Neurotoxic Effects of Local Anesthetics on Developing Motor Neurons in a Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture (Isolation and Culture of Primary Motor Neurons)
2.2. Experimental Groups
2.3. Cell Viability Tests
2.4. Cytotoxicity Assay
2.5. Measurement of Intracellular ROS
2.6. Caspase-Glo 3/7 Assay
2.7. Statistical Analysis
3. Results
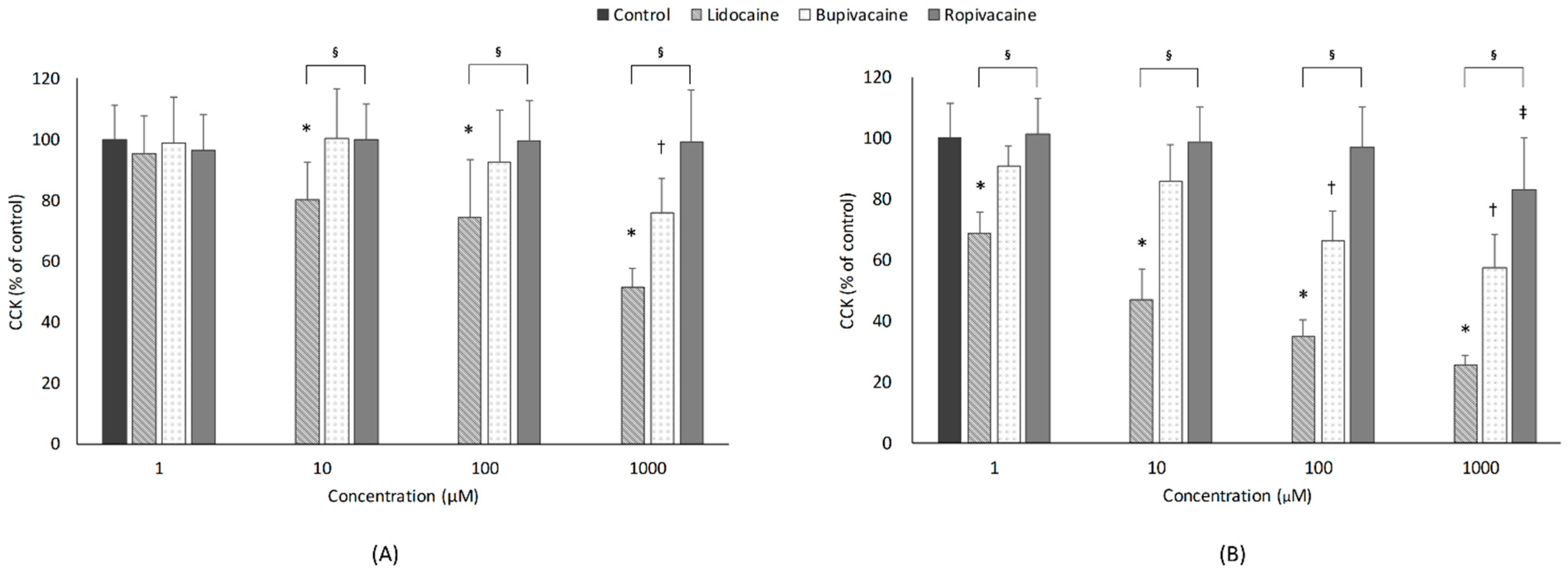
3.1. Cell Viability
3.1.1. Concentration
3.1.2. Type of LAs
3.1.3. Exposure Time
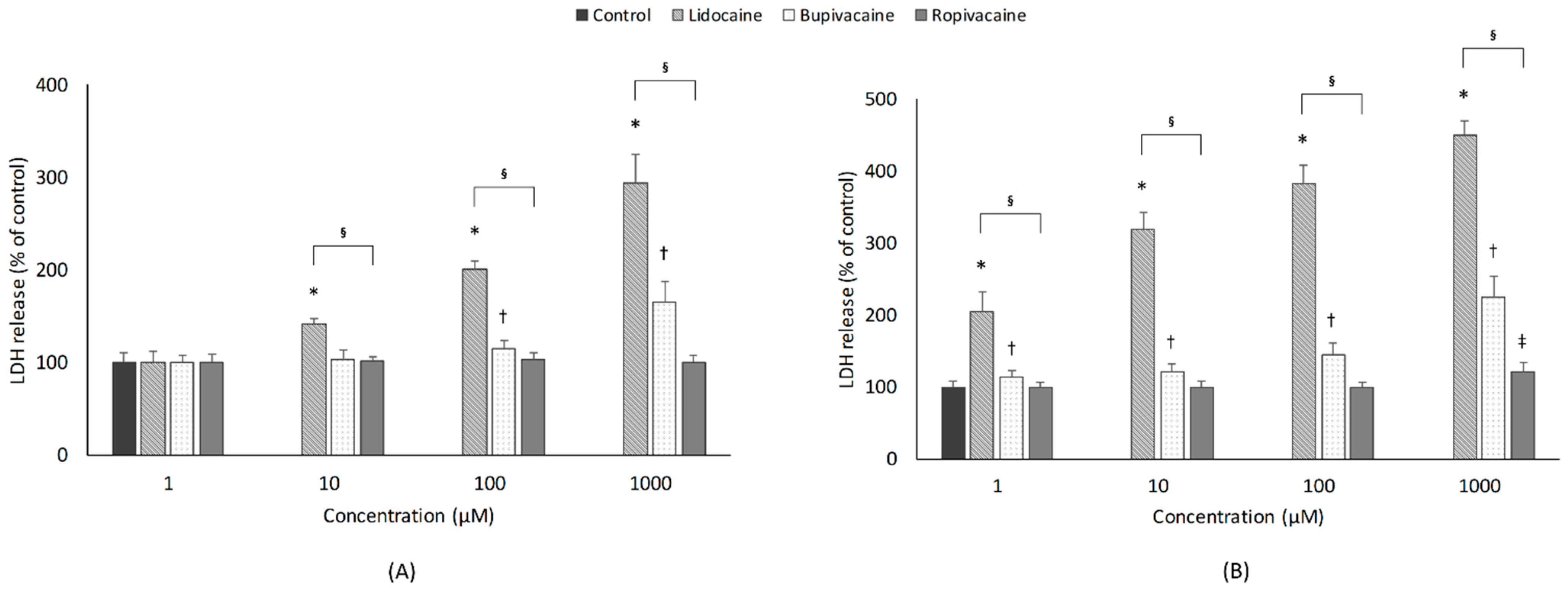
3.2. Cytotoxicity
3.2.1. Concentration
3.2.2. Type of LAs
3.2.3. Exposure Time
3.3. ROS
3.3.1. Concentration
3.3.2. Type of LAs
3.3.3. Exposure Time
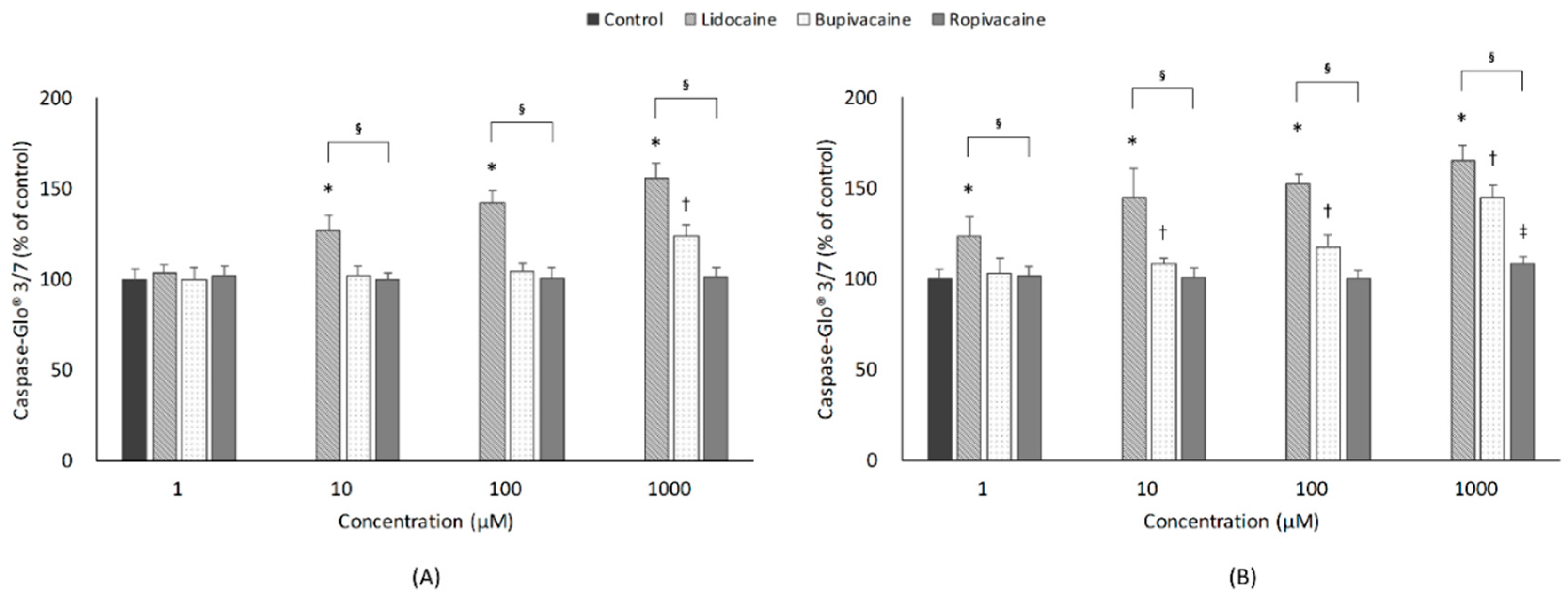
3.4. Apoptosis
3.4.1. Concentration
3.4.2. Type of LAs
3.4.3. Exposure Time
4. Discussion
4.1. Cell Viability and Cytotoxicity
4.2. ROS
4.3. Apoptosis
4.4. Other Findings
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hodgson, P.S.; Neal, J.M.; Pollock, J.E.; Liu, S.S. The neurotoxicity of drugs given intrathecally (spinal). Anesth. Analg. 1999, 88, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Matsumoto, M.; Tsuruta, S.; Hirata, T.; Gondo, T.; Sakabe, T. The nerve root entry zone is highly vulnerable to intrathecal tetracaine in rabbits. Anesth. Analg. 2005, 101, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Selander, D. Neurotoxicity of local anesthetics: Animal data. Reg. Anesth. 1993, 18, 461–468. [Google Scholar]
- Saito, S.; Radwan, I.; Obata, H.; Takahashi, K.; Goto, F. Direct neurotoxicity of tetracaine on growth cones and neurites of growing neurons in vitro. Anesthesiology 2001, 95, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Malet, A.; Faure, M.O.; Deletage, N.; Pereira, B.; Haas, J.; Lambert, G. The comparative cytotoxic effects of different local anesthetics on a human neuroblastoma cell line. Anesth. Analg. 2015, 120, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Mete, M.; Aydemir, I.; Tuglu, I.M.; Selcuki, M. Neurotoxic effects of local anesthetics on the mouse neuroblastoma NB2a cell line. Biotech. Histochem. 2015, 90, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Perez-Castro, R.; Patel, S.; Garavito-Aguilar, Z.V.; Rosenberg, A.; Recio-Pinto, E.; Zhang, J.; Blanck, T.J.; Xu, F. Cytotoxicity of local anesthetics in human neuronal cells. Anesth. Analg. 2009, 108, 997–1007. [Google Scholar] [CrossRef]
- Werdehausen, R.; Fazeli, S.; Braun, S.; Hermanns, H.; Essmann, F.; Hollmann, M.W.; Bauer, I.; Stevens, M.F. Apoptosis induction by different local anaesthetics in a neuroblastoma cell line. Br. J. Anaesth. 2009, 103, 711–718. [Google Scholar] [CrossRef]
- Doan, L.V.; Eydlin, O.; Piskoun, B.; Kline, R.P.; Recio-Pinto, E.; Rosenberg, A.D.; Blanck, T.J.; Xu, F. Despite differences in cytosolic calcium regulation, lidocaine toxicity is similar in adult and neonatal rat dorsal root ganglia in vitro. Anesthesiology 2014, 120, 50–61. [Google Scholar] [CrossRef]
- Johnson, M.E.; Saenz, J.A.; DaSilva, A.D.; Uhl, C.B.; Gores, G.J. Effect of local anesthetic on neuronal cytoplasmic calcium and plasma membrane lysis (necrosis) in a cell culture model. Anesthesiology 2002, 97, 1466–1476. [Google Scholar] [CrossRef]
- Puljak, L.; Kojundzic, S.L.; Hogan, Q.H.; Sapunar, D. Lidocaine injection into the rat dorsal root ganglion causes neuroinflammation. Anesth. Analg. 2009, 108, 1021–1026. [Google Scholar] [CrossRef]
- Radwan, I.A.; Saito, S.; Goto, F. The neurotoxicity of local anesthetics on growing neurons: A comparative study of lidocaine, bupivacaine, mepivacaine, and ropivacaine. Anesth. Analg. 2002, 94, 319–324. [Google Scholar]
- Ohtake, K.; Matsumoto, M.; Wakamatsu, H.; Kawai, K.; Nakakimura, K.; Sakabe, T. Glutamate release and neuronal injury after intrathecal injection of local anesthetics. Neuroreport 2000, 11, 1105–1109. [Google Scholar] [CrossRef]
- Rigler, M.L.; Drasner, K.; Krejcie, T.C.; Yelich, S.J.; Scholnick, F.T.; DeFontes, J.; Bohner, D. Cauda equina syndrome after continuous spinal anesthesia. Anesth. Analg. 1991, 72, 275–281. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Fu, S.L.; Nie, B.M.; Li, Y.; Lin, L.; Yin, L.; Wang, Y.X.; Lu, P.H.; Xu, X.M. Methods for isolating highly-enriched embryonic spinal cord neurons: A comparison between enzymatic and mechanical dissociations. J. Neurosci. Methods 2006, 158, 13–18. [Google Scholar] [CrossRef]
- Tominaga, H.; Ishiyama, M.; Ohseto, F.; Sasamoto, K.; Hamamoto, T.; Suzuki, K.; Watanabe, M. A water-soluble tetrazolium salt useful for colorimetric cell viability assay. Anal. Commun. 1999, 36, 47–50. [Google Scholar] [CrossRef]
- Burd, J.F.; Usategui-Gomez, M. A colorimetric assay for serum lactate dehydrogenase. Clin. Chim. Acta 1973, 46, 223–227. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; de Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef]
- Yamashita, A.; Matsumoto, M.; Matsumoto, S.; Itoh, M.; Kawai, K.; Sakabe, T. A comparison of the neurotoxic effects on the spinal cord of tetracaine, lidocaine, bupivacaine, and ropivacaine administered intrathecally in rabbits. Anesth. Analg. 2003, 97, 512–519. [Google Scholar] [CrossRef]
- Friederich, P.; Schmitz, T.P. Lidocaine-induced cell death in a human model of neuronal apoptosis. Eur. J. Anaesthesiol. 2002, 19, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Kasaba, T.; Onizuka, S.; Takasaki, M. Procaine and mepivacaine have less toxicity in vitro than other clinically used local anesthetics. Anesth. Analg. 2003, 97, 85–90. [Google Scholar] [CrossRef]
- Castelli, V.; Piroli, A.; Marinangeli, F.; d’Angelo, M.; Benedetti, E.; Ippoliti, R.; Zis, P.; Varrassi, G.; Giordano, A.; Paladini, A.; et al. Local anesthetics counteract cell proliferation and migration of human triple-negative breast cancer and melanoma cells. J. Cell Physiol. 2020, 235, 3474–3484. [Google Scholar] [CrossRef]
- Chang, Y.C.; Hsu, Y.C.; Liu, C.L.; Huang, S.Y.; Hu, M.C.; Cheng, S.P. Local anesthetics induce apoptosis in human thyroid cancer cells through the mitogen-activated protein kinase pathway. PLoS ONE 2014, 9, e89563. [Google Scholar] [CrossRef]
- Chang, Y.C.; Liu, C.L.; Chen, M.J.; Hsu, Y.W.; Chen, S.N.; Lin, C.H.; Chen, C.M.; Yang, F.M.; Hu, M.C. Local anesthetics induce apoptosis in human breast tumor cells. Anesth. Analg. 2014, 118, 116–124. [Google Scholar] [CrossRef]
- Piegeler, T.; Votta-Velis, E.G.; Liu, G.; Place, A.T.; Schwartz, D.E.; Beck-Schimmer, B.; Minshall, R.D.; Borgeat, A. Antimetastatic potential of amide-linked local anesthetics: Inhibition of lung adenocarcinoma cell migration and inflammatory Src signaling independent of sodium channel blockade. Anesthesiology 2012, 117, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Cela, O.; Piccoli, C.; Scrima, R.; Quarato, G.; Marolla, A.; Cinnella, G.; Dambrosio, M.; Capitanio, N. Bupivacaine uncouples the mitochondrial oxidative phosphorylation, inhibits respiratory chain complexes I and III and enhances ROS production: Results of a study on cell cultures. Mitochondrion 2010, 10, 487–496. [Google Scholar] [CrossRef]
- Lu, J.; Xu, S.Y.; Zhang, Q.G.; Lei, H.Y. Bupivacaine induces reactive oxygen species production via activation of the AMP-activated protein kinase-dependent pathway. Pharmacology 2011, 87, 121–129. [Google Scholar] [CrossRef]
- Okamoto, A.; Tanaka, M.; Sumi, C.; Oku, K.; Kusunoki, M.; Nishi, K.; Matsuo, Y.; Takenaga, K.; Shingu, K.; Hirota, K. The antioxidant N-acetyl cysteine suppresses lidocaine-induced intracellular reactive oxygen species production and cell death in neuronal SH-SY5Y cells. BMC Anesthesiol. 2016, 16, 104. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.E.; Uhl, C.B.; Spittler, K.H.; Wang, H.; Gores, G.J. Mitochondrial injury and caspase activation by the local anesthetic lidocaine. Anesthesiology 2004, 101, 1184–1194. [Google Scholar] [CrossRef]
- Suresh, S.; De Oliveira, G.S., Jr. Local anaesthetic dosage of peripheral nerve blocks in children: Analysis of 40 121 blocks from the Pediatric Regional Anesthesia Network database. Br. J. Anaesth. 2018, 120, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Ready, L.B.; Plumer, M.H.; Haschke, R.H.; Austin, E.; Sumi, S.M. Neurotoxicity of intrathecal local anesthetics in rabbits. Anesthesiology 1985, 63, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.M.; Datta, S.; Flanagan, H.; Covino, B.G. Comparison of bupivacaine- and ropivacaine-induced conduction blockade in the isolated rabbit vagus nerve. Anesth. Analg. 1989, 68, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Kole, A.J.; Annis, R.P.; Deshmukh, M. Mature neurons: Equipped for survival. Cell Death Dis. 2013, 4, e689. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koo, C.-H.; Baik, J.; Shin, H.-J.; Kim, J.-H.; Ryu, J.-H.; Han, S.-H. Neurotoxic Effects of Local Anesthetics on Developing Motor Neurons in a Rat Model. J. Clin. Med. 2021, 10, 901. https://doi.org/10.3390/jcm10050901
Koo C-H, Baik J, Shin H-J, Kim J-H, Ryu J-H, Han S-H. Neurotoxic Effects of Local Anesthetics on Developing Motor Neurons in a Rat Model. Journal of Clinical Medicine. 2021; 10(5):901. https://doi.org/10.3390/jcm10050901
Chicago/Turabian StyleKoo, Chang-Hoon, Jiseok Baik, Hyun-Jung Shin, Jin-Hee Kim, Jung-Hee Ryu, and Sung-Hee Han. 2021. "Neurotoxic Effects of Local Anesthetics on Developing Motor Neurons in a Rat Model" Journal of Clinical Medicine 10, no. 5: 901. https://doi.org/10.3390/jcm10050901
APA StyleKoo, C.-H., Baik, J., Shin, H.-J., Kim, J.-H., Ryu, J.-H., & Han, S.-H. (2021). Neurotoxic Effects of Local Anesthetics on Developing Motor Neurons in a Rat Model. Journal of Clinical Medicine, 10(5), 901. https://doi.org/10.3390/jcm10050901





