Clinical Significance of Indeterminate QuantiFERON-TB Gold Plus Assay Results in Hospitalized COVID-19 Patients with Severe Hyperinflammatory Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Statistical Analysis
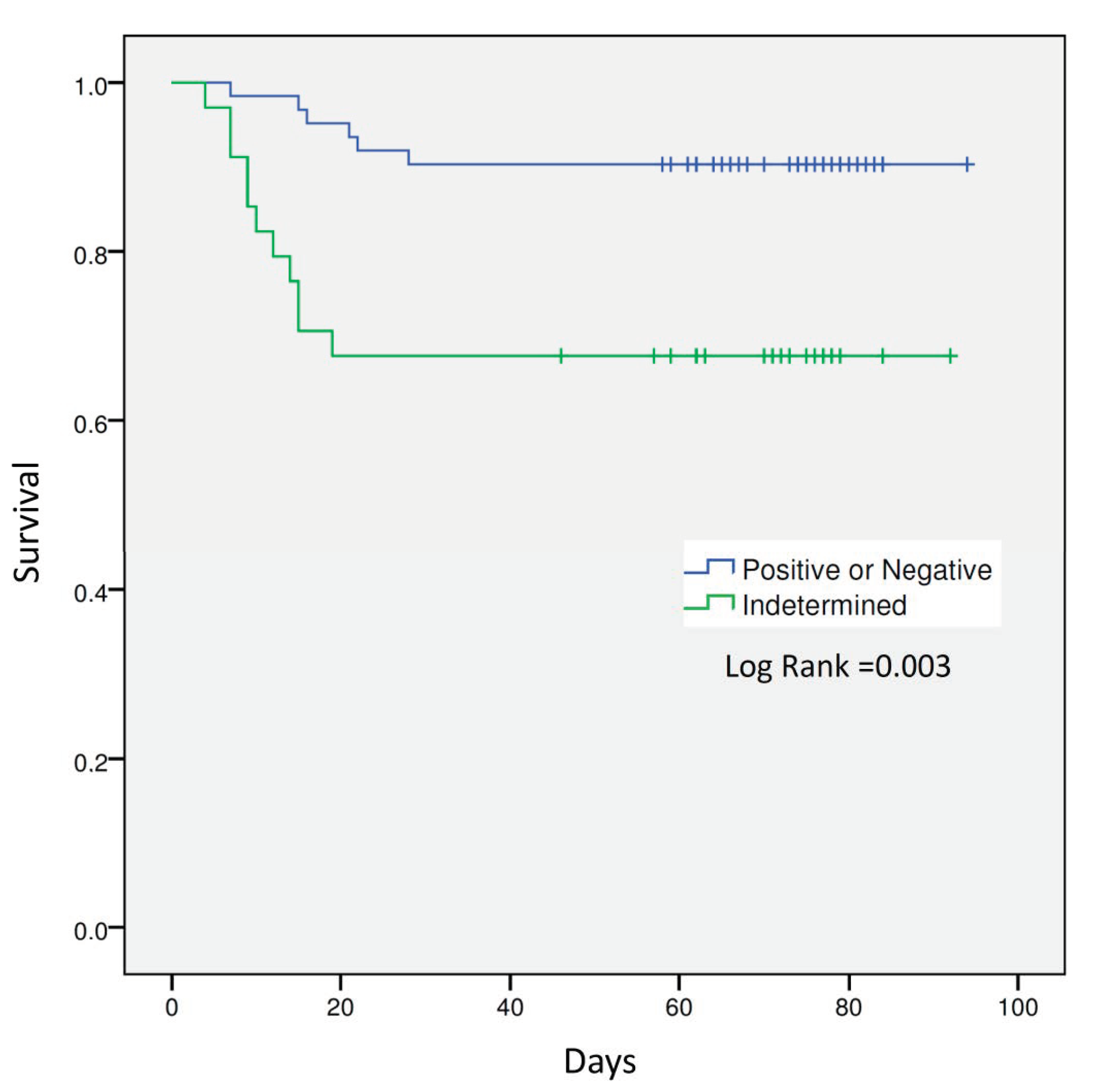
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lai, C.C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Ye, D.; Liu, Q. Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence. Int. J. Antimicrob. Agents 2020, 55, 105948. [Google Scholar] [CrossRef] [PubMed]
- WHO. Director-General’s Opening Remarks at the Media Briefing on COVID-19-11. March 2020. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19 (accessed on 11 March 2020).
- Rubio-Rivas, M.; Ronda, M.; Padulles, A.; Mitjavila, F.; Riera-Mestre, A.; García-Forero, C.; Iriarte, A.; Mora, J.M.; Padulles, N.; Gonzalez, M.; et al. Beneficial effect of corticosteroids in preventing mortality in patients receiving tocilizumab to treat severe COVID-19 illness. Int. J. Infect. Dis. 2020, 101, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Farina, N.; Campochiaro, C.; De Luca, G.; Della-Torre, E.; Tomelleri, A.; Dagna, L. Repurposing of Biologic and Targeted Synthetic Anti-Rheumatic Drugs in COVID-19 and Hyper-Inflammation: A Comprehensive Review of Available and Emerging Evidence at the Peak of the Pandemic. Front. Pharmacol. 2020, 11, 598308. [Google Scholar] [CrossRef] [PubMed]
- Słomka, A.; Kowalewski, M.; Żekanowska, E. Coronavirus Disease 2019 (COVID-19): A Short Review on Hematological Manifestations. Pathogens 2020, 9, 493. [Google Scholar] [CrossRef] [PubMed]
- Solanich, X.; Antolí, A.; Padullés, N.; Fanlo-Maresma, M.; Iriarte, A.; Mitjavila, F.; Capdevila, O.; Molina, M.; Sabater, J.; Bas, J.; et al. Pragmatic, open-label, single-center, randomized, phase II clinical trial to evaluate the efficacy and safety of methylprednisolone pulses and tacrolimus in patients with severe pneumonia secondary to COVID-19: The TACROVID trial protocol. Contemp. Clin. Trials Commun. 2021, 21, 100716. [Google Scholar] [CrossRef] [PubMed]
- Qiagen. QuantiFERON-TB Gold Plus ELISA Package Insert. 2014. Available online: https://www.quantiferon.com/products/quantiferon-tb-gold-plus-qft-plus/package-inserts/ (accessed on 5 February 2021).
- Hakimian, S.; Popov, Y.; Rupawala, A.H.; Salomon-Escoto, K.; Hatch, S.; Pellish, R. The conundrum of indeterminate QuantiFERON-TB Gold results before anti-tumor necrosis factor initiation. Biologics 2018, 12, 61–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torre, A.; Aliberti, S.; Castellotti, P.F.; Cirillo, D.M.; Grisolia, A.; Mangioni, D.; Marchetti, G.; Rossotti, R.; Santus, P.; Besozzi, G.; et al. Preliminary observations on IGRA testing for TB infection in patients with severe COVID-19 eligible for immunosuppressive therapy. Respir. Med. 2020, 175, 106204. [Google Scholar] [CrossRef]
- Pandharipande, P.P.; Shintani, A.K.; Hagerman, H.E.; St Jacques, P.J.; Rice, T.W.; Sanders, N.W.; Ware, L.B.; Bernard, G.R.; Ely, E.W. Derivation and validation of Spo2/Fio2 ratio to impute for Pao2/Fio2 ratio in the respiratory component of the Sequential Organ Failure Assessment score. Crit. Care Med. 2009, 37, 1317–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO R&D Blueprint. Novel Coronavirus. COVID-19 Therapeutic Trial Synopsis. Available online: https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf (accessed on 5 February 2021).
- Casas, S.; Muñoz, L.; Moure, R.; Castellote, J.; Guerra, M.R.; Gonzalez, L.; Andreu, A.; Rafecas, A.G.; Alcaide, F.; Santin, M. Comparison of the 2-step tuberculin skin test and the quantiFERON-TB Gold In-Tube Test for the screening of tuberculosis infection before liver transplantation. Liver Transplant. 2011, 17, 1205–1211. [Google Scholar] [CrossRef]
- Casas, S.; Andreu, A.; Juanola, X.; Bordas, X.; Alcaide, F.; Moure, R.; Anibarro, L.; Cuchí, E.; Esteve, M.; Ortiz, V.; et al. Diagnosis of tuberculosis infection by tuberculin skin test and a whole-blood interferon-γ release assay in patients considered for anti-tumor necrosis factor-α therapy. Diagn. Microbiol. Infect. Dis. 2011, 71, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Santin, M.; Casas, S.; Saumoy, M.; Andreu, A.; Moure, R.; Alcaide, F.; Ferrer, E.; Podzamczer, D. Detection of latent tuberculosis by the tuberculin skin test and a whole-blood interferon-γ release assay, and the development of active tuberculosis in HIV-seropositive persons. Diagn. Microbiol. Infect. Dis. 2011, 69, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, H.K.; Mehra, M.R. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J. Hear. Lung Transplant. 2020, 39, 405–407. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Vabret, N.; Britton, G.J.; Gruber, C.; Hegde, S.; Kim, J.; Kuksin, M.; Levantovsky, R.; Malle, L.; Moreira, A.; Park, M.D.; et al. Immunology of COVID-19: Current State of the Science. Immunity 2020, 52, 910–941. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Péré, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Liu, C.; Su, L.; Zhang, D.; Fan, J.; Yang, Y.; Xiao, M.; Xie, J.; Xu, Y.; et al. IP-10 and MCP-1 as biomarkers associated with disease severity of COVID-19. Mol. Med. 2020, 26, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Remy, K.E.; Mazer, M.; Striker, D.A.; Ellebedy, A.H.; Walton, A.H.; Unsinger, J.; Blood, T.M.; Mudd, P.A.; Yi, D.J.; Mannion, D.A.; et al. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI Insight 2020, 5, e140329. [Google Scholar] [CrossRef] [PubMed]
| Indeterminate QTF-Plus (n = 34) | Interpretable QTF-Plus (n = 62) | Unadjusted Model | Adjusted Model | ||||
|---|---|---|---|---|---|---|---|
| p-Value | OR (95%CI) | p-Value | OR (95%CI) | p-Value | |||
| Gender (male) | 22 (64.7%) | 45 (72.6%) | 0.422 | 0.693 (0.282–1.700) | 0.422 | ||
| Age (years) | 70.5 (56.9–76.2) | 63.7 (49.2–74.9) | 0.237 | 1.020 (0.990–1.051) | 0.203 | ||
| Comorbidities | |||||||
| Hypertension | 16 (47.1%) | 34 (54.8%) | 0.466 | 0.732 (0.316–1.694) | 0.466 | ||
| Dyslipidemia | 19 (55.9%) | 28 (45.2%) | 0.315 | 1.538 (0.663–3.569) | 0.315 | ||
| Diabetes | 11 (32.4%) | 12 (19.4%) | 0.154 | 1.993 (0.766–5.182) | 0.154 | ||
| Cardiovascular disease | 7 (20.6%) | 12 (19.4%) | 0.885 | 1.080 (0.381–3.066) | 0.885 | ||
| Lung disease | 6 (17.6%) | 9 (14.5%) | 0.686 | 1.262 (0.408–3.906) | 0.686 | ||
| Immunocompromised patient | 4 (11.8%) | 3 (4.8%) | 0.212 | 2.622 (0.551–12.480) | 0.212 | ||
| Candidate for invasive measures (yes) | 25 (73.5%) | 52 (83.9%) | 0.224 | 0.534 (0.193–1.480) | 0.224 | ||
| COVID-19 onset to admission (days) | 7.5 (5.75–12.25) | 8 (7–10.25) | 0.401 | 1.013 (0.928–1.105) | 0.778 | ||
| Laboratory tests (*) | |||||||
| Leukocytes (×109/L) | 8.6 (6.3–13.9) | 6.8 (5.6–9.6) | 0.016 | 1.131 (1.024–1.248) | 0.015 | ||
| Neutrophils (×109/L) | 7.3 (5.4–12.4) | 5.5 (3.5–8.3) | 0.002 | 1.147 (1.032–1.274) | 0.011 | ||
| Lymphocytes (×109/L) | 0.64 (0.48–1.15) | 0.88 (0.64–1.27) | 0.017 | 0.287 (0.105–0.783) | 0.015 | ||
| Serum ferritin (μg/L) (n = 92) | 1756 (1081–2574) | 1444 (692–2233) | 0.159 | 1.000 (1.000–1.001) | 0.201 | ||
| LDH (U/L) (n = 95) | 412 (330–517) | 317 (265–424) | 0.003 | 1.005 (1.001–1.008) | 0.005 | 1.005 (1.002–1.008) | 0.003 |
| C-reactive protein (mg/L) | 79 (29–197) | 87 (27–160) | 0.654 | 1.002 (0.997–1.006) | 0.447 | ||
| IL-6 (ng/L),(n = 52) | 100 (20–1044) | 99 (55–193) | 0.955 | 1.001 (1.000–1.012) | 0.130 | ||
| Troponin (ng/L), (n = 69) | 10.0 (7.0–25.0) | 10.5 (6.0–18.2) | 0.546 | 1.000 (0.991–1.008) | 0.926 | ||
| D-dimer (μg/L), (n = 93) | 636 (353–2913) | 361 (250–946) | 0.025 | 1.000 (1.000–1.001) | 0.016 | ||
| Hypoxemia | 0.015 | ||||||
| None | 4 (11.8%) | 21 (33.8%) | - | ||||
| Mild | 6 (17.6%) | 20 (32.3%) | 1.557 (0.386–6.423) | 0.536 | |||
| Moderate | 22 (64.7%) | 20 (32.3%) | 5.775 (1.690–19.734) | 0.005 | |||
| Severe | 2 (5.9%) | 1 (1.6%) | 10.5 (0.758–145.359) | 0.079 | |||
| COVID-19 onset to QFT-Plus (days) | 11 (9–18) | 12 (10-16) | 0.797 | 1.005 (0.937–1.077) | 0.893 | ||
| Severe WHO 8-OS on QFT-time | 9 (26.5) | 5 (8.1) | 0.015 | 4.104 (1.248–13.491) | 0.015 | ||
| IS prior to QFT-Plus | |||||||
| None | 5 (14.7%) | 28 (45.1%) | 0.003 | 0.209 (0.072–0.612) | 0.003 | ||
| Corticosteroids | 10 (29.4%) | 6 (9.7%) | 0.013 | 3.889 (1.270–11.912) | 0.013 | 4.477 (1.397–14.345) | 0.012 |
| IL-6 blockage | 3 (8.8%) | 5 (8.1%) | 0.898 | 1.103 (0.247–4.928) | 0.898 | ||
| Corticosteroids and IL-6 blockage | 16 (47.1%) | 23 (37.1%) | 0.342 | 1.507 (0.646–3.519) | 0.342 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solanich, X.; Fernández-Huerta, M.; Basaez, C.; Antolí, A.; Rocamora-Blanch, G.; Corbella, X.; Santin, M.; Alcaide, F. Clinical Significance of Indeterminate QuantiFERON-TB Gold Plus Assay Results in Hospitalized COVID-19 Patients with Severe Hyperinflammatory Syndrome. J. Clin. Med. 2021, 10, 918. https://doi.org/10.3390/jcm10050918
Solanich X, Fernández-Huerta M, Basaez C, Antolí A, Rocamora-Blanch G, Corbella X, Santin M, Alcaide F. Clinical Significance of Indeterminate QuantiFERON-TB Gold Plus Assay Results in Hospitalized COVID-19 Patients with Severe Hyperinflammatory Syndrome. Journal of Clinical Medicine. 2021; 10(5):918. https://doi.org/10.3390/jcm10050918
Chicago/Turabian StyleSolanich, Xavier, Miguel Fernández-Huerta, Celeste Basaez, Arnau Antolí, Gemma Rocamora-Blanch, Xavier Corbella, Miguel Santin, and Fernando Alcaide. 2021. "Clinical Significance of Indeterminate QuantiFERON-TB Gold Plus Assay Results in Hospitalized COVID-19 Patients with Severe Hyperinflammatory Syndrome" Journal of Clinical Medicine 10, no. 5: 918. https://doi.org/10.3390/jcm10050918
APA StyleSolanich, X., Fernández-Huerta, M., Basaez, C., Antolí, A., Rocamora-Blanch, G., Corbella, X., Santin, M., & Alcaide, F. (2021). Clinical Significance of Indeterminate QuantiFERON-TB Gold Plus Assay Results in Hospitalized COVID-19 Patients with Severe Hyperinflammatory Syndrome. Journal of Clinical Medicine, 10(5), 918. https://doi.org/10.3390/jcm10050918








