Platelet Phenotyping and Function Testing in Thrombocytopenia
Abstract
:1. Introduction
2. Indications for Testing Platelet Phenotype and Function in Thrombocytopenia
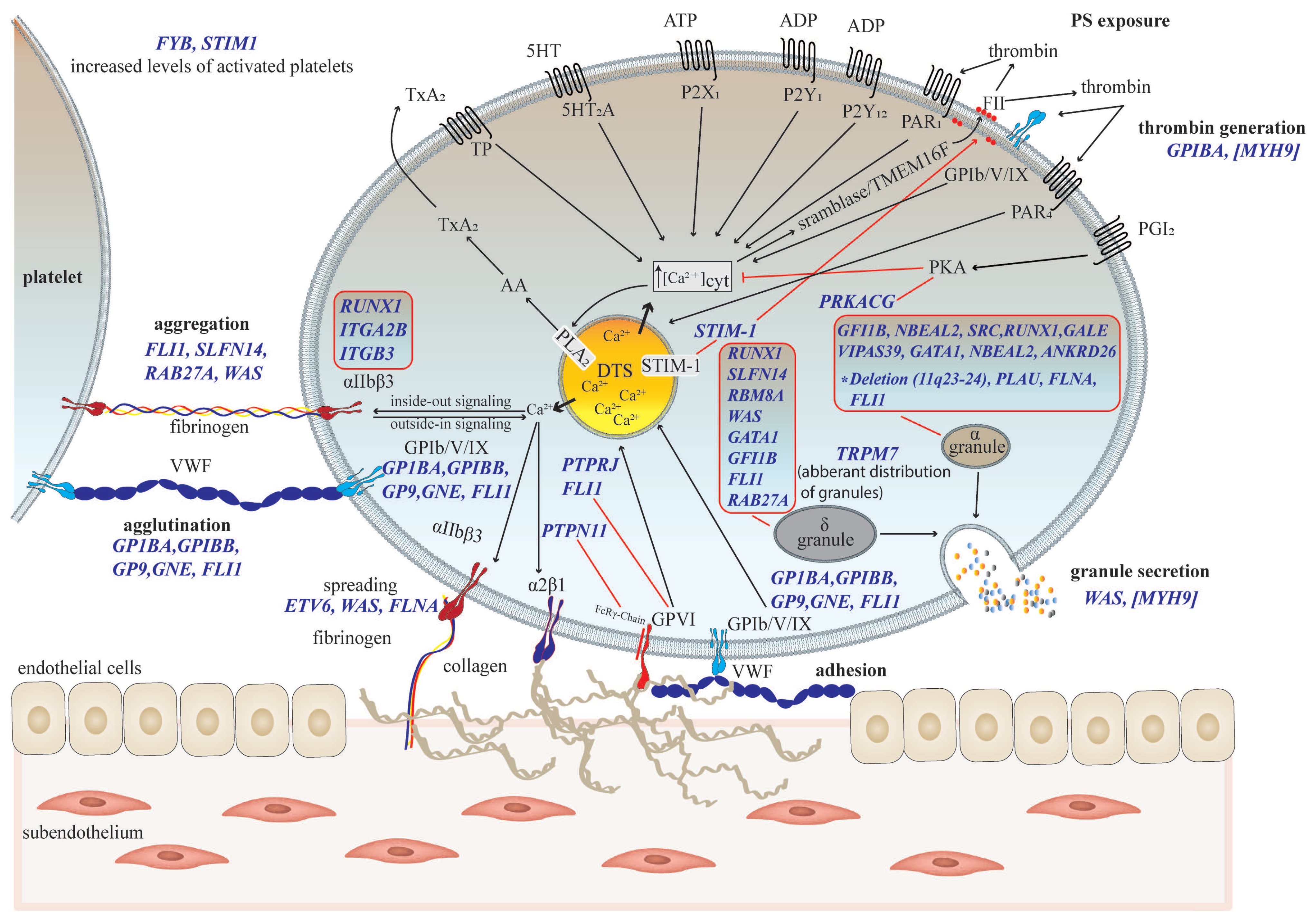
3. Platelet Phenotyping in Thrombocytopenia
3.1. Bleeding Assessment Tool (BAT)
3.2. Laboratory Assessment of Platelet Count, Size and Global Morphology
3.3. Blood Smear Analysis by Immunofluorescence Microscopy
3.4. Quantitation of Immature/Reticulated Platelets
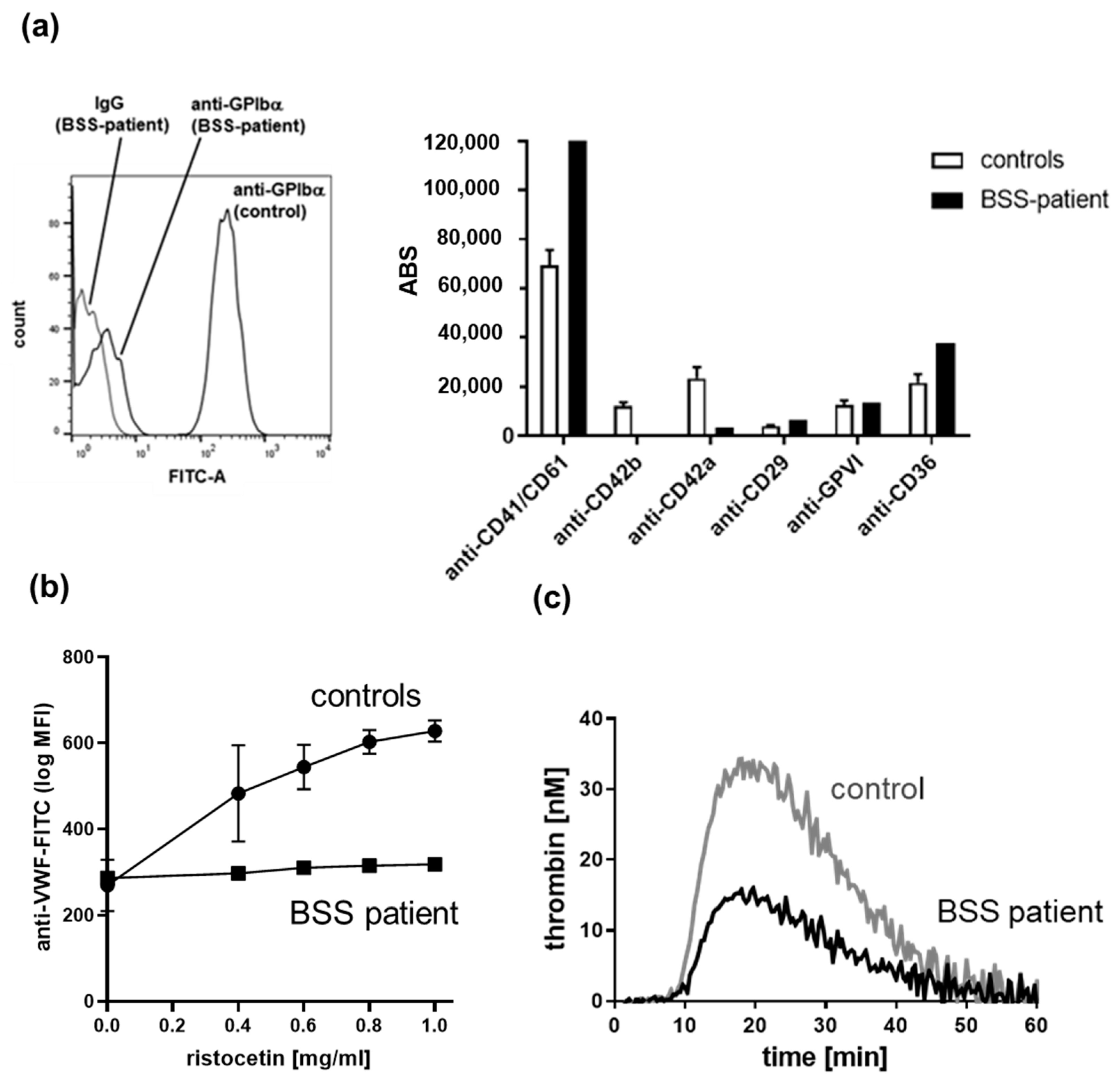
3.5. Flow Cytometry
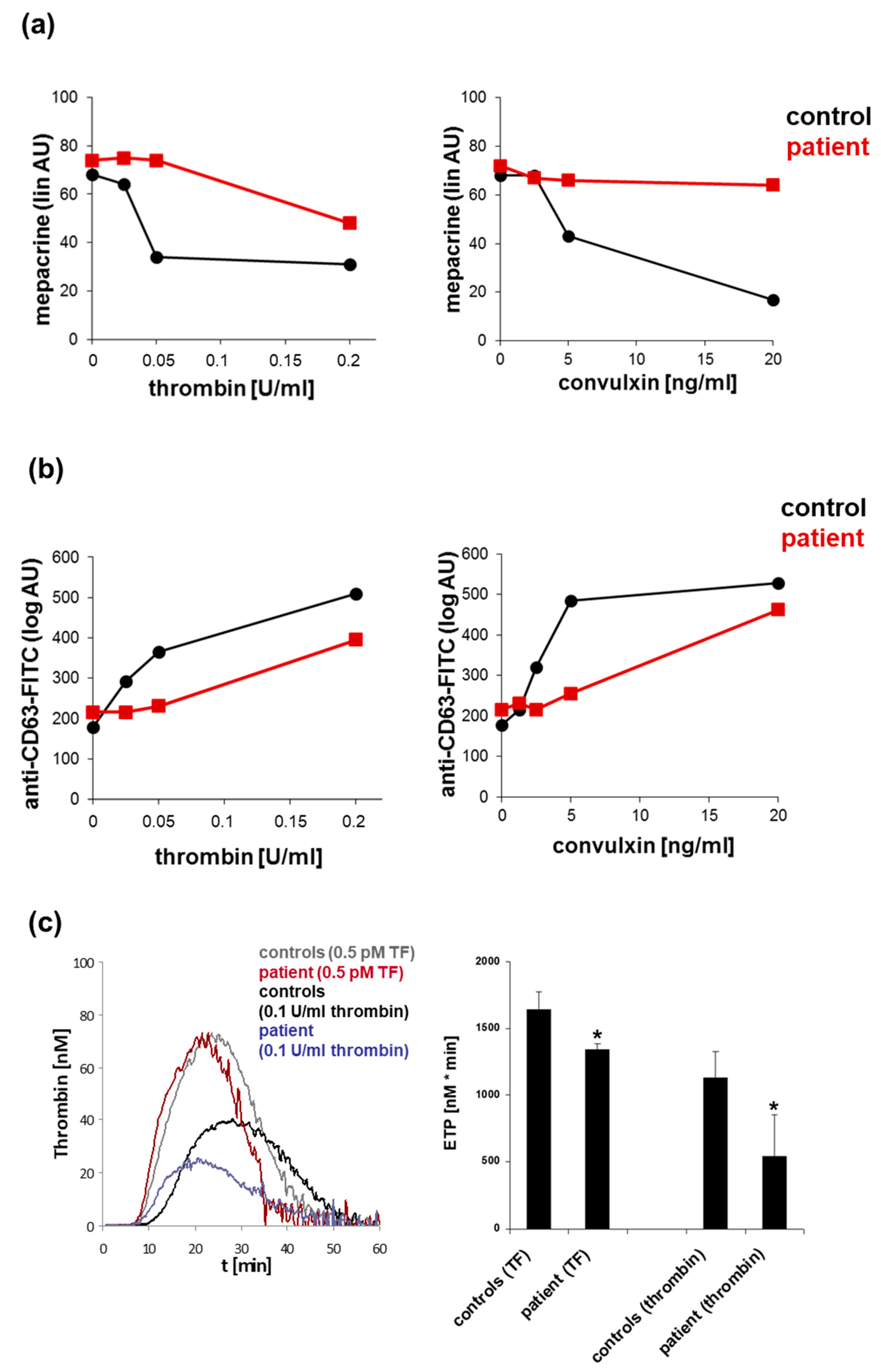
3.6. Phenotyping of Platelet Granule Defects by Electron Microscopy and ELISA
4. Point-of-Care-Related Platelet Function Tests
4.1. Impedance-Based Aggregometry—Multiplate® Analyzer
4.2. Platelet Function Analyzer (PFA)
4.3. Impact-R™ System
4.4. Thromboelastography/Metry
5. Specialized Platelet Function Tests
5.1. Light Transmission Aggregometry in Platelet-Rich Plasma (LTA)
5.2. Lumi-Aggregometry in Platelet-Rich Plasma
5.3. Flow Cytometry (Whole Blood, Platelet-Rich Plasma)
5.4. Platelet-Based Thrombin Generation Tests (Platelet-Rich Plasma)
5.5. Microfluidics (Whole Blood)
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mazzano, D.; Pereira, J. Approach to the patient with platelet-related bleeding. In Platelets in Thrombotic and Non-Thrombotic Disorders: Pathophysiology, Pharmacology and Therapeutics: An Update; Gresele, P., Kleiman, N.S., Lopez, J.A., Page, C.P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 717–725. [Google Scholar]
- Harrison, P.; Mackie, I.; Mumford, A.; Briggs, C.; Liesner, R.; Winter, M.; Machin, S.; British Committee for Standards in Haematology. Guidelines for the laboratory investigation of heritable disorders of platelet function. Br. J. Haematol. 2011, 155, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Gresele, P.; Subcommittee on Platelet Physiology of the International Society on Thrombosis and Hemostasis. Diagnosis of inherited platelet function disorders: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2015, 13, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Hayward, C.P.M.; Moffat, K.A.; Brunet, J.; Carlino, S.A.; Plumhoff, E.; Meijer, P.; Zehnder, J.L. Update on diagnostic testing for platelet function disorders: What is practical and useful? Int. J. Lab. Hematol. 2019, 41 (Suppl. 1), 26–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gresele, P.; Bury, L.; Falcinelli, E. Inherited Platelet Function Disorders: Algorithms for Phenotypic and Genetic Investigation. Semin. Thromb. Hemost. 2016, 42, 292–305. [Google Scholar] [CrossRef]
- Slichter, S.J.; Kaufman, R.M.; Assmann, S.F.; McCullough, J.; Triulzi, D.J.; Strauss, R.G.; Gernsheimer, T.B.; Ness, P.M.; Brecher, M.E.; Josephson, C.D.; et al. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N. Engl. J. Med. 2010, 362, 600–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, M.P. Platelets in liver and renal disease. Hematol. Am. Soc. Hematol. Educ. Program. 2016, 2016, 251–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, P.; Cook, D.J.; Lim, W.; Fraser, G.A.; Arnold, D.M. The frequency and clinical significance of thrombocytopenia complicating critical illness: A systematic review. Chest 2011, 139, 271–278. [Google Scholar] [CrossRef]
- Vinholt, P.J. The role of platelets in bleeding in patients with thrombocytopenia and hematological disease. Clin. Chem. Lab. Med. 2019, 57, 1808–1817. [Google Scholar] [CrossRef]
- Witters, P.; Freson, K.; Verslype, C.; Peerlinck, K.; Hoylaerts, M.; Nevens, F.; Van Geet, C.; Cassiman, D. Review article: Blood platelet number and function in chronic liver disease and cirrhosis. Aliment. Pharmacol. Ther. 2008, 27, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Assinger, A.; Schrottmaier, W.C.; Salzmann, M.; Rayes, J. Platelets in Sepsis: An Update on Experimental Models and Clinical Data. Front. Immunol. 2019, 10, 1687. [Google Scholar] [CrossRef]
- Lutz, P.; Jurk, P. Platelets in Advanced Chronic Kidney Disease: Two Sides of the Coin. Semin. Thromb. Hemost. 2020, 46, 342–356. [Google Scholar] [CrossRef]
- Scharf, R.E. Acquired disorders of platelet function. In Platelets in Thrombotic and Non-Thrombotic Disorders: Pathophysiology, Pharmacology and Therapeutics: An Update; Gresele, P., Kleiman, N.S., Lopez, J.A., Page, C.P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 951–973. [Google Scholar]
- Thorup, C.V.; Christensen, S.; Hvas, A.M. Immature Platelets As a Predictor of Disease Severity and Mortality in Sepsis and Septic Shock: A Systematic Review. Semin. Thromb. Hemost. 2020, 46, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Danese, E.; Montagnana, M.; Favaloro, E.J.; Lippi, G. Drug-Induced Thrombocytopenia: Mechanisms and Laboratory Diagnostics. Semin. Thromb. Hemost. 2020, 46, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Bakchoul, T.; Marini, I. Drug-associated thrombocytopenia. Hematol. Am. Soc. Hematol. Educ. Program. 2018, 2018, 576–583. [Google Scholar] [CrossRef] [Green Version]
- Schlagenhauf, A.; Kalbhenn, J.; Geisen, U.; Beyersdorf, F.; Zieger, B. Acquired von Willebrand Syndrome and Platelet Function Defects during Extracorporeal Life Support (Mechanical Circulatory Support). Hamostaseologie 2020, 40, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Balduini, C.L.; Pecci, A. Inherited thrombocytopenias. In Platelets in Thrombotic and Non-Thrombotic Disorders: Pathophysiology, Pharmacology and Therapeutics: An Update; Gresele, P., Kleiman, N.S., Lopez, J.A., Page, C.P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 727–747. [Google Scholar]
- Pecci, A.; Balduini, C.L. Inherited thrombocytopenias: An updated guide for clinicians. Blood Rev. 2020, 100784. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim-Kosta, M.; Alessi, M.C.; Hezard, N. Laboratory Techniques Used to Diagnose Constitutional Platelet Dysfunction. Hamostaseologie 2020, 40, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Almazni, I.; Stapley, R.; Morgan, N.V. Inherited Thrombocytopenia: Update on Genes and Genetic Variants Which may be Associated With Bleeding. Front. Cardiovasc. Med. 2019, 6, 80. [Google Scholar] [CrossRef] [Green Version]
- Johnson, B.; Lowe, G.C.; Futterer, J.; Lordkipanidze, M.; MacDonald, D.; Simpson, M.A.; Sanchez-Guiu, I.; Drake, S.; Bem, D.; Leo, V.; et al. Whole exome sequencing identifies genetic variants in inherited thrombocytopenia with secondary qualitative function defects. Haematologica 2016, 101, 1170–1179. [Google Scholar] [CrossRef] [Green Version]
- Jurk, K.; Greinacher, A.; Walter, U.; Scharrer, I. May-Hegglin anomaly with MYH9 gene E1841K mutation is associated with major platelet defects in granule secretion and thrombin generation. In Proceedings of the 57th Annual Meeting of the Society of Thrombosis and Haemostasis Research, Munich, Germany. P-2-61.
- Elbaz, C.; Sholzberg, M. An illustrated review of bleeding assessment tools and common coagulation tests. Res. Pract Thromb. Haemost. 2020, 4, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Gresele, P.; Orsini, S.; Noris, P.; Falcinelli, E.; Alessi, M.C.; Bury, L.; Borhany, M.; Santoro, C.; Glembotsky, A.C.; Cid, A.R.; et al. Validation of the ISTH/SSC bleeding assessment tool for inherited platelet disorders: A communication from the Platelet Physiology SSC. J. Thromb. Haemost. 2020, 18, 732–739. [Google Scholar] [CrossRef]
- Nurden, A.T.; Nurden, P. Should any genetic defect affecting alpha-granules in platelets be classified as gray platelet syndrome? Am. J. Hematol. 2016, 91, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Abdulhay, N.J.; Fiorini, C.; Verboon, J.M.; Ludwig, L.S.; Ulirsch, J.C.; Zieger, B.; Lareau, C.A.; Mi, X.; Roy, A.; Obeng, E.A.; et al. Impaired human hematopoiesis due to a cryptic intronic GATA1 splicing mutation. J. Exp. Med. 2019, 216, 1050–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Althaus, K.; Greinacher, A. MYH9-related platelet disorders. Semin. Thromb. Hemost. 2009, 35, 189–203. [Google Scholar] [CrossRef] [Green Version]
- Greinacher, A.; Pecci, A.; Kunishima, S.; Althaus, K.; Nurden, P.; Balduini, C.L.; Bakchoul, T. Diagnosis of inherited platelet disorders on a blood smear: A tool to facilitate worldwide diagnosis of platelet disorders. J. Thromb. Haemost. 2017, 15, 1511–1521. [Google Scholar] [CrossRef] [Green Version]
- Zaninetti, C.; Greinacher, A. Diagnosis of Inherited Platelet Disorders on a Blood Smear. J. Clin. Med. 2020, 9, 539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corpataux, N.; Franke, K.; Kille, A.; Valina, C.M.; Neumann, F.J.; Nuhrenberg, T.; Hochholzer, W. Reticulated Platelets in Medicine: Current Evidence and Further Perspectives. J. Clin. Med. 2020, 9, 3737. [Google Scholar] [CrossRef] [PubMed]
- Skipper, M.T.; Rubak, P.; Stentoft, J.; Hvas, A.M.; Larsen, O.H. Evaluation of platelet function in thrombocytopenia. Platelets 2018, 29, 270–276. [Google Scholar] [CrossRef]
- Hedley, B.D.; Llewellyn-Smith, N.; Lang, S.; Hsia, C.C.; MacNamara, N.; Rosenfeld, D.; Keeney, M. Combined accurate platelet enumeration and reticulated platelet determination by flow cytometry. Cytom. B Clin. Cytom. 2015, 88, 330–337. [Google Scholar] [CrossRef]
- Hille, L.; Cederqvist, M.; Hromek, J.; Stratz, C.; Trenk, D.; Nuhrenberg, T.G. Evaluation of an Alternative Staining Method Using SYTO 13 to Determine Reticulated Platelets. Thromb. Haemost. 2019, 119, 779–785. [Google Scholar] [CrossRef] [Green Version]
- Dupuis, A.; Bordet, J.C.; Eckly, A.; Gachet, C. Platelet delta-Storage Pool Disease: An Update. J. Clin. Med. 2020, 9, 2508. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, H.F.; Korporaal, S.J. Platelet morphology and Ultrastructure. In Platelets in Thrombotic and Non-Thrombotic Disorders: Pathophysiology, Pharmacology and Therapeutics: An Update; Gresele, P., Kleiman, N.S., Lopez, J.A., Page, C.P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 21–37. [Google Scholar]
- Hayward, C.P.; Moffat, K.A.; Spitzer, E.; Timleck, M.; Plumhoff, E.; Israels, S.J.; White, J.; NASCOLA Working Group on Platelet Dense Granule Deficiency. Results of an external proficiency testing exercise on platelet dense-granule deficiency testing by whole mount electron microscopy. Am. J. Clin. Pathol. 2009, 131, 671–675. [Google Scholar] [CrossRef] [Green Version]
- Brunet, J.G.; Iyer, J.K.; Badin, M.S.; Graf, L.; Moffat, K.A.; Timleck, M.; Spitzer, E.; Hayward, C.P.M. Electron microscopy examination of platelet whole mount preparations to quantitate platelet dense granule numbers: Implications for diagnosing suspected platelet function disorders due to dense granule deficiency. Int. J. Lab. Hematol. 2018, 40, 400–407. [Google Scholar] [CrossRef]
- Eckly, A.; Rinckel, J.Y.; Proamer, F.; Ulas, N.; Joshi, S.; Whiteheart, S.W.; Gachet, C. Respective contributions of single and compound granule fusion to secretion by activated platelets. Blood 2016, 128, 2538–2549. [Google Scholar] [CrossRef] [Green Version]
- Gresele, P.; Bury, L.; Mezzasoma, A.M.; Falcinelli, E. Platelet function assays in diagnosis: An update. Expert Rev. Hematol. 2019, 12, 29–46. [Google Scholar] [CrossRef]
- Rubak, P.; Villadsen, K.; Hvas, A.M. Reference intervals for platelet aggregation assessed by multiple electrode platelet aggregometry. Thromb. Res. 2012, 130, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Toth, O.; Calatzis, A.; Penz, S.; Losonczy, H.; Siess, W. Multiple electrode aggregometry: A new device to measure platelet aggregation in whole blood. Thromb. Haemost. 2006, 96, 781–788. [Google Scholar] [PubMed]
- Michelson, A.D. Methods for the measurement of platelet function. Am. J. Cardiol. 2009, 103, 20A–26A. [Google Scholar] [CrossRef]
- Awidi, A.; Maqablah, A.; Dweik, M.; Bsoul, N.; Abu-Khader, A. Comparison of platelet aggregation using light transmission and multiple electrode aggregometry in Glanzmann thrombasthenia. Platelets 2009, 20, 297–301. [Google Scholar] [CrossRef]
- Albanyan, A.; Al-Musa, A.; AlNounou, R.; Al Zahrani, H.; Nasr, R.; AlJefri, A.; Saleh, M.; Malik, A.; Masmali, H.; Owaidah, T. Diagnosis of Glanzmann thrombasthenia by whole blood impedance analyzer (MEA) vs. light transmission aggregometry. Int. J. Lab. Hematol. 2015, 37, 503–508. [Google Scholar] [CrossRef]
- Al Ghaithi, R.; Drake, S.; Watson, S.P.; Morgan, N.V.; Harrison, P. Comparison of multiple electrode aggregometry with lumi-aggregometry for the diagnosis of patients with mild bleeding disorders. J. Thromb. Haemost. 2017, 15, 2045–2052. [Google Scholar] [CrossRef] [Green Version]
- Moenen, F.; Vries, M.J.A.; Nelemans, P.J.; van Rooy, K.J.M.; Vranken, J.; Verhezen, P.W.M.; Wetzels, R.J.H.; Ten Cate, H.; Schouten, H.C.; Beckers, E.A.M.; et al. Screening for platelet function disorders with Multiplate and platelet function analyzer. Platelets 2019, 30, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Seyfert, U.T.; Haubelt, H.; Vogt, A.; Hellstern, P. Variables influencing Multiplate(TM) whole blood impedance platelet aggregometry and turbidimetric platelet aggregation in healthy individuals. Platelets 2007, 18, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Femia, E.A.; Scavone, M.; Lecchi, A.; Cattaneo, M. Effect of platelet count on platelet aggregation measured with impedance aggregometry (Multiplate analyzer) and with light transmission aggregometry. J. Thromb. Haemost. 2013, 11, 2193–2196. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, M.; Lecchi, A.; Zighetti, M.L.; Lussana, F. Platelet aggregation studies: Autologous platelet-poor plasma inhibits platelet aggregation when added to platelet-rich plasma to normalize platelet count. Haematologica 2007, 92, 694–697. [Google Scholar] [CrossRef] [Green Version]
- Tiedemann Skipper, M.; Rubak, P.; Halfdan Larsen, O.; Hvas, A.M. Thrombocytopenia model with minimal manipulation of blood cells allowing whole blood assessment of platelet function. Platelets 2016, 27, 295–300. [Google Scholar] [CrossRef]
- Franchini, M. The platelet function analyzer (PFA-100): An update on its clinical use. Clin. Lab. 2005, 51, 367–372. [Google Scholar]
- Favaloro, E.J. Clinical utility of closure times using the platelet function analyzer-100/200. Am. J. Hematol. 2017, 92, 398–404. [Google Scholar] [CrossRef] [Green Version]
- Hayward, C.P.; Harrison, P.; Cattaneo, M.; Ortel, T.L.; Rao, A.K.; Platelet Physiology Subcommittee of the SSC; Standardization Committee of the International Society Society on Thrombosis and Hemostasis. Platelet function analyzer (PFA)-100 closure time in the evaluation of platelet disorders and platelet function. J. Thromb. Haemost. 2006, 4, 312–319. [Google Scholar] [CrossRef]
- Quiroga, T.; Goycoolea, M.; Munoz, B.; Morales, M.; Aranda, E.; Panes, O.; Pereira, J.; Mezzano, D. Template bleeding time and PFA-100 have low sensitivity to screen patients with hereditary mucocutaneous hemorrhages: Comparative study in 148 patients. J. Thromb. Haemost. 2004, 2, 892–898. [Google Scholar] [CrossRef]
- Sladky, J.L.; Klima, J.; Grooms, L.; Kerlin, B.A.; O’Brien, S.H. The PFA-100 (R) does not predict delta-granule platelet storage pool deficiencies. Haemoph. Off. J. World Fed. Hemoph. 2012, 18, 626–629. [Google Scholar] [CrossRef]
- Kaufmann, J.; Adler, M.; Alberio, L.; Nagler, M. Utility of the Platelet Function Analyzer in Patients with Suspected Platelet Function Disorders: Diagnostic Accuracy Study. TH Open 2020, 4, e427–e436. [Google Scholar] [CrossRef] [PubMed]
- Savion, N.; Varon, D. Impact--the cone and plate(let) analyzer: Testing platelet function and anti-platelet drug response. Pathophysiol. Haemost. Thromb. 2006, 35, 83–88. [Google Scholar] [CrossRef]
- Panzer, S.; Eichelberger, B.; Koren, D.; Kaufmann, K.; Male, C. Monitoring survival and function of transfused platelets in Bernard-Soulier syndrome by flow cytometry and a cone and plate(let) analyzer (Impact-R). Transfusion 2007, 47, 103–106. [Google Scholar] [CrossRef]
- Shenkman, B.; Budde, U.; Angerhaus, D.; Lubetsky, A.; Savion, N.; Seligsohn, U.; Varon, D. ADAMTS-13 regulates platelet adhesion under flow. A new method for differentiation between inherited and acquired thrombotic thrombocytopenic purpura. Thromb. Haemost. 2006, 96, 160–166. [Google Scholar]
- Kenet, G.; Lubetsky, A.; Shenkman, B.; Tamarin, I.; Dardik, R.; Rechavi, G.; Barzilai, A.; Martinowitz, U.; Savion, N.; Varon, D. Cone and platelet analyser (CPA): A new test for the prediction of bleeding among thrombocytopenic patients. Br. J. Haematol. 1998, 101, 255–259. [Google Scholar] [CrossRef]
- Vinholt, P.J.; Hvas, A.M.; Nybo, M. An overview of platelet indices and methods for evaluating platelet function in thrombocytopenic patients. Eur. J. Haematol. 2014, 92, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Bolliger, D.; Seeberger, M.D.; Tanaka, K.A. Principles and practice of thromboelastography in clinical coagulation management and transfusion practice. Transfus. Med. Rev. 2012, 26, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, H.H.; Heemskerk, J.W.; Levi, M.; Reitsma, P.H. New fundamentals in hemostasis. Physiol. Rev. 2013, 93, 327–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colucci, M.; Semeraro, N.; Semerao, F. Platelets and fibrinolysis. In Platelets in Thrombotic and Non-Thrombotic Disorders: Pathophysiology, Pharmacology and Therapeutics: An Update; Gresele, P., Kleiman, N.S., Lopez, J.A., Page, C.P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 463–487. [Google Scholar]
- Ranucci, M.; Baryshnikova, E. Sensitivity of Viscoelastic Tests to Platelet Function. J. Clin. Med. 2020, 9, 189. [Google Scholar] [CrossRef] [Green Version]
- Castellino, F.J.; Liang, Z.; Davis, P.K.; Balsara, R.D.; Musunuru, H.; Donahue, D.L.; Smith, D.L.; Sandoval-Cooper, M.J.; Ploplis, V.A.; Walsh, M. Abnormal whole blood thrombi in humans with inherited platelet receptor defects. PLoS ONE 2012, 7, e52878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunduz, E.; Akay, O.M.; Bal, C.; Gulbas, Z. Can thrombelastography be a new tool to assess bleeding risk in patients with idiopathic thrombocytopenic purpura? Platelets 2011, 22, 516–520. [Google Scholar] [CrossRef]
- Greene, L.A.; Chen, S.; Seery, C.; Imahiyerobo, A.M.; Bussel, J.B. Beyond the platelet count: Immature platelet fraction and thromboelastometry correlate with bleeding in patients with immune thrombocytopenia. Br. J. Haematol. 2014, 166, 592–600. [Google Scholar] [CrossRef]
- Estcourt, L.J.; Stanworth, S.J.; Harrison, P.; Powter, G.; McClure, M.; Murphy, M.F.; Mumford, A.D. Prospective observational cohort study of the association between thromboelastometry, coagulation and platelet parameters and bleeding in patients with haematological malignancies- the ATHENA study. Br. J. Haematol. 2014, 166, 581–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, J.D.; Lopez-Espina, C.G.; Bliden, K.; Gurbel, P.; Hartmann, J.; Achneck, H.E. TEG(R)6s system measures the contributions of both platelet count and platelet function to clot formation at the site-of-care. Platelets 2020, 31, 932–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Born, G.V. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature 1962, 194, 927–929. [Google Scholar] [CrossRef] [PubMed]
- Beck, F.; Geiger, J.; Gambaryan, S.; Solari, F.A.; Dell’Aica, M.; Loroch, S.; Mattheij, N.J.; Mindukshev, I.; Potz, O.; Jurk, K.; et al. Temporal quantitative phosphoproteomics of ADP stimulation reveals novel central nodes in platelet activation and inhibition. Blood 2017, 129, e1–e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pielsticker, C.; Brodde, M.F.; Raum, L.; Jurk, K.; Kehrel, B.E. Plasmin-Induced Activation of Human Platelets Is Modulated by Thrombospondin-1, Bona Fide Misfolded Proteins and Thiol Isomerases. Int. J. Mol. Sci. 2020, 21, 8851. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, M.; Cerletti, C.; Harrison, P.; Hayward, C.P.; Kenny, D.; Nugent, D.; Nurden, P.; Rao, A.K.; Schmaier, A.H.; Watson, S.P.; et al. Recommendations for the Standardization of Light Transmission Aggregometry: A Consensus of the Working Party from the Platelet Physiology Subcommittee of SSC/ISTH. J. Thromb. Haemost. 2013. [Google Scholar] [CrossRef] [PubMed]
- Hayward, C.P.; Moffat, K.A.; Raby, A.; Israels, S.; Plumhoff, E.; Flynn, G.; Zehnder, J.L. Development of North American consensus guidelines for medical laboratories that perform and interpret platelet function testing using light transmission aggregometry. Am. J. Clin. Pathol. 2010, 134, 955–963. [Google Scholar] [CrossRef] [Green Version]
- Alessi, M.C.; Sie, P.; Payrastre, B. Strengths and Weaknesses of Light Transmission Aggregometry in Diagnosing Hereditary Platelet Function Disorders. J. Clin. Med. 2020, 9, 763. [Google Scholar] [CrossRef] [Green Version]
- Althaus, K.; Zieger, B.; Bakchoul, T.; Jurk, K.; THROMKID-Plus Studiengruppe der Gesellschaft für Thrombose- und Hämostaseforschung (GTH) und der Gesellschaft für Pädiatrische Onkologie und Hämatologie (GPOH). Standardization of Light Transmission Aggregometry for Diagnosis of Platelet Disorders: An Inter-Laboratory External Quality Assessment. Thromb. Haemost. 2019, 119, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Gresele, P.; Harrison, P.; Bury, L.; Falcinelli, E.; Gachet, C.; Hayward, C.P.; Kenny, D.; Mezzano, D.; Mumford, A.D.; Nugent, D.; et al. Diagnosis of suspected inherited platelet function disorders: Results of a worldwide survey. J. Thromb. Haemost. 2014, 12, 1562–1569. [Google Scholar] [CrossRef]
- Cattaneo, M.; Hayward, C.P.; Moffat, K.A.; Pugliano, M.T.; Liu, Y.; Michelson, A.D. Results of a worldwide survey on the assessment of platelet function by light transmission aggregometry: A report from the platelet physiology subcommittee of the SSC of the ISTH. J. Thromb. Haemost. 2009, 7, 1029. [Google Scholar] [CrossRef] [PubMed]
- Hayward, C.P.; Pai, M.; Liu, Y.; Moffat, K.A.; Seecharan, J.; Webert, K.E.; Cook, R.J.; Heddle, N.M. Diagnostic utility of light transmission platelet aggregometry: Results from a prospective study of individuals referred for bleeding disorder assessments. J. Thromb. Haemost. 2009, 7, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, J.; Mullier, F.; Vayne, C.; Lordkipanidze, M. Advances in Platelet Function Testing-Light Transmission Aggregometry and Beyond. J. Clin. Med. 2020, 9, 2636. [Google Scholar] [CrossRef]
- Dawood, B.B.; Lowe, G.C.; Lordkipanidze, M.; Bem, D.; Daly, M.E.; Makris, M.; Mumford, A.; Wilde, J.T.; Watson, S.P. Evaluation of participants with suspected heritable platelet function disorders including recommendation and validation of a streamlined agonist panel. Blood 2012, 120, 5041–5049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cattaneo, M. Light transmission aggregometry and ATP release for the diagnostic assessment of platelet function. Semin. Thromb. Hemost. 2009, 35, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Badin, M.S.; Graf, L.; Iyer, J.K.; Moffat, K.A.; Seecharan, J.L.; Hayward, C.P. Variability in platelet dense granule adenosine triphosphate release findings amongst patients tested multiple times as part of an assessment for a bleeding disorder. Int. J. Lab. Hematol. 2016, 38, 648–657. [Google Scholar] [CrossRef]
- Durrant, T.N.; van den Bosch, M.T.; Hers, I. Integrin alphaIIbbeta3 outside-in signaling. Blood 2017, 130, 1607–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Asten, I.; Schutgens, R.E.G.; Baaij, M.; Zandstra, J.; Roest, M.; Pasterkamp, G.; Huisman, A.; Korporaal, S.J.A.; Urbanus, R.T. Validation of flow cytometric analysis of platelet function in patients with a suspected platelet function defect. J. Thromb. Haemost. 2018, 16, 689–698. [Google Scholar] [CrossRef] [Green Version]
- Navred, K.; Martin, M.; Ekdahl, L.; Zetterberg, E.; Andersson, N.G.; Strandberg, K.; Norstrom, E. A simplified flow cytometric method for detection of inherited platelet disorders-A comparison to the gold standard light transmission aggregometry. PLoS ONE 2019, 14, e0211130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurk, K. Analysis of platelet function and dysfunction. Hamostaseologie 2015, 35, 60–72. [Google Scholar] [CrossRef]
- Baaten, C.; Ten Cate, H.; van der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet populations and priming in hematological diseases. Blood Rev. 2017, 31, 389–399. [Google Scholar] [CrossRef]
- Schwarz, U.R.; Geiger, J.; Walter, U.; Eigenthaler, M. Flow cytometry analysis of intracellular VASP phosphorylation for the assessment of activating and inhibitory signal transduction pathways in human platelets--definition and detection of ticlopidine/clopidogrel effects. Thromb. Haemost. 1999, 82, 1145–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spurgeon, B.E.J.; Naseem, K.M. Phosphoflow cytometry and barcoding in blood platelets: Technical and analytical considerations. Cytom. B Clin. Cytom. 2020, 98, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Blair, T.A.; Michelson, A.D.; Frelinger, A.L., 3rd. Mass Cytometry Reveals Distinct Platelet Subtypes in Healthy Subjects and Novel Alterations in Surface Glycoproteins in Glanzmann Thrombasthenia. Sci. Rep. 2018, 8, 10300. [Google Scholar] [CrossRef]
- Dovlatova, N.; Lordkipanidze, M.; Lowe, G.C.; Dawood, B.; May, J.; Heptinstall, S.; Watson, S.P.; Fox, S.C.; Group, U.G.S. Evaluation of a whole blood remote platelet function test for the diagnosis of mild bleeding disorders. J. Thromb. Haemost. 2014, 12, 660–665. [Google Scholar] [CrossRef] [Green Version]
- Linden, M.D.; Frelinger, A.L., 3rd; Barnard, M.R.; Przyklenk, K.; Furman, M.I.; Michelson, A.D. Application of flow cytometry to platelet disorders. Semin. Thromb. Hemost. 2004, 30, 501–511. [Google Scholar] [CrossRef]
- van Asten, I.; Schutgens, R.E.G.; Urbanus, R.T. Toward Flow Cytometry Based Platelet Function Diagnostics. Semin. Thromb. Hemost. 2018, 44, 197–205. [Google Scholar] [CrossRef]
- Boknas, N.; Macwan, A.S.; Sodergren, A.L.; Ramstrom, S. Platelet function testing at low platelet counts: When can you trust your analysis? Res. Pract. Thromb. Haemost. 2019, 3, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Loroch, S.; Trabold, K.; Gambaryan, S.; Reiss, C.; Schwierczek, K.; Fleming, I.; Sickmann, A.; Behnisch, W.; Zieger, B.; Zahedi, R.P.; et al. Alterations of the platelet proteome in type I Glanzmann thrombasthenia caused by different homozygous delG frameshift mutations in ITGA2B. Thromb. Haemost. 2017, 117, 556–569. [Google Scholar] [CrossRef] [PubMed]
- van Bladel, E.R.; Laarhoven, A.G.; van der Heijden, L.B.; Heitink-Polle, K.M.; Porcelijn, L.; van der Schoot, C.E.; de Haas, M.; Roest, M.; Vidarsson, G.; de Groot, P.G.; et al. Functional platelet defects in children with severe chronic ITP as tested with 2 novel assays applicable for low platelet counts. Blood 2014, 123, 1556–1563. [Google Scholar] [CrossRef] [Green Version]
- Frelinger, A.L., 3rd; Grace, R.F.; Gerrits, A.J.; Berny-Lang, M.A.; Brown, T.; Carmichael, S.L.; Neufeld, E.J.; Michelson, A.D. Platelet function tests, independent of platelet count, are associated with bleeding severity in ITP. Blood 2015, 126, 873–879. [Google Scholar] [CrossRef] [Green Version]
- Leinoe, E.B.; Hoffmann, M.H.; Kjaersgaard, E.; Nielsen, J.D.; Bergmann, O.J.; Klausen, T.W.; Johnsen, H.E. Prediction of haemorrhage in the early stage of acute myeloid leukaemia by flow cytometric analysis of platelet function. Br. J. Haematol. 2005, 128, 526–532. [Google Scholar] [CrossRef]
- Gunay-Aygun, M.; Falik-Zaccai, T.C.; Vilboux, T.; Zivony-Elboum, Y.; Gumruk, F.; Cetin, M.; Khayat, M.; Boerkoel, C.F.; Kfir, N.; Huang, Y.; et al. NBEAL2 is mutated in gray platelet syndrome and is required for biogenesis of platelet alpha-granules. Nat. Genet. 2011, 43, 732–734. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Mullier, F.; Frotscher, B.; Briquel, M.E.; Toussaint, M.; Massin, F.; Lecompte, T.; Latger-Cannard, V. Usefulness of Flow Cytometric Mepacrine Uptake/Release Combined with CD63 Assay in Diagnosis of Patients with Suspected Platelet Dense Granule Disorder. Semin. Thromb. Hemost. 2016, 42, 282–291. [Google Scholar] [CrossRef]
- van Asten, I.; Blaauwgeers, M.; Granneman, L.; Heijnen, H.F.G.; Kruip, M.; Beckers, E.A.M.; Coppens, M.; Eikenboom, J.; Tamminga, R.Y.J.; Pasterkamp, G.; et al. Flow cytometric mepacrine fluorescence can be used for the exclusion of platelet dense granule deficiency. J. Thromb. Haemost. 2020, 18, 706–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monroe, D.M.; Hoffman, M.; Roberts, H.R. Platelets and thrombin generation. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1381–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomaiuolo, M.; Brass, L.F.; Stalker, T.J. Regulation of Platelet Activation and Coagulation and Its Role in Vascular Injury and Arterial Thrombosis. Interv. Cardiol. Clin. 2017, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemker, H.C.; Giesen, P.; Al Dieri, R.; Regnault, V.; de Smedt, E.; Wagenvoord, R.; Lecompte, T.; Beguin, S. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol. Haemost. Thromb. 2003, 33, 4–15. [Google Scholar] [CrossRef]
- Panova-Noeva, M.; van der Meijden, P.E.J.; Ten Cate, H. Clinical Applications, Pitfalls, and Uncertainties of Thrombin Generation in the Presence of Platelets. J. Clin. Med. 2019, 9, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, H.J. Impaired platelet procoagulant mechanisms in patients with bleeding disorders. Semin. Thromb. Hemost. 2009, 35, 233–241. [Google Scholar] [CrossRef]
- Hemker, H.C.; Al Dieri, R.; De Smedt, E.; Beguin, S. Thrombin generation, a function test of the haemostatic-thrombotic system. Thromb. Haemost. 2006, 96, 553–561. [Google Scholar] [PubMed]
- van der Meijden, P.E.; Feijge, M.A.; Swieringa, F.; Gilio, K.; Nergiz-Unal, R.; Hamulyak, K.; Heemskerk, J.W. Key role of integrin alpha(IIb)beta (3) signaling to Syk kinase in tissue factor-induced thrombin generation. Cell. Mol. Life Sci. CMLS 2012, 69, 3481–3492. [Google Scholar] [CrossRef] [Green Version]
- Dohrmann, M.; Makhoul, S.; Gross, K.; Krause, M.; Pillitteri, D.; von Auer, C.; Walter, U.; Lutz, J.; Volf, I.; Kehrel, B.E.; et al. CD36-fibrin interaction propagates FXI-dependent thrombin generation of human platelets. FASEB J. 2020, 34, 9337–9357. [Google Scholar] [CrossRef] [PubMed]
- Estevez, B.; Kim, K.; Delaney, M.K.; Stojanovic-Terpo, A.; Shen, B.; Ruan, C.; Cho, J.; Ruggeri, Z.M.; Du, X. Signaling-mediated cooperativity between glycoprotein Ib-IX and protease-activated receptors in thrombin-induced platelet activation. Blood 2016, 127, 626–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subcommittee on Control of Anticoagulation of the SSC of the ISTHTowards a recommendation for the standardization of the measurement of platelet-dependent thrombin generation. J. Thromb. Haemost. 2011, 9, 1859–1861. [CrossRef]
- Vanschoonbeek, K.; Feijge, M.A.; Van Kampen, R.J.; Kenis, H.; Hemker, H.C.; Giesen, P.L.; Heemskerk, J.W. Initiating and potentiating role of platelets in tissue factor-induced thrombin generation in the presence of plasma: Subject-dependent variation in thrombogram characteristics. J. Thromb. Haemost. 2004, 2, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Roest, M.; Reininger, A.; Zwaginga, J.J.; King, M.R.; Heemskerk, J.W. Flow chamber-based assays to measure thrombus formation in vitro: Requirements for standardization. J. Thromb. Haemost. 2011, 9, 2322–2324. [Google Scholar] [CrossRef]
- Neeves, K.B.; McCarty, O.J.; Reininger, A.J.; Sugimoto, M.; King, M.R. Biorheology Subcommittee of the SSC of the ISTHFlow-dependent thrombin and fibrin generation in vitro: Opportunities for standardization: Communication from SSC of the ISTH. J. Thromb. Haemost. 2014, 12, 418–420. [Google Scholar] [CrossRef]
- Diamond, S.L. Systems Analysis of Thrombus Formation. Circ. Res. 2016, 118, 1348–1362. [Google Scholar] [CrossRef] [PubMed]
- Nagy, M.; Heemskerk, J.W.M.; Swieringa, F. Use of microfluidics to assess the platelet-based control of coagulation. Platelets 2017, 28, 441–448. [Google Scholar] [CrossRef]
- de Witt, S.M.; Swieringa, F.; Cavill, R.; Lamers, M.M.; van Kruchten, R.; Mastenbroek, T.; Baaten, C.; Coort, S.; Pugh, N.; Schulz, A.; et al. Identification of platelet function defects by multi-parameter assessment of thrombus formation. Nat. Commun. 2014, 5, 4257. [Google Scholar] [CrossRef] [Green Version]
- Nagy, M.; Mastenbroek, T.G.; Mattheij, N.J.A.; de Witt, S.; Clemetson, K.J.; Kirschner, J.; Schulz, A.S.; Vraetz, T.; Speckmann, C.; Braun, A.; et al. Variable impairment of platelet functions in patients with severe, genetically linked immune deficiencies. Haematologica 2018, 103, 540–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Geffen, J.P.; Brouns, S.L.N.; Batista, J.; McKinney, H.; Kempster, C.; Nagy, M.; Sivapalaratnam, S.; Baaten, C.; Bourry, N.; Frontini, M.; et al. High-throughput elucidation of thrombus formation reveals sources of platelet function variability. Haematologica 2019, 104, 1256–1267. [Google Scholar] [CrossRef]
- Coenen, D.M.; Mastenbroek, T.G.; Cosemans, J. Platelet interaction with activated endothelium: Mechanistic insights from microfluidics. Blood 2017, 130, 2819–2828. [Google Scholar] [CrossRef] [PubMed]
- Jurk, K.; Kehrel, B.E. Pathophysiology and biochemistry of platelets. Der Internist 2010, 51, 1086, 1088–1092, 1094. [Google Scholar] [CrossRef] [PubMed]
- Brouns, S.L.N.; Provenzale, I.; van Geffen, J.P.; van der Meijden, P.E.J.; Heemskerk, J.W.M. Localized endothelial-based control of platelet aggregation and coagulation under flow: A proof-of-principle vessel-on-a-chip study. J. Thromb. Haemost. 2020, 18, 931–941. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurk, K.; Shiravand, Y. Platelet Phenotyping and Function Testing in Thrombocytopenia. J. Clin. Med. 2021, 10, 1114. https://doi.org/10.3390/jcm10051114
Jurk K, Shiravand Y. Platelet Phenotyping and Function Testing in Thrombocytopenia. Journal of Clinical Medicine. 2021; 10(5):1114. https://doi.org/10.3390/jcm10051114
Chicago/Turabian StyleJurk, Kerstin, and Yavar Shiravand. 2021. "Platelet Phenotyping and Function Testing in Thrombocytopenia" Journal of Clinical Medicine 10, no. 5: 1114. https://doi.org/10.3390/jcm10051114
APA StyleJurk, K., & Shiravand, Y. (2021). Platelet Phenotyping and Function Testing in Thrombocytopenia. Journal of Clinical Medicine, 10(5), 1114. https://doi.org/10.3390/jcm10051114






