Walk and Run Test in Patients with Degenerative Compression of the Cervical Spinal Cord
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
- age ≥18 years;
- ability to walk at least 10 m without the assistance of another person.
- Patients or subjects were excluded if they were affected by any of the following: severe respiratory or cardiac disease hindering walking abilities or safe mobilization;
- history of any other neurological disorders with persistent deficit;
- symptomatic musculoskeletal problems affecting gait, especially coxarthrosis or gonarthrosis;
- symptomatic lumbar spinal stenosis (MRI of the lumbar spine performed only in patients with symptoms or signs suspected of lumbar spinal stenosis);
- previous surgical decompression to alleviate DCM.
2.3. MRI Examination and Assessment of Cervical Cord Compression
2.4. mJOA Score
2.5. Gait Assessment
2.6. Statistics
3. Results
3.1. Participant Demography
3.2. Gait Analysis
3.2.1. Healthy Controls
3.2.2. NMDCC
3.2.3. DCM
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rhee, J.M.; Heflin, J.A.; Hamasaki, T.; Freedman, B. Prevalence of Physical Signs in Cervical Myelopathy: A Prospective, Controlled Study. Spine 2009, 34, 890–895. [Google Scholar] [CrossRef]
- Badhiwala, J.H.; Ahuja, C.S.; Akbar, M.A.; Witiw, C.D.; Nassiri, F.; Furlan, J.C.; Curt, A.; Wilson, J.R.; Fehlings, M.G. Degenerative Cervical Myelopathy—Update and Future Directions. Nat. Rev. Neurol. 2020, 16, 108–124. [Google Scholar] [CrossRef]
- Keřkovský, M.; Bednařík, J.; Jurová, B.; Dušek, L.; Kadaňka, Z.; Kadaňka, Z.; Němec, M.; Kovaľová, I.; Šprláková-Puková, A.; Mechl, M. Spinal Cord MR Diffusion Properties in Patients with Degenerative Cervical Cord Compression. J. Neuroimaging 2017, 27, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Nouri, A.; Tetreault, L.; Singh, A.; Karadimas, S.K.; Fehlings, M.G. Degenerative Cervical Myelopathy: Epidemiology, Genetics, and Pathogenesis. Spine 2015, 40, E675–E693. [Google Scholar] [CrossRef] [PubMed]
- Kalsi-Ryan, S.; Karadimas, S.K.; Fehlings, M.G. Cervical Spondylotic Myelopathy: The Clinical Phenomenon and the Current Pathobiology of an Increasingly Prevalent and Devastating Disorder. Neuroscientist 2013, 19, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Tetreault, L.; Goldstein, C.L.; Arnold, P.; Harrop, J.; Hilibrand, A.; Nouri, A.; Fehlings, M.G. Degenerative Cervical Myelopathy: A Spectrum of Related Disorders Affecting the Aging Spine. Neurosurgery 2015, 77 (Suppl. 4), S51–S67. [Google Scholar] [CrossRef]
- Harrop, J.S.; Naroji, S.; Maltenfort, M.; Anderson, D.G.; Albert, T.; Ratliff, J.K.; Ponnappan, R.K.; Rihn, J.A.; Smith, H.E.; Hilibrand, A.; et al. Cervical Myelopathy: A Clinical and Radiographic Evaluation and Correlation to Cervical Spondylotic Myelopathy. Spine 2010, 35, 620–624. [Google Scholar] [CrossRef]
- Tracy, J.A.; Bartleson, J.D. Cervical Spondylotic Myelopathy. Neurologist 2010, 16, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.M.; Mowforth, O.D.; Smith, E.K.; Kotter, M.R. Degenerative Cervical Myelopathy. BMJ 2018, 360. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.S.; Stewart, M.E.; Davies, B.M.; Kotter, M.R.N. The Prevalence of Asymptomatic and Symptomatic Spinal Cord Compression on Magnetic Resonance Imaging: A Systematic Review and Meta-Analysis. Glob. Spine J. 2020, 2192568220934496. [Google Scholar] [CrossRef]
- Kovalova, I.; Kerkovsky, M.; Kadanka, Z.; Kadanka, Z.; Nemec, M.; Jurova, B.; Dusek, L.; Jarkovsky, J.; Bednarik, J. Prevalence and Imaging Characteristics of Nonmyelopathic and Myelopathic Spondylotic Cervical Cord Compression. Spine 2016, 41, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- Bednarik, J.; Kadanka, Z.; Dusek, L.; Kerkovsky, M.; Vohanka, S.; Novotny, O.; Urbanek, I.; Kratochvilova, D. Presymptomatic Spondylotic Cervical Myelopathy: An Updated Predictive Model. Eur. Spine J. 2008, 17, 421–431. [Google Scholar] [CrossRef]
- Labounek, R.; Valošek, J.; Horák, T.; Svátková, A.; Bednařík, P.; Vojtíšek, L.; Horáková, M.; Nestrašil, I.; Lenglet, C.; Cohen-Adad, J.; et al. HARDI-ZOOMit Protocol Improves Specificity to Microstructural Changes in Presymptomatic Myelopathy. Sci. Rep. 2020, 10, 17529. [Google Scholar] [CrossRef]
- Kalsi-Ryan, S.; Rienmueller, A.C.; Riehm, L.; Chan, C.; Jin, D.; Martin, A.R.; Badhiwala, J.H.; Akbar, M.A.; Massicotte, E.M.; Fehlings, M.G. Quantitative Assessment of Gait Characteristics in Degenerative Cervical Myelopathy: A Prospective Clinical Study. J. Clin. Med. 2020, 9, 752. [Google Scholar] [CrossRef] [PubMed]
- Kuhtz-Buschbeck, J.P.; Jöhnk, K.; Mäder, S.; Stolze, H.; Mehdorn, M. Analysis of Gait in Cervical Myelopathy. Gait Posture 1999, 9, 184–189. [Google Scholar] [CrossRef]
- Singh, A.; Choi, D.; Crockard, A. Use of Walking Data in Assessing Operative Results for Cervical Spondylotic Myelopathy: Long-Term Follow-up and Comparison with Controls. Spine 2009, 34, 1296–1300. [Google Scholar] [CrossRef]
- Zheng, C.-F.; Liu, Y.-C.; Hu, Y.-C.; Xia, Q.; Miao, J.; Zhang, J.-D.; Zhang, K. Correlations of Japanese Orthopaedic Association Scoring Systems with Gait Parameters in Patients with Degenerative Spinal Diseases. Orthop. Surg. 2016, 8, 447–453. [Google Scholar] [CrossRef]
- Gorter, K. Influence of Laminectomy on the Course of Cervical Myelopathy. Acta Neurochir. 1976, 33, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Lunsford, L.D.; Bissonette, D.J.; Zorub, D.S. Anterior Surgery for Cervical Disc Disease. Part 2: Treatment of Cervical Spondylotic Myelopathy in 32 Cases. J. Neurosurg. 1980, 53, 12–19. [Google Scholar] [CrossRef]
- Kim, C.R.; Yoo, J.Y.; Lee, S.H.; Lee, D.H.; Rhim, S.C. Gait Analysis for Evaluating the Relationship between Increased Signal Intensity on T2-Weighted Magnetic Resonance Imaging and Gait Function in Cervical Spondylotic Myelopathy. Arch. Phys. Med. Rehabil. 2010, 91, 1587–1592. [Google Scholar] [CrossRef]
- Martin, A.R.; De Leener, B.; Cohen-Adad, J.; Cadotte, D.W.; Nouri, A.; Wilson, J.R.; Tetreault, L.; Crawley, A.P.; Mikulis, D.J.; Ginsberg, H.; et al. Can Microstructural MRI Detect Subclinical Tissue Injury in Subjects with Asymptomatic Cervical Spinal Cord Compression? A Prospective Cohort Study. BMJ Open 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Kadanka, Z.; Adamova, B.; Kerkovsky, M.; Kadanka, Z.; Dusek, L.; Jurova, B.; Vlckova, E.; Bednarik, J. Predictors of Symptomatic Myelopathy in Degenerative Cervical Spinal Cord Compression. Brain Behav. 2017, 7, e00797. [Google Scholar] [CrossRef] [PubMed]
- Benzel, E.C.; Lancon, J.; Kesterson, L.; Hadden, T. Cervical Laminectomy and Dentate Ligament Section for Cervical Spondylotic Myelopathy. J. Spinal Disord. 1991, 4, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Ruina, A. Computer Optimization of a Minimal Biped Model Discovers Walking and Running. Nature 2006, 439, 72–75. [Google Scholar] [CrossRef]
- Moorthy, R.K.; Bhattacharji, S.; Thayumanasamy, G.; Rajshekhar, V. Quantitative Changes in Gait Parameters after Central Corpectomy for Cervical Spondylotic Myelopathy. J. Neurosurg. Spine 2005, 2, 418–424. [Google Scholar] [CrossRef]
- Malone, A.; Meldrum, D.; Bolger, C. Three-Dimensional Gait Analysis Outcomes at 1 Year Following Decompressive Surgery for Cervical Spondylotic Myelopathy. Eur. Spine J. 2015, 24, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Morris, M.E.; Schache, A.; McCrory, P. Observational Gait Analysis in Traumatic Brain Injury: Accuracy of Clinical Judgment. Gait Posture 2009, 29, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Crockard, H.A. Quantitative Assessment of Cervical Spondylotic Myelopathy by a Simple Walking Test. Lancet 1999, 354, 370–373. [Google Scholar] [CrossRef]
- Yavuzer, G.; Oken, O.; Elhan, A.; Stam, H.J. Repeatability of Lower Limb Three-Dimensional Kinematics in Patients with Stroke. Gait Posture 2008, 27, 31–35. [Google Scholar] [CrossRef]
- Nishimura, H.; Endo, K.; Suzuki, H.; Tanaka, H.; Shishido, T.; Yamamoto, K. Gait Analysis in Cervical Spondylotic Myelopathy. Asian Spine J. 2015, 9, 321–326. [Google Scholar] [CrossRef]
- Cappellini, G.; Ivanenko, Y.P.; Poppele, R.E.; Lacquaniti, F. Motor Patterns in Human Walking and Running. J. Neurophysiol. 2006, 95, 3426–3437. [Google Scholar] [CrossRef] [PubMed]
- Novacheck, T.F. The Biomechanics of Running. Gait Posture 1998, 7, 77–95. [Google Scholar] [CrossRef]
- Bednařík, J.; Sládková, D.; Kadaňka, Z.; Dušek, L.; Keřkovský, M.; Voháňka, S.; Novotný, O.; Urbánek, I.; Němec, M. Are Subjects with Spondylotic Cervical Cord Encroachment at Increased Risk of Cervical Spinal Cord Injury after Minor Trauma? J. Neurol. Neurosurg. Psychiatry 2011, 82, 779–781. [Google Scholar] [CrossRef]
- Wilson, J.R.; Barry, S.; Fischer, D.J.; Skelly, A.C.; Arnold, P.M.; Riew, K.D.; Shaffrey, C.I.; Traynelis, V.C.; Fehlings, M.G. Frequency, Timing, and Predictors of Neurological Dysfunction in the Nonmyelopathic Patient with Cervical Spinal Cord Compression, Canal Stenosis, and/or Ossification of the Posterior Longitudinal Ligament. Spine 2013, 38 (Suppl. 1), S37–S54. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Tetreault, L.A.; Riew, K.D.; Middleton, J.W.; Aarabi, B.; Arnold, P.M.; Brodke, D.S.; Burns, A.S.; Carette, S.; Chen, R.; et al. A Clinical Practice Guideline for the Management of Patients with Degenerative Cervical Myelopathy: Recommendations for Patients With Mild, Moderate, and Severe Disease and Nonmyelopathic Patients With Evidence of Cord Compression. Global Spine J. 2017, 7 (Suppl. 3), 70S–83S. [Google Scholar] [CrossRef] [PubMed]
- Adamova, B.; Bednarik, J.; Andrasinova, T.; Kovalova, I.; Kopacik, R.; Jabornik, M.; Kerkovsky, M.; Jakubcova, B.; Jarkovsky, J. Does Lumbar Spinal Stenosis Increase the Risk of Spondylotic Cervical Spinal Cord Compression? Eur. Spine J. 2015, 24, 2946–2953. [Google Scholar] [CrossRef] [PubMed]
| HC (N = 100) | Correlation with Age: Spearman’s Rank Correlation Coefficient: r (p) | Comparison between Sexes: Chi-Square Test: p | |
|---|---|---|---|
| Age | Sex | ||
| Time/Velocity (cm/s) | Walk | 0.610/−0.610 (<0.001) | 0.006 ‡ |
| Run | 0.657/−0.657 (<0.001) | 0.001 ‡ | |
| Number of steps | Walk | 0.497 (<0.001) | <0.001 ‡ |
| Run | 0.353 (<0.001) | <0.001 ‡ | |
| Cadence (steps/min) | Walk | −0.268 (0.007) | 0.659 † |
| Run | −0.564 (<0.001) | 0.707 † | |
| Healthy Controls (N = 100): Subgroups | Parameters: 10 m Walk | |||
| Time (s)/ Velocity (cm/s) | Number of Steps/ Cadence (Steps/min) | |||
| X ± SD | Normal limits Time: X+2SD/95.perc. Velocity: X-2SD/5.perc. | X ± SD | Normal Limits N.steps: X+2SD/95.perc. Cadence: X-2SD/5.perc. | |
| Men ≤ 60 years N = 27 | 4.2 ± 0.5/ 238.3 ± 30.9 | 5.2/5.3 176.5/186.9 | 10.7 ± 1.2 153.6 ± 22.8 | 13.1/13.0 108.0/125.2 |
| Men > 60 years N = 21 | 5.0 ± 0.8/ 198.8 ± 37.3 | 6.6/6.4 124.2/145.0 | 12.8 ± 2.1 158.3 ± 32.3 | 17.0/16.0 93.7/103.9 |
| Women ≤ 60 years N = 31 | 4.5 ± 0.6/ 221.3 ± 28.6 | 5.7/5.6 164.1/178.5 | 12.2 ± 1.3 162.3 ± 25.1 | 14.8/14.5 112.1/129.4 |
| Women > 60 years N = 21 | 6.1 ± 1.0/ 165.8 ± 30.2 | 8.1/8.1 105.4/110.0 | 14.6 ± 2.4 143.8 ± 24.4 | 19.4/18.0 95.0/101.5 |
| Healthy Controls (N = 100): Subgroups | Parameters: 10 m Run | |||
| Time (s)/ Velocity (cm/s) | Number of Steps/ Cadence (Steps/min) | |||
| X ± SD | Normal Limits Time: X+2SD/95.perc. Velocity: X-2SD/5.perc. | X ± SD | Normal Limits N.steps: X+2SD/95.perc. Cadence: X-2SD/5.perc. | |
| Men ≤ 60 years N = 27 | 2.6 ± 0.3/ 383.7 ± 58.7 | 3.2/3.3 266.3/304.0 | 8.7 ± 1.3 199.1 ± 27.6 | 11.3/11.0 143.9/151.3 |
| Men > 60 years N = 21 | 3.4 ± 0.7/ 296.8 ± 55.8 | 4.8/4.2 185.2/237.0 | 9.4 ± 1.0 167.9 ± 29.5 | 11.4/11.2 108.9/116.9 |
| Women ≤ 60 years N = 31 | 3.0 ± 0.4/ 336.2 ± 40.2 | 3.8/3.6 255.8/279.0 | 9.7 ± 1.2 193.6 ± 21.2 | 12.1/12.0 151.2/158.4 |
| Women > 60 years N = 21 | 4.2 ± 1.0/ 238.2 ± 51.0 | 6.2/6.3 136.2/158.0 | 10.6 ± 1.0 155.9 ± 31.2 | 12.6/12.0 93.5/96.8 |
| 10 m Walk—Number (Proportion) of Abnormal Values & | |||
| Group | NMDCC (N = 126) | DCM (N = 45) | Comparison of the groups: chi-square test (p) |
| Parameter | |||
| Time | 57 (45.2%)/60 (47.6%) | 31 (68.9%)/32 (71.1%) | 0.006/0.007 |
| Velocity | 57 (45.2%)/60 (47.6%) | 31 (68.9%)/32 (71.1%) | 0.006/0.007 |
| Number of steps | 21 (16.7%)/22 (17.5%) | 14 (31.1%)/23 (51.1%) | 0.04/<0.001 |
| Cadence | 6 (4.8%)/33 (26.2%) | 5 (11.1%)/20 (44.4%) | 0.136/0.02 |
| Any abnormality (walk) | 59 (46.8%)/66 (52.4%) | 32 (71.1%)/34 (75.5%) | 0.005/0.007 |
| 10 m Run—Number (Proportion) of Abnormal Values & | |||
| Group | NMDCC (N = 126) | DCM (N = 34) # | Comparison of the groups: chi-square test (p) |
| Parameter | |||
| Time | 53 (42.1%)/59 (46.8%) | 23 (67.6%)/24 (70.6%) | 0.008/0.014 |
| Velocity | 53 (42.1%)/59 (46.8%) | 23 (67.6%)/24 (70.6%) | 0.008/0.014 |
| Number of steps | 41 (32.5%)/41 (32.5%) | 22 (64.7%)/22 (64.7%) | <0.001/<0.001 |
| Cadence | 24 (19.0%)/42 (33.3%) | 8 (23.5%)/15 (44.1%) | 0.562/0.244 |
| Any abnormality (run) | 72 (57.1%)/82 (65.1%) | 27 (79.4%)/28 (82.4%) | 0.018/0.054 |
| Any abnormality (walk and/or run) | 84 (66.7%)/91 (72.2%) | 38 (84.4%)/40 (88.9%) | 0.024/0.023 |
| Parameters | Groups | HC | NMDCC | DCM | Kruskal–Wallis p Value * |
|---|---|---|---|---|---|
| X (SD); Median (Min.–Max.) | |||||
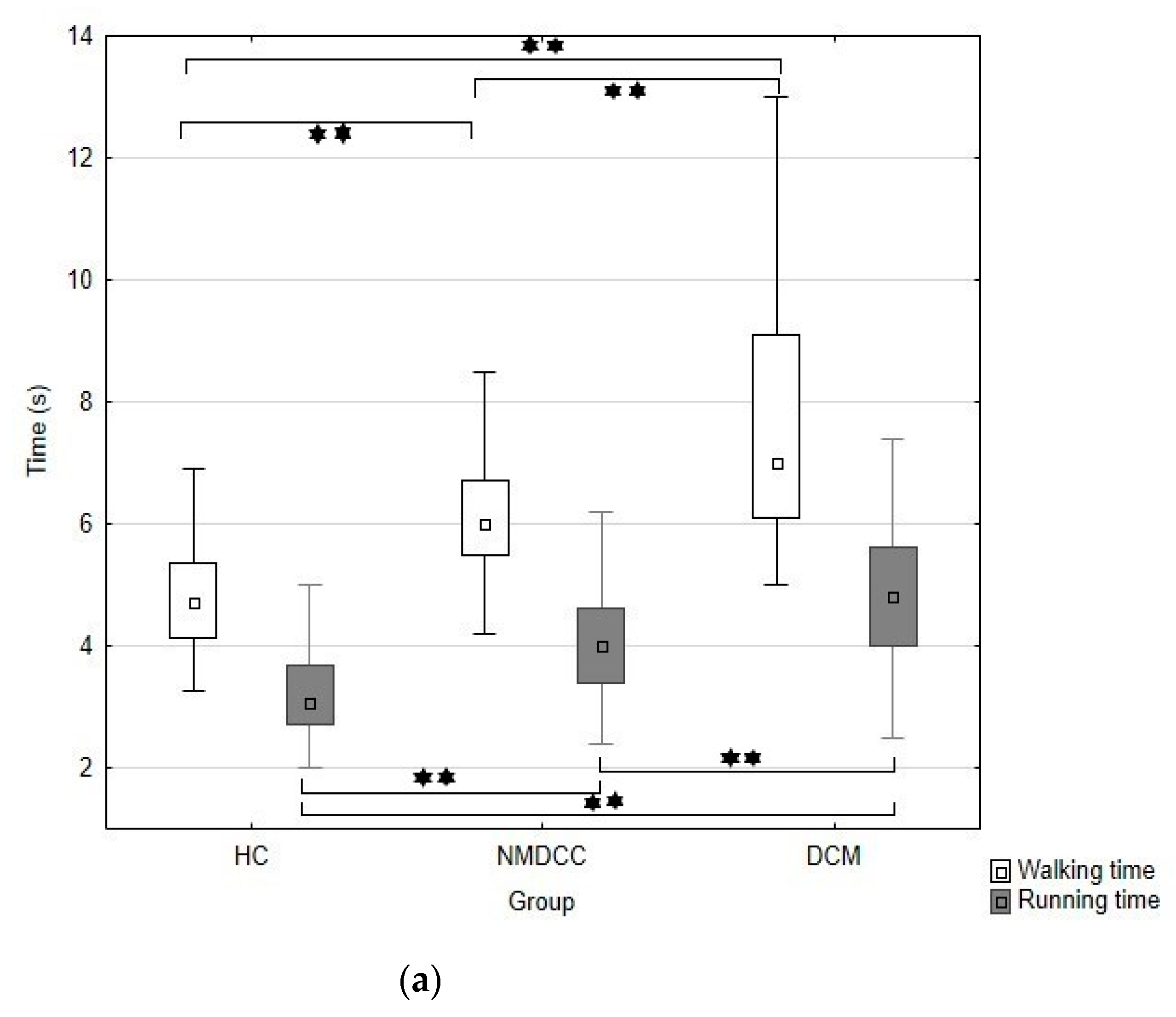
| Walk time (s) | 4.9 (1.3); 4.7 (3.3–13.6) a | 6.2 (1.1); 6.0 (4.2–9.9) b | 7.2 (2.5); 7.0 (5.0–18.0) c | <0.001 | |
| Walk velocity (cm/s) | 209.0 (42.5); 212.5 (73–306) a | 165.9 (27.2); 167 (101–238) b | 139.6 (34.2); 150 (56–200) c | <0.001 | |
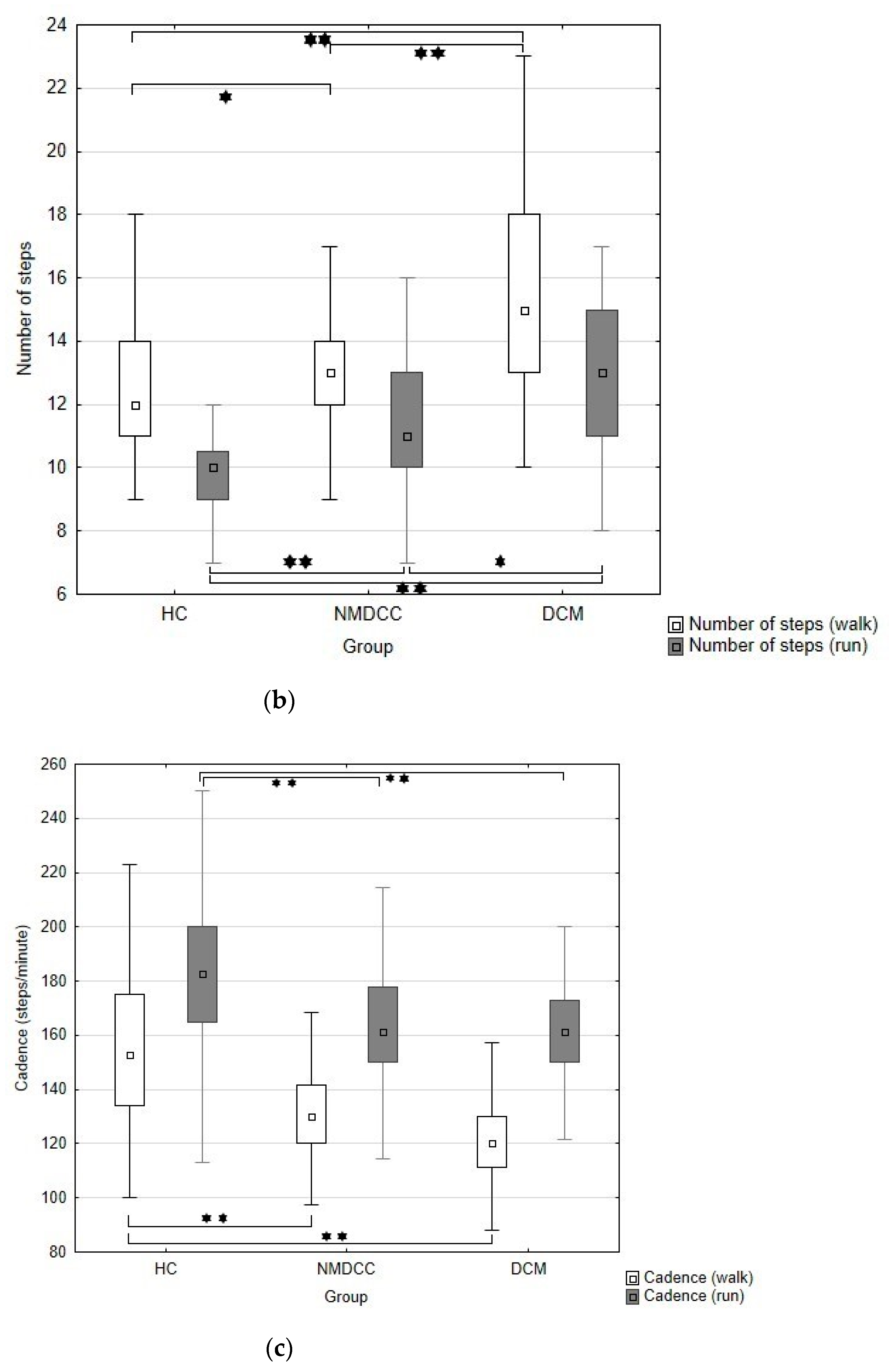
| Walk steps (No.) | 12.4 (2.2); 12 (9–23) a | 13.2 (1.9); 13 (8–18) b | 14.8 (2.9); 15 (10–23) c | <0.001 | |
| Walk cadence (steps/min.) | 155.2 (29.2); 152.9 (100.0–263.4) a | 130.7 (20.6); 130 (53.3–228.6) b | 120.7 (18.0); 120 (63.3–159.4) b | <0.001 | |
| Run time | 3.3 (0.9); 3.1 (2–8) a | 4.1 (0.9); 4.0 (2.4–6.7) b | 4.6 (1.4); 4.8 (2.5–9.4) c | <0.001 | |
| Run velocity (cm/s) | 320.1 (74.1); 323.5 (125–497) a | 255.9 (56.5); 250 (149–416) b | 219.0 (61.7); 221 (150–400) c | <0.001 | |
| Run steps (No.) | 9.6 (1.4); 10 (6–12) a | 11.3 (2.4); 11 (7–18) b | 12.8 (3.0); 13 (8–22) c | <0.001 | |
| Run cadence (steps/min.) | 181.8 (33.3); 182.6 (75.0–264.0) a | 167.1 (25.9); 169.4 (114.3–266.7) b | 160.2 (20.0); 161.2 (108.5–200.0) b | <0.001 | |
| DCM Patients (N = 45) | Spearman’s Rank Correlation Coefficient r (p) | ||
|---|---|---|---|
| mJOA: | mJOA LE | ||
| Time (s) | Walk | −0.766 (<<0.001) | −0.790 (<<0.001) |
| Run | −0.505 (0.002) | −0.568 (<0.001) | |
| Velocity (cm/s) | Walk | 0.766 (<<0.001) | 0.790 (<<0.001) |
| Run | 0.505 (0.002) | 0.568 (<0.001) | |
| Number of steps | Walk | −0.589 (<0.001) | −0.649 (<<0.001) |
| Run | −0.485 (0.004) | −0.471 (0.005) | |
| Cadence (steps/min) | Walk | 0.514 (<0.001) | 0.483 (<0.001) |
| Run | 0.173 (0.329) | 0.239 (0.173) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadanka, Z., Jr.; Kadanka, Z., Sr.; Skutil, T.; Vlckova, E.; Bednarik, J. Walk and Run Test in Patients with Degenerative Compression of the Cervical Spinal Cord. J. Clin. Med. 2021, 10, 927. https://doi.org/10.3390/jcm10050927
Kadanka Z Jr., Kadanka Z Sr., Skutil T, Vlckova E, Bednarik J. Walk and Run Test in Patients with Degenerative Compression of the Cervical Spinal Cord. Journal of Clinical Medicine. 2021; 10(5):927. https://doi.org/10.3390/jcm10050927
Chicago/Turabian StyleKadanka, Zdenek, Jr., Zdenek Kadanka, Sr., Tomas Skutil, Eva Vlckova, and Josef Bednarik. 2021. "Walk and Run Test in Patients with Degenerative Compression of the Cervical Spinal Cord" Journal of Clinical Medicine 10, no. 5: 927. https://doi.org/10.3390/jcm10050927
APA StyleKadanka, Z., Jr., Kadanka, Z., Sr., Skutil, T., Vlckova, E., & Bednarik, J. (2021). Walk and Run Test in Patients with Degenerative Compression of the Cervical Spinal Cord. Journal of Clinical Medicine, 10(5), 927. https://doi.org/10.3390/jcm10050927






