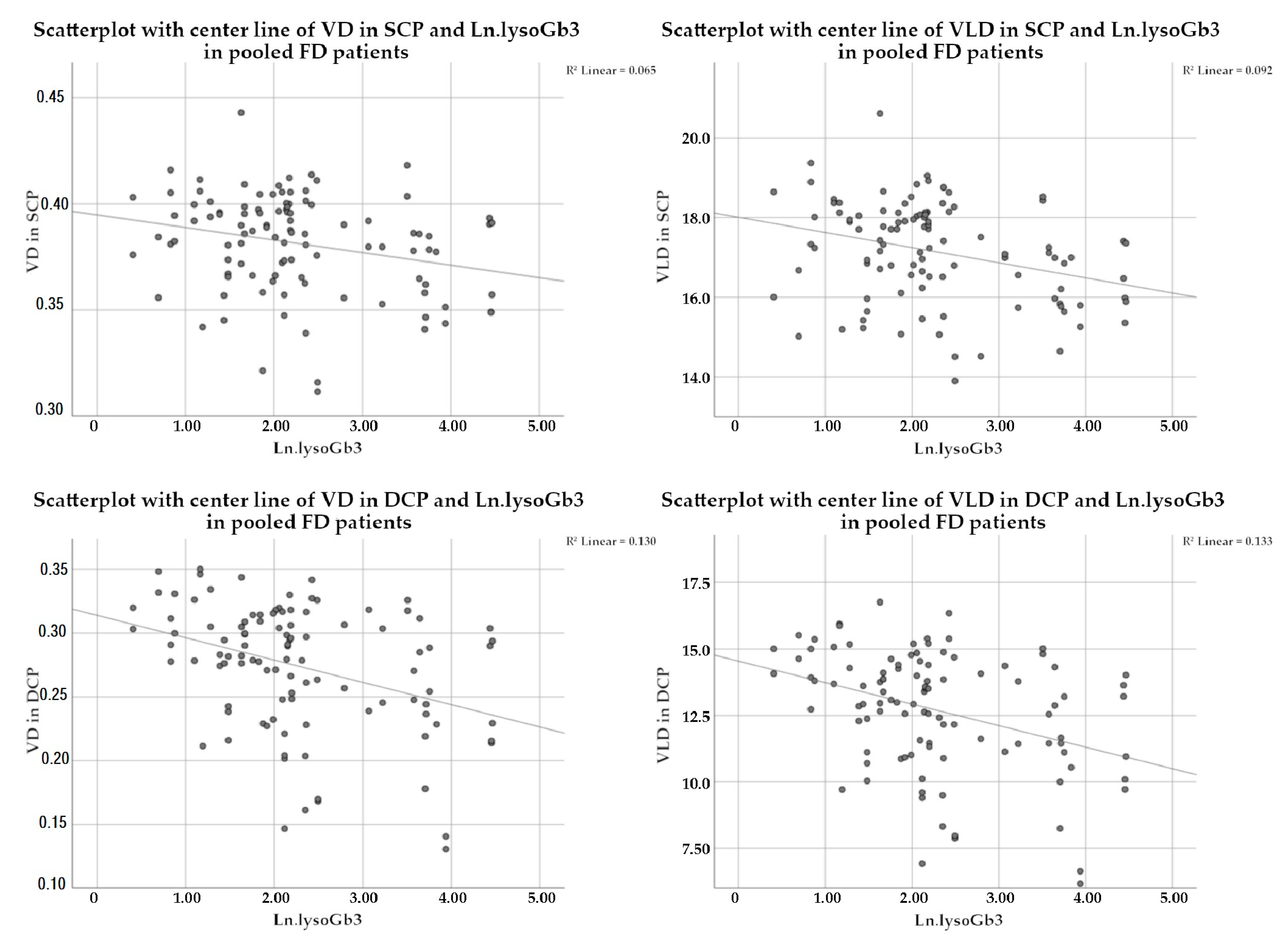
| OCTA Parameter | Regression Estimator | SE | p-Value * | |
|---|---|---|---|---|
| pooled FD group | ||||
| SCP | VD | −0.000338 | 0.000155 | 0.034 |
| VLD | −0.020157 | 0.008128 | 0.017 | |
| DCP | VD | −0.000714 | 0.000297 | 0.020 |
| VLD | −0.032385 | 0.013286 | 0.018 | |
| male FD subgroup | ||||
| SCP | VD | −0.000349 | 0.000129 | 0.014 |
| VLD | −0.021716 | 0.006728 | 0.005 | |
| DCP | VD | −0.000655 | 0.000359 | 0.084 |
| VLD | −0.030220 | 0.015725 | 0.070 | |
| female FD subgroup | ||||
| SCP | VD | −0.000287 | 0.001157 | 0.806 |
| VLD | −0.018082 | 0.060351 | 0.766 | |
| DCP | VD | −0.001237 | 0.001745 | 0.484 |
| VLD | −0.053284 | 0.080509 | 0.513 | |
| classic phenotype FD subgroup | ||||
| SCP | VD | −0.000142 | 0.000234 | 0.547 |
| VLD | −0.009219 | 0.011554 | 0.430 | |
| DCP | VD | −0.000333 | 0.000413 | 0.426 |
| VLD | −0.014732 | 0.018550 | 0.432 | |
| later-onset phenotype FD subgroup | ||||
| SCP | VD | −0.001530 | 0.002049 | 0.471 |
| VLD | −0.051812 | 0.143124 | 0.725 | |
| DCP | VD | −0.004082 | 0.005504 | 0.474 |
| VLD | −0.128910 | 0.243568 | 0.607 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiest, M.R.J.; Toro, M.D.; Nowak, A.; Baur, J.; Fasler, K.; Hamann, T.; Al-Sheikh, M.; Zweifel, S.A. Globotrioasylsphingosine Levels and Optical Coherence Tomography Angiography in Fabry Disease Patients. J. Clin. Med. 2021, 10, 1093. https://doi.org/10.3390/jcm10051093
Wiest MRJ, Toro MD, Nowak A, Baur J, Fasler K, Hamann T, Al-Sheikh M, Zweifel SA. Globotrioasylsphingosine Levels and Optical Coherence Tomography Angiography in Fabry Disease Patients. Journal of Clinical Medicine. 2021; 10(5):1093. https://doi.org/10.3390/jcm10051093
Chicago/Turabian StyleWiest, Maximilian Robert Justus, Mario Damiano Toro, Albina Nowak, Joel Baur, Katrin Fasler, Timothy Hamann, Mayss Al-Sheikh, and Sandrine Anne Zweifel. 2021. "Globotrioasylsphingosine Levels and Optical Coherence Tomography Angiography in Fabry Disease Patients" Journal of Clinical Medicine 10, no. 5: 1093. https://doi.org/10.3390/jcm10051093
APA StyleWiest, M. R. J., Toro, M. D., Nowak, A., Baur, J., Fasler, K., Hamann, T., Al-Sheikh, M., & Zweifel, S. A. (2021). Globotrioasylsphingosine Levels and Optical Coherence Tomography Angiography in Fabry Disease Patients. Journal of Clinical Medicine, 10(5), 1093. https://doi.org/10.3390/jcm10051093







