Factors Predicting the Success of Adhesiolysis Using a Steerable Catheter in Lumbar Failed Back Surgery Syndrome: A Retrospective Study
Abstract
:1. Introduction
2. Experimental Section
2.1. Subjects
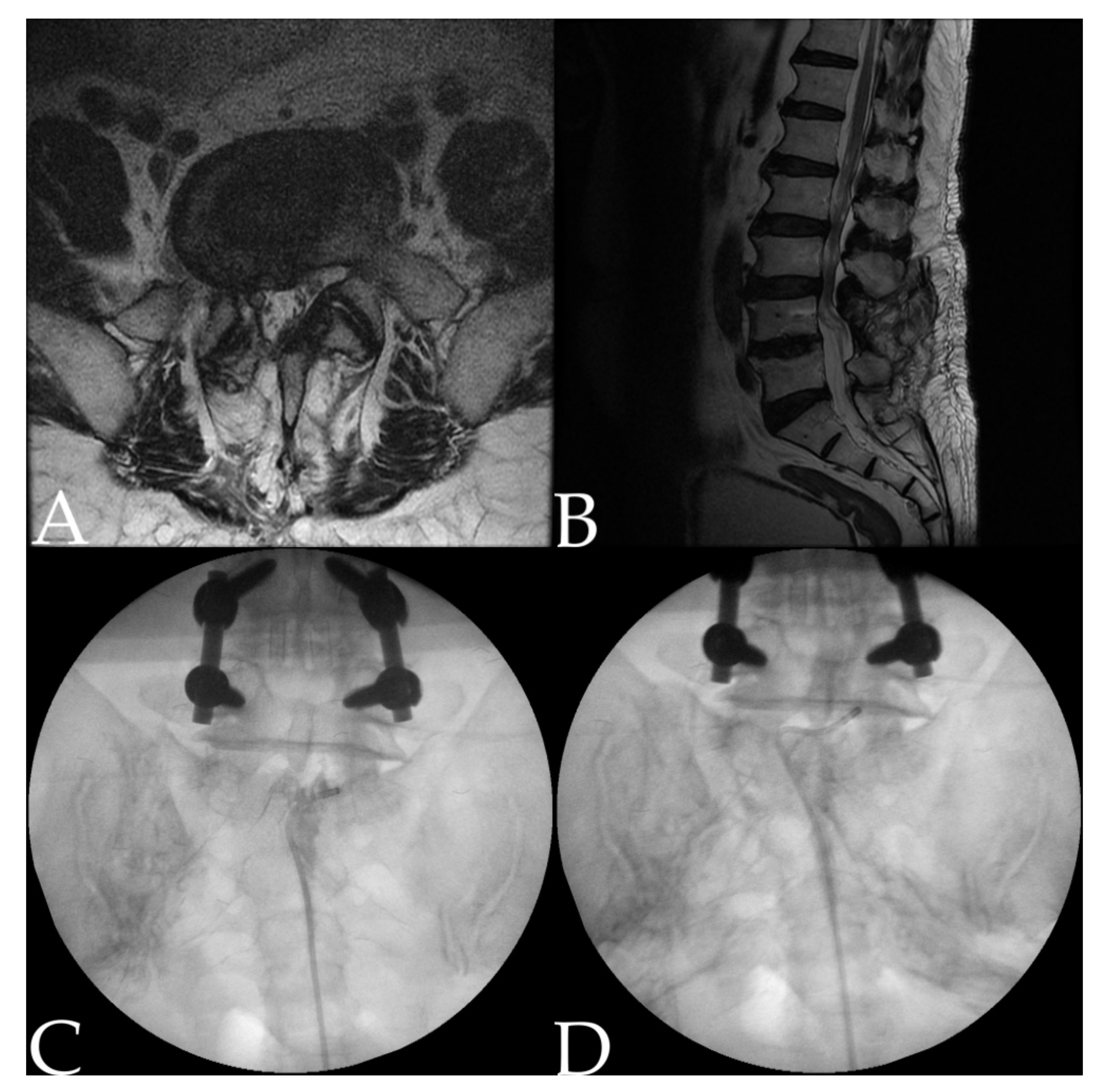
2.2. Procedure: Adhesiolysis Using a Steerable Catheter
2.3. Data Collection
2.4. Definition of Treatment Response
2.5. Statistical Analysis
3. Results
3.1. Demographics
3.2. Characteristics of the Responders and Nonresponders
3.3. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomson, S. Failed back surgery syndrome—Definition, epidemiology and demographics. Br. J. Pain 2013, 7, 56–59. [Google Scholar] [CrossRef] [Green Version]
- Oster, B.A.; Kikanloo, S.R.; Levine, N.L.; Lian, J.; Cho, W. Systematic Review of Outcomes Following 10-year Mark of Spine Patient Outcomes Research Trial (SPORT) for Spinal Stenosis. Spine 2020, 45, 832–836. [Google Scholar] [CrossRef]
- Golinvaux, N.S.; Bohl, D.D.; Basques, B.A.; Yacob, A.; Grauer, J.N. Comparison of the lumbar disc herniation patients randomized in SPORT to 6846 discectomy patients from NSQIP: Demographics, perioperative variables, and complications correlate well. Spine J. 2015, 15, 685–691. [Google Scholar] [CrossRef]
- Lurie, J.D.; Tosteson, T.D.; Tosteson, A.N.; Zhao, W.; Morgan, T.S.; Abdu, W.A.; Herkowitz, H.; Weinstein, J.N. Surgical versus nonoperative treatment for lumbar disc herniation: Eight-year results for the spine patient outcomes research trial. Spine 2014, 39, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.N.; Tosteson, T.D.; Lurie, J.D.; Tosteson, A.N.; Blood, E.; Hanscom, B.; Herkowitz, H.; Cammisa, F.; Albert, T.; Boden, S.D.; et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N. Engl. J. Med. 2008, 358, 794–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weir, S.; Samnaliev, M.; Kuo, T.-C.; Ni Choitir, C.; Tierney, T.S.; Cumming, D.; Bruce, J.; Manca, A.; Taylor, R.S.; Eldabe, S. The incidence and healthcare costs of persistent postoperative pain following lumbar spine surgery in the UK: A cohort study using the Clinical Practice Research Datalink (CPRD) and Hospital Episode Statistics (HES). BMJ Open 2017, 7, e017585. [Google Scholar] [CrossRef] [Green Version]
- Rigoard, P.; Gatzinsky, K.; Deneuville, J.-P.; Duyvendak, W.; Naiditch, N.; Van Buyten, J.-P.; Eldabe, S. Optimizing the management and outcomes of failed back surgery syndrome: A consensus statement on definition and outlines for patient assessment. Pain Res. Manag. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- North, R.B.; Campbell, J.N.; James, C.S.; Conover-Walker, M.K.; Wang, H.; Piantadosi, S.; Rybock, J.D.; Long, D.M. Failed back surgery syndrome: 5-year follow-up in 102 patients undergoing repeated operation. Neurosurgery 1991, 28, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Van Buyten, J.-P.; Linderoth, B. “The failed back surgery syndrome”: Definition and therapeutic algorithms—An update. Eur. J. Pain Suppl. 2010, 4, 273–286. [Google Scholar] [CrossRef]
- Rigoard, P.; Desai, M.; Taylor, R.S. Failed back surgery syndrome: What’s in a name? A proposal to replace “FBSS” by “POPS”…. Neurochirurgie 2015, 61, S16–S21. [Google Scholar] [CrossRef]
- Fritsch, E.W.; Heisel, J.; Rupp, S. The Failed Back Surgery Syndrome: Reasons, Intraoperative Findings, and Long-term Results: A Report of 182 Operative Treatments. Spine 1996, 21, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Long, D.M. Failed back surgery syndrome. Neurosurg. Clin. N. Am. 1991, 2, 899–919. [Google Scholar] [CrossRef]
- Gatzinsky, K.; Eldabe, S.; Deneuville, J.-P.; Duyvendak, W.; Naiditch, N.; Van Buyten, J.-P.; Rigoard, P. Optimizing the Management and Outcomes of Failed Back Surgery Syndrome: A Proposal of a Standardized Multidisciplinary Team Care Pathway. Pain Res. Manag. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Nava, A.; Rigoard, P.; Shah, B.; Taylor, R.S. Optimal medical, rehabilitation and behavioral management in the setting of failed back surgery syndrome. Neurochirurgie 2015, 61, S66–S76. [Google Scholar] [CrossRef] [PubMed]
- Meints, S.; Hollingshead, N.; Hirsh, A. Factors influencing providers’ treatment decisions for chronic low back pain. J. Pain 2013, 14, S101. [Google Scholar] [CrossRef]
- Sebaaly, A.; Lahoud, M.-J.; Rizkallah, M.; Kreichati, G.; Kharrat, K. Etiology, Evaluation, and Treatment of Failed Back Surgery Syndrome. Asian Spine J. 2018, 12, 574–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, B.; Gatchel, R.J.; Lou, L.; Stowell, A.; Robinson, R.; Polatin, P.B. Interdisciplinary Treatment of Failed Back Surgery Syndrome (FBSS): A Comparison of FBSS and Non-FBSS Patients. Pain Pract. 2005, 5, 190–202. [Google Scholar] [CrossRef]
- Amirdelfan, K.; Webster, L.; Poree, L.; Sukul, V.; McRoberts, P. Treatment options for failed back surgery syndrome patients with refractory chronic pain: An evidence based approach. Spine 2017, 42, S41–S52. [Google Scholar] [CrossRef]
- Ross, J.S.; Robertson, J.T.; Frederickson, R.C.; Petrie, J.L.; Obuchowski, N.; Modic, M.T.; de Tribolet, N. Association between peridural scar and recurrent radicular pain after lumbar discectomy: Magnetic resonance evaluation. Neurosurgery 1996, 38, 855–863. [Google Scholar] [CrossRef] [Green Version]
- Maliszewski, M.; Tymowski, M.; Lelek, P.; Bierzyńska-Macyszyn, G.; Majchrzak, H. An attempt to use Gore-Tex surgical membrane in lumbar disc surgery. Neurol. Neurochir. Pol. 2004, 38, 271–277. [Google Scholar]
- Kayaoglu, C.; Calikoğlu, C.; Binler, S. Re-operation after lumbar disc surgery: Results in 85 cases. J. Int. Med Res. 2003, 31, 318–323. [Google Scholar] [CrossRef] [Green Version]
- Manchikanti, L.; Singh, V. Epidural lysis of adhesions and myeloscopy. Curr. Pain Headache Rep. 2002, 6, 427–435. [Google Scholar] [CrossRef]
- Chan, C.-W.; Peng, P. Failed back surgery syndrome. Pain Med. 2011, 12, 577–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahimzadeh, P.; Sharma, V.; Imani, F.; Faiz, H.R.; Ghodraty, M.R.; Nikzad-Jamnani, A.R.; Nader, N.D. Adjuvant hyaluronidase to epidural steroid improves the quality of analgesia in failed back surgery syndrome: A prospective randomized clinical trial. Pain Physician 2014, 17, E75–E82. [Google Scholar]
- Rapčan, R.; Kočan, L.; Mláka, J.; Burianek, M.; Kočanová, H.; Rapčanová, S.; Hess, M.; Hammond, A.; Griger, M.; Venglarčík, M.; et al. A Randomized, Multicenter, Double-Blind, Parallel Pilot Study Assessing the Effect of Mechanical Adhesiolysis vs. Adhesiolysis with Corticosteroid and Hyaluronidase Administration into the Epidural Space During Epiduroscopy. Pain Med. 2018, 19, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Brito-García, N.; García-Pérez, L.; Kovacs, F.M.; del Pino-Sedeno, T.; Perez-Ramos, J.; Imaz-Iglesia, I.; Serrano-Aguilar, P. Efficacy, effectiveness, safety, and cost-effectiveness of epidural adhesiolysis for treating failed back surgery syndrome. A systematic review. Pain Med. 2019, 20, 692–706. [Google Scholar] [CrossRef]
- Cho, J.H.; Lee, J.H.; Song, K.S.; Hong, J.Y.; Joo, Y.S.; Lee, D.H.; Hwang, C.J.; Lee, C.S. Treatment Outcomes for Patients with Failed Back Surgery. Pain Physician 2017, 20, E29–E43. [Google Scholar] [CrossRef] [PubMed]
- Epter, R.S.; Helm, S.; Hayek, S.M.; Benyamin, R.M.; Smith, H.S.; Abdi, S. Systematic review of percutaneous adhesiolysis and management of chronic low back pain in post lumbar surgery syndrome. Pain Physician 2009, 12, 361–378. [Google Scholar] [PubMed]
- Hayek, S.M.; Helm, S.; Benyamin, R.M.; Singh, V.; Bryce, D.A.; Smith, H.S. Effectiveness of spinal endoscopic adhesiolysis in post lumbar surgery syndrome: A systematic review. Pain Physician 2009, 12, 419–435. [Google Scholar] [PubMed]
- Lee, G.Y.; Lee, J.W.; Choi, H.S.; Oh, K.J.; Kang, H.S. A new grading system of lumbar central canal stenosis on MRI: An easy and reliable method. Skelet. Radiol. 2011, 40, 1033–1039. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Lee, J.W.; Yeom, J.S.; Kim, K.J.; Kim, H.J.; Chung, S.K.; Kang, H.S. A practical MRI grading system for lumbar foraminal stenosis. AJR Am. J. Roentgenol 2010, 194, 1095–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eunjoo Choi, M.; Nahm, F.S.; Lee, P.-B. Evaluation of prognostic predictors of percutaneous adhesiolysis using a Racz catheter for post lumbar surgery syndrome or spinal stenosis. Pain Physician 2013, 16, E531–E536. [Google Scholar]
- Lee, J.H.; Lee, S.-H. Clinical effectiveness of percutaneous adhesiolysis and predictive factors of treatment efficacy in patients with lumbosacral spinal stenosis. Pain Med. 2013, 14, 1497–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Lee, S.H. Clinical effectiveness of percutaneous adhesiolysis using Navicath for the management of chronic pain due to lumbosacral disc herniation. Pain Physician 2012, 15, 213–221. [Google Scholar] [PubMed]
- Oh, Y.; Kim, D.-H.; Park, J.-Y.; Ji, G.Y.; Shin, D.A.; Lee, S.W.; Park, J.K.; Shin, J.-W.; Choi, S.-S. Factors Associated with Successful Response to Balloon Decompressive Adhesiolysis Neuroplasty in Patients with Chronic Lumbar Foraminal Stenosis. J. Clin. Med. 2019, 8, 1766. [Google Scholar] [CrossRef] [Green Version]
- Park, C.H.; Lee, S.H.; Jung, J.Y. Dural sac cross-sectional area does not correlate with efficacy of percutaneous adhesiolysis in single level lumbar spinal stenosis. Pain Physician 2011, 14, 377–382. [Google Scholar] [PubMed]
- Oh, Y.; Shin, D.A.; Kim, D.J.; Cho, W.; Na, T.; Leem, J.-G.; Shin, J.-W.; Kim, D.-H.; Hahm, K.-D.; Choi, S.-S. Effectiveness of and Factors Associated with Balloon Adhesiolysis in Patients with Lumbar Post-Laminectomy Syndrome: A Retrospective Study. J. Clin. Med. 2020, 9, 1144. [Google Scholar] [CrossRef]

| Parameters | n = 150 |
|---|---|
| Age (years) | 66.7 ± 12.6 |
| Sex (male/female) | 63 (58%)/87 (42%) |
| Body mass index (kg/m2) | 24.3 ± 3.6 |
| Duration of pain (months) | 72.1 ± 69.6 |
| Pre-procedural NRS | 7.0 ± 1.6 |
| Number of previous spine surgeries: 1/2/3 or more | 117 (78%)/23 (15.3%)/10 (6.7%) |
| Concurrent disease: Diabetes/hypertension | 38 (25.3%)/71 (47.3%) |
| Spondylolisthesis | 29 (19.3%) |
| Outer diameter of catheter: 1.7mm/2.1mm | 81 (54%)/69 (46%) |
| Central stenosis: mild/moderate/severe | 54 (36%)/24 (16%)/23 (15.3%) |
| Foraminal stenosis: mild/moderate/severe | 55 (36.7%)/32 (21.3%)/14 (9.3%) |
| Nerve blocks during f/u period after the procedure: Y/N | 131 (87.3%)/19 (12.7%) |
| Included Group (n = 150) | Follow up Loss Group (n = 39) | p-Value | |
|---|---|---|---|
| Age (years) | 66.7 ± 12.5 | 67.2 ± 13.3 | 0.827 |
| Sex (male/female) | 63 (42%)/87 (58%) | 13 (33.3%)/26 (66.7%) | 0.325 |
| Body mass index (kg/m2) | 24.3 ± 3.6 | 24.9 ± 2.6 | 0.444 |
| Duration of pain (months) | 72.5 ± 69.4 | 74.2 ± 110.5 | 0.907 |
| Number of previous spine surgeries: 1/2/3 or more | 116 (77.3%)/24 (16%)/10 (6.7%) | 27 (69.2%)/7 (17.9%)/5 (12.8%) | 0.403 |
| Diabetes | 38 (25.3%) | 10 (25.6%) | 0.969 |
| Hypertension | 72 (48%) | 13 (33.3%) | 0.101 |
| Spondylolisthesis | 53 (35.3%) | 19 (51.3%) | 0.125 |
| Outer diameter of catheter: | 0.135 | ||
| 1.7 mm/2.1 mm | 80 (53.5%)/70 (46.7%) | 26 (66.7%)/13 (33.3%) | |
| Central stenosis, n (%): | 0.299 | ||
| Mild/moderate/severe | 43 (43.4%)/28 (28.3%)/28 (28.3%) | 5 (25%)/7( 35%)/8 (40%) | |
| Foraminal stenosis, n (%): | 0.710 | ||
| Mild/moderate/severe | 48 (49%)/36 (36.7%)/14 (14.3%) | 8 (40%)/8 (40%)/4 (20%) |
| Nonresponders (n = 81) | Successful Responders (n = 69) | p-Value | |
|---|---|---|---|
| Age (years) | 66.6 ± 13.1 | 67.9 ± 11.9 | 0.265 |
| Sex (male/female) | 33 (40.7%)/48 (59.3%) | 30 (43.5%)/39 (56.5%) | 0.735 |
| Body mass index (kg/m2) | 24.1 ± 3.4 | 24.4 ± 3.9 | 0.750 |
| Pre-procedural NRS | 7.1 ± 1.6 | 7.0 ± 1.6 | 0.778 |
| Duration of pain (months) | 78.5 ± 69.4 | 64.5 ± 69.6 | 0.220 |
| Number of previous spine surgeries: 1/2/3 or more | 67 (82.7%)/5 (6.2%)/9 (11.1%) | 50 (72.5%)/18 (26.1%)/1 (1.4%) | 0.951 |
| Diabetes | 20 (24.7%) | 18 (26.1%) | 0.845 |
| Hypertension | 37 (45.7%) | 34 (49.3%) | 0.660 |
| Spondylolisthesis | 20 (24.7%) | 9 (13%) | 0.072 |
| Outer diameter of catheter: | 0.017 * | ||
| 1.7 mm/2.1 mm | 51 (63%)/30 (37%) | 30 (43.5%)/39 (56.5%) | |
| Central stenosis, n (%): | 0.428 | ||
| Mild/moderate/severe | 27 (50.9%)/12 (22.6%)/14 (26.4%) | 27 (56.3%)/12 (25%)/9 (18.8%) | |
| Foraminal stenosis, n (%): | 0.039 * | ||
| Mild/moderate/severe | 24 (45.3%)/19 (35.8%)/10 (18.9%) | 31 (64.6%)/13 (27.1%)/4 (8.3%) | |
| Treatment level: 1/2/3 or more | 5 (6.2%)/20 (24.7%)/56 (69.1%) | 8 (11.6%)/18 (26.1%)/43 (62.3%) | 0.249 |
| Treatment location: | 0.548 | ||
| Central only | 1 (1.2%) | 0 (0%) | |
| Central with both foramina | 77 (95.1%) | 65 (94.2%) | |
| Central with a unilateral foramen (left/right) | 3 (3.7%) | 4 (5.8%) | |
| Nerve blocks during f/u period after the procedure: Y/N | 72 (88.9%)/9 (11.1%) | 59 (85.5%)/10 (14.5%) | 0.535 |
| Complication | Number |
|---|---|
| Suspected dura puncture | 13 |
| Subdural injection | 2 |
| Temporary motor weakness | 0 |
| Vascular injection | 1 |
| Disc injection | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.Y.; Lee, Y.H.; Yoo, S.; Kim, J.Y.; Joo, M.; Park, H.J. Factors Predicting the Success of Adhesiolysis Using a Steerable Catheter in Lumbar Failed Back Surgery Syndrome: A Retrospective Study. J. Clin. Med. 2021, 10, 913. https://doi.org/10.3390/jcm10050913
Kim JY, Lee YH, Yoo S, Kim JY, Joo M, Park HJ. Factors Predicting the Success of Adhesiolysis Using a Steerable Catheter in Lumbar Failed Back Surgery Syndrome: A Retrospective Study. Journal of Clinical Medicine. 2021; 10(5):913. https://doi.org/10.3390/jcm10050913
Chicago/Turabian StyleKim, Ji Yeong, Yong Ho Lee, Subin Yoo, Ji Young Kim, Mina Joo, and Hue Jung Park. 2021. "Factors Predicting the Success of Adhesiolysis Using a Steerable Catheter in Lumbar Failed Back Surgery Syndrome: A Retrospective Study" Journal of Clinical Medicine 10, no. 5: 913. https://doi.org/10.3390/jcm10050913
APA StyleKim, J. Y., Lee, Y. H., Yoo, S., Kim, J. Y., Joo, M., & Park, H. J. (2021). Factors Predicting the Success of Adhesiolysis Using a Steerable Catheter in Lumbar Failed Back Surgery Syndrome: A Retrospective Study. Journal of Clinical Medicine, 10(5), 913. https://doi.org/10.3390/jcm10050913






