Effect of Insulin Pump Use on Diabetic Ketoacidosis in Type 1 Diabetes Mellitus: A Matched Cohort Study
Abstract
:1. Introduction
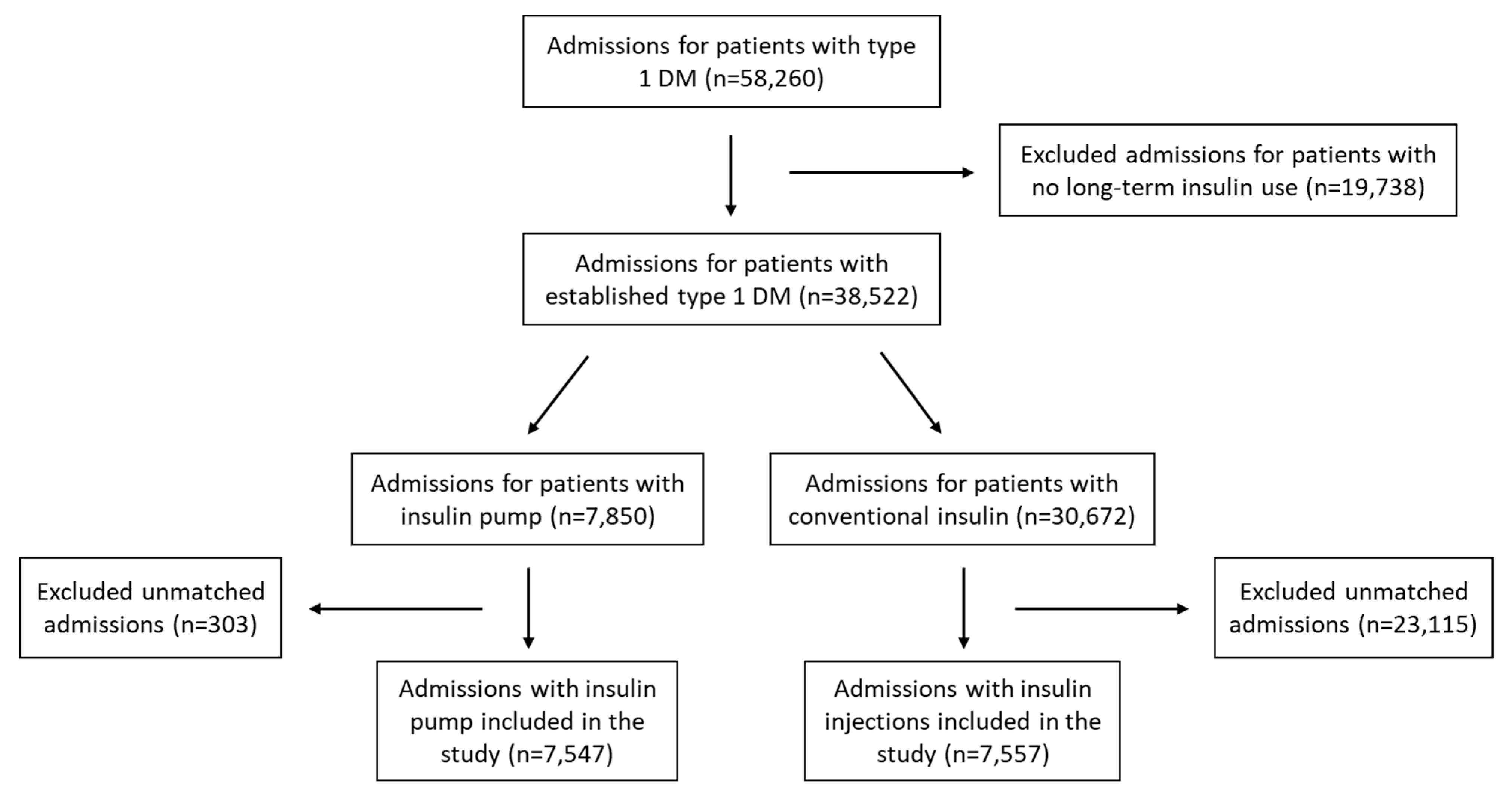
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Umpierrez, G.E.; Kitabchi, A.E. Diabetic ketoacidosis: Risk factors and management strategies. Treat Endocrinol. 2003, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Fayfman, M.; Pasquel, F.J.; Umpierrez, G.E. Management of Hyperglycemic Crises: Diabetic Ketoacidosis and Hyperglycemic Hyperosmolar State. Med. Clin. N. Am. 2017, 101, 587–606. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, F.S.; Brodovicz, K.; Soleymanlou, N.; Marquard, J.; Wissinger, E.; Maiese, B.A. Incidence and prevalence of diabetic ketoacidosis (DKA) among adults with type 1 diabetes mellitus (T1D): A systematic literature review. BMJ Open 2017, 7, e016587. [Google Scholar]
- Ellemann, K.; Soerensen, J.N.; Pedersen, L.; Edsberg, B.; Andersen, O.O. Epidemiology and Treatment of Diabetic Ketoacidosis in a Community Population. Diabetes Care 1984, 7, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, W.; Kumar, N.; Kumar, S.; Rizwan, A. Precipitating Risk Factors, Clinical Presentation, and Outcome of Diabetic Ketoacidosis in Patients with Type 1 Diabetes. Cureus 2019, 11, e4789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanas, R.; Ludvigsson, J. Hypoglycemia and ketoacidosis with insulin pump therapy in children and adolescents. Pediatr. Diabetes 2006, 7, 32–38. [Google Scholar] [CrossRef]
- Weissberg-Benchell, J.; Antisdel-Lomaglio, J.; Seshadri, R. Insulin pump therapy: A meta-analysis. Diabetes Care 2003, 26, 1079–1087. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Rincon, A.; Hincapie-García, J.; Arango, C.M.; Aristizabal, N.; Castillo, E.; Hincapie, G.; Zapata, E.; Cuesta, D.P.; Delgado, M.; Abad, V.; et al. Clinical Outcomes After 1 Year of Augmented Insulin Pump Therapy in Patients with Diabetes in a Specialized Diabetes Center in Medellín, Colombia. Diabetes Technol. Ther. 2016, 18, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Karges, B.M.; Schwandt, A.; Heidtmann, B.; Kordonouri, O.; Binder, E.; Schierloh, U.; Boettcher, C.; Kapellen, T.; Rosenbauer, J.; Holl, R.W. Association of Insulin Pump Therapy vs Insulin Injection Therapy with Severe Hypoglycemia, Ketoacidosis, and Glycemic Control Among Children, Adolescents, and Young Adults with Type 1 Diabetes. JAMA 2017, 318, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Fernandez, J.; Gomez, F.J.; Pinés, P.; González, J.; López, J.; López, L.M.; Blanco, B.; Roa, C.; Herranz, S.; Muñoz-Rodríguez, J.R. Continuous Subcutaneous Insulin Infusion in Adult Type 1 Diabetes Mellitus Patients: Results from a Public Health System. Diabetes Technol. Ther. 2019, 21, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Pearce, N. Analysis of matched case-control studies. BMJ 2016, 352, i969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nimri, R.; Nir, J.; Phillip, M. Insulin Pump Therapy. Am. J. Ther. 2020, 27, e30–e41. [Google Scholar] [CrossRef] [PubMed]
- Graham, C. Continuous Glucose Monitoring and Global Reimbursement: An Update. Diabetes Technol. Ther. 2017, 19, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinemann, L.; Fleming, G.A.; Petrie, J.R.; Holl, R.W.; Bergenstal, R.M.; Peters, A.L. Insulin Pump Risks and Benefits: A Clinical Appraisal of Pump Safety Standards, Adverse Event Reporting, and Research Needs: A joint statement of the European As-sociation for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetes Care 2015, 38, 716–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulton, A.J.; Del Prato, S. Regulation of medical devices used in diabetology in Europe. Time for a Reform? Diabetologia 2012, 55, 2295–2297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Insulin Pump Group | Conventional Insulin Group | p-Value | |
|---|---|---|---|
| Median (IQR) or N (%) | Median (IQR) or N (%) | ||
| Age (years) | 42 (14–70) | 36 (15–57) | |
| Sex | <0.001 | ||
| Male | 2925 (37.3) | 14,625 (47.7) | |
| Female | 4925 (62.7) | 16,047 (52.3) | |
| Race | <0.001 | ||
| White | 6559 (83.6) | 19,080 (62.2) | |
| Black | 670 (8.5) | 7002 (22.8) | |
| Hispanic | 399 (5.1) | 3230 (10.5) | |
| Asian or Pacific Islander | 58 (0.7) | 423 (1.4) | |
| Native American | 33 (0.4) | 277 (0.9) | |
| Other | 131 (1.7) | 660 (2.2) | |
| Type of Insurance | <0.001 | ||
| Medicare | 2760 (35.2) | 11,478 (37.4) | |
| Medicaid | 1253 (16) | 9750 (31.8) | |
| Private Insurance | 3575 (45.5) | 6862 (22.4) | |
| Self-pay | 108 (1.4) | 1718 (5.6) | |
| No charge | 8 (0.1) | 142 (0.5) | |
| Other | 146 (1.9) | 722 (2.4) | |
| Total | 7850 | 30,672 |
| Matched Cohort | p Value * | Unmatched Cohort | p Value * | |||
|---|---|---|---|---|---|---|
| Median (IQR) or N (%) | Median (IQR) or N (%) | |||||
| IP Group | CI Group | IP Group | CI Group | |||
| Age (years) | 46 (31–60) | 46 (31–60) | 0.894 | |||
| Sex | 0.959 | <0.001 | ||||
| Male | 2857 (37.9) | 2862 (37.9) | 66 (22.1) | 11,762 (50.9) | ||
| Female | 4690 (62.1) | 4695 (62.1) | 232 (77.9) | 11,353 (49.1) | ||
| Race | <0.001 | |||||
| White | 6379 (84.5) | 6382 (84.4) | 1 | 176 (59.1) | 12,708 (55) | |
| Black | 658 (8.7) | 662 (8.8) | 12 (4) | 6324 (27.4) | ||
| Hispanic | 371 (4.9) | 374 (5) | 26 (8.7) | 2853 (12.3) | ||
| Asian or Pacific Islander | 35 (0.5) | 35 (0.4) | 23 (7.7) | 389 (1.7) | ||
| Native American | 17 (0.2) | 17 (0.2) | 16 (5.4) | 264 (1.2) | ||
| Other | 87 (1.2) | 87 (1.2) | 45 (15.1) | 577 (2.4) | ||
| Type of Insurance | <0.001 | |||||
| Medicare | 2741 (36.3) | 2742 (36.3) | 0.999 | 17 (5.7) | 8735 (37.8) | |
| Medicaid | 1231 (16.3) | 1235 (16.3) | 22 (7.4) | 8512 (36.8) | ||
| Private Insurance | 3366 (44.6) | 3370 (44.6) | 207 (69.4) | 3493 (15.1) | ||
| Self-pay | 97 (1.3) | 98 (1.3) | 10 (3.4) | 1623 (7) | ||
| No charge | 4 (0.1) | 4 (0.1) | 4 (1.3) | 138 (0.6) | ||
| Other | 109 (1.4) | 109 (1.4) | 38 (12.8) | 614 (2.7) | ||
| Total | 7547 | 7557 | 298 | 23,115 | ||
| OR | 95% CI for OR | p-Value | |
|---|---|---|---|
| Age | 0.985 | 0.982–0.987 | <0.001 |
| Female Sex | 1.160 | 1.065–1.263 | 0.001 |
| Insurance–Medicare | 1 | Ref | |
| Insurance–Medicaid | 1.236 | 1.085–1.408 | 0.001 |
| Insurance–Private insurance | 0.706 | 0.635–0.785 | <0.001 |
| Insurance–Self-pay | 2.009 | 1.476–2.735 | <0.001 |
| Insurance–No charge | 0.543 | 0.065–4.526 | 0.57 |
| Insurance–Other | 0.78 | 0.541–1.124 | 0.18 |
| Race–white | 1 | Ref | |
| Race–black | 1.172 | 1.018–1.348 | 0.03 |
| Race–Hispanic | 0.853 | 0.707–1.029 | 0.10 |
| Race–Asian or Pacific Islander | 0.987 | 0.504–1.933 | 0.97 |
| Race–Native American | 0.758 | 0.308–1.868 | 0.55 |
| Race–Other | 0.724 | 0.478–1.097 | 0.13 |
| Insulin pump | 1.038 | 0.957–1.125 | 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshami, A.; Purewal, T.; Douedi, S.; Alazzawi, M.; Hossain, M.A.; Ong, R.; Sen, S.; Cheng, J.; Patel, S. Effect of Insulin Pump Use on Diabetic Ketoacidosis in Type 1 Diabetes Mellitus: A Matched Cohort Study. J. Clin. Med. 2021, 10, 898. https://doi.org/10.3390/jcm10050898
Alshami A, Purewal T, Douedi S, Alazzawi M, Hossain MA, Ong R, Sen S, Cheng J, Patel S. Effect of Insulin Pump Use on Diabetic Ketoacidosis in Type 1 Diabetes Mellitus: A Matched Cohort Study. Journal of Clinical Medicine. 2021; 10(5):898. https://doi.org/10.3390/jcm10050898
Chicago/Turabian StyleAlshami, Abbas, Tiffany Purewal, Steven Douedi, Mohammed Alazzawi, Mohammad A. Hossain, Raquel Ong, Shuvendu Sen, Jennifer Cheng, and Swapnil Patel. 2021. "Effect of Insulin Pump Use on Diabetic Ketoacidosis in Type 1 Diabetes Mellitus: A Matched Cohort Study" Journal of Clinical Medicine 10, no. 5: 898. https://doi.org/10.3390/jcm10050898






