Lung Transplantation for Pleuroparenchymal Fibroelastosis
Abstract
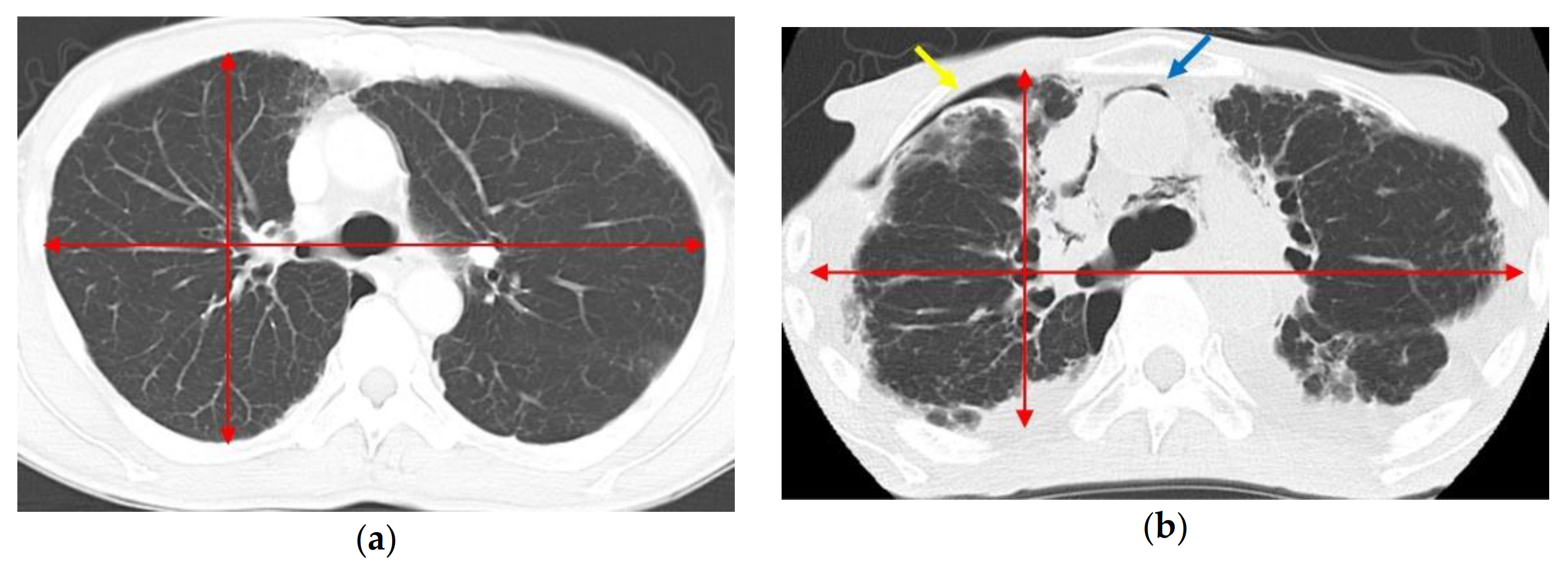
1. Introduction
2. Prevalence and Incidence of PPFE
3. Clinical Characteristics of PPFE
3.1. BMI
3.2. Flat Chest
3.3. Pneumothorax and ALS
4. Optimal Timing of LT
5. Post-LT Course
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amitani, R.; Niimi, A.; Kuse, F. Idiopathic pulmonary upper lobe fibrosis. Kokyu 1992, 11, 693–699. [Google Scholar]
- Frankel, S.K.; Cool, C.D.; Lynch, D.A.; Brown, K.K. Idiopathic pleuroparenchymal fibroelastosis: Description of a novel clinicopathologic entity. Chest 2004, 126, 2007–2013. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef]
- Bonifazi, M.; Montero, M.A.; Renzoni, E.A. Idiopathic Pleuroparenchymal Fibroelastosis. Curr. Pulmonol. Rep. 2017, 6, 9–15. [Google Scholar] [CrossRef]
- Harada, T.; Yoshida, Y.; Kitasato, Y.; Tsuruta, N.; Wakamatsu, K.; Hirota, T.; Tanaka, M.; Tashiro, N.; Ishii, H.; Shiraishi, M.; et al. The thoracic cage becomes flattened in the progression of pleuroparenchymal fibroelastosis. Eur. Respir. Rev. 2014, 23, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Vogel, M.; Brodoefel, H.; Bethge, W.; Faul, C.; Hartmann, J.; Schimmel, H.; Wehrmann, M.; Claussen, C.D.; Horger, M. Spontaneous thoracic air-leakage syndrome in patients following allogeneic hematopoietic stem cell transplantation: Causes, CT-follow up and patient outcome. Eur. J. Radiol. 2006, 60, 392–397. [Google Scholar] [CrossRef]
- Franquet, T.; Rodríguez, S.; Hernández, J.M.; Martino, R.; Giménez, A.; Hidalgo, A.; Domingo, P. Air-leak syndromes in hematopoietic stem cell transplant recipients with chronic GVHD: High-resolution CT findings. J. Thorac. Imaging 2007, 22, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Sakai, R.; Kanamori, H.; Nakaseko, C.; Yoshiba, F.; Fujimaki, K.; Sakura, T.; Fujisawa, S.; Kawai, N.; Onoda, M.; Matsushima, T.; et al. Air-leak syndrome following allo-SCT in adult patients: Report from the Kanto Study Group for Cell Therapy in Japan. Bone Marrow Transpl. 2011, 46, 379–384. [Google Scholar] [CrossRef]
- Ishii, T.; Bando, S.; Kanaji, N.; Tadokoro, A.; Watanabe, N.; Imataki, O.; Dobashi, H.; Kushida, Y.; Haba, R.; Yokomise, H. Air-leak syndrome by pleuroparenchymal fibroelastosis after bone marrow transplantation. Intern. Med. 2016, 55, 105–111. [Google Scholar] [CrossRef][Green Version]
- Oda, T.; Ogura, T.; Kitamura, H.; Hagiwara, E.; Baba, T.; Enomoto, Y.; Iwasawa, T.; Okudela, K.; Takemura, T.; Sakai, F.; et al. Distinct characteristics of pleuroparenchymal fibroelastosis with usual interstitial pneumonia compared with idiopathic pulmonary fibrosis. Chest 2014, 146, 1248–1255. [Google Scholar] [CrossRef]
- Nakatani, T.; Arai, T.; Kitaichi, M.; Akira, M.; Tachibana, K.; Sugimoto, C.; Hirooka, A.; Tsuji, T.; Minomo, S.; Hayashi, S.; et al. Pleuroparenchymal fibroelastosis from a consecutive database: A rare disease entity? Eur. Respir. J. 2015, 45, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Shioya, M.; Otsuka, M.; Yamada, G.; Umeda, Y.; Ikeda, K.; Nishikiori, H.; Kuronuma, K.; Chiba, H.; Takahashi, H. Poorer Prognosis of Idiopathic Pleuroparenchymal Fibroelastosis Compared with Idiopathic Pulmonary Fibrosis in Advanced Stage. Can. Respir. J. 2018, 2018, 6043053. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Chae, E.J.; Song, J.S.; Lee, J.H.; Song, J.W. Pleuroparenchymal fibroelastosis in patients with idiopathic pulmonary fibrosis. Respirology 2020, 25, 1046–1052. [Google Scholar] [CrossRef]
- Tanizawa, K.; Handa, T.; Kubo, T.; Chen-Yoshikawa, T.F.; Aoyama, A.; Motoyama, H.; Hijiya, K.; Yoshizawa, A.; Oshima, Y.; Ikezoe, K.; et al. Clinical significance of radiological pleuroparenchymal fibroelastosis pattern in interstitial lung disease patients registered for lung transplantation: A retrospective cohort study. Respir. Res. 2018, 19, 162. [Google Scholar] [CrossRef] [PubMed]
- Shiiya, H.; Tian, D.; Sato, M.; Karasaki, T.; Kitano, K.; Nagayama, K.; Anraku, M.; Kaga, K.; Matsui, Y.; Nakajima, J. Differences Between Patients with Idiopathic Pleuroparenchymal Fibroelastosis and Those with Other Types of Idiopathic Interstitial Pneumonia in Candidates for Lung Transplants. Transplant. Proc. 2019, 51, 2014–2021. [Google Scholar] [CrossRef] [PubMed]
- Mariani, F.; Gatti, B.; Rocca, A.; Bonifazi, F.; Cavazza, A.; Fanti, S.; Tomassetti, S.; Piciucchi, S.; Poletti, V.; Zompatori, M. Pleuroparenchymal fibroelastosis: The prevalence of secondary forms in hematopoietic stem cell and lung transplantation recipients. Diagn. Interv. Radiol. 2016, 22, 400–406. [Google Scholar] [CrossRef]
- Enomoto, Y.; Nakamura, Y.; Colby, T.V.; Johkoh, T.; Sumikawa, H.; Nishimoto, K.; Yoshimura, K.; Matsushima, S.; Oyama, Y.; Hozumi, H.; et al. Radiologic pleuroparenchymal fibroelastosis-like lesion in connective tissue disease-related interstitial lung disease. PLoS ONE 2017, 12, e0180283. [Google Scholar] [CrossRef]
- Bonifazi, M.; Sverzellati, N.; Negri, E.; Jacob, J.; Egashira, R.; Moser, J.; Piciucchi, S.; Mei, F.; De Lauretis, A.; Visca, D.; et al. Pleuroparenchymal fibroelastosis in systemic sclerosis: Prevalence and prognostic impact. Eur. Respir. J. 2020, 56, 1902135. [Google Scholar] [CrossRef]
- Ofek, E.; Sato, M.; Saito, T.; Wagnetz, U.; Roberts, H.C.; Chaparro, C.; Waddell, T.K.; Singer, L.G.; Hutcheon, M.A.; Keshavjee, S.; et al. Restrictive allograft syndrome post lung transplantation is characterized by pleuroparenchymal fibroelastosis. Mod. Pathol. 2013, 26, 350–356. [Google Scholar] [CrossRef]
- Royer, P.J.; Olivera-Botello, G.; Koutsokera, A.; Aubert, J.D.; Bernasconi, E.; Tissot, A.; Pison, C.; Nicod, L.; Boissel, J.P.; Magnan, A. Chronic lung allograft dysfunction: A systematic review of mechanisms. Transplantation 2016, 100, 1803–1814. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Waddell, T.K.; Wagnetz, U.; Roberts, H.C.; Hwang, D.M.; Haroon, A.; Wagnetz, D.; Chaparro, C.; Singer, L.G.; Hutcheon, M.A.; et al. Restrictive allograft syndrome (RAS): A novel form of chronic lung allograft dysfunction. J. Heart Lung Transplant. 2011, 30, 735–742. [Google Scholar] [CrossRef]
- Watanabe, K. Pleuroparenchymal Fibroelastosis: Its Clinical Characteristics. Curr. Respir. Med. Rev. 2013, 9, 229–237. [Google Scholar] [CrossRef]
- Chua, F.; Desai, S.R.; Nicholson, A.G.; Devaraj, A.; Renzoni, E.; Rice, A.; Wells, A.U. Pleuroparenchymal fibroelastosis a review of clinical, radiological, and pathological characteristics. Ann. Am. Thorac. Soc. 2019, 16, 1351–1359. [Google Scholar] [CrossRef]
- Upala, S.; Panichsillapakit, T.; Wijarnpreecha, K.; Jaruvongvanich, V.; Sanguankeo, A. Underweight and obesity increase the risk of mortality after lung transplantation: A systematic review and meta-analysis. Transpl. Int. 2016, 29, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Singer, J.P.; Peterson, E.R.; Snyder, M.E.; Katz, P.P.; Golden, J.A.; D’Ovidio, F.; Bacchetta, M.; Sonett, J.R.; Kukreja, J.; Shah, L.; et al. Body composition and mortality after adult lung transplantation in the United States. Am. J. Respir. Crit. Care Med. 2014, 190, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.; Safaeinili, N.; Kurihara, C.; Odell, D.D.; Jain, M.; DeCamp, M.M.; Budinger, G.R.S.; Bharat, A. Association of body mass index with lung transplantation survival in the United States following implementation of the lung allocation score. J. Thorac. Cardiovasc. Surg. 2018, 155, 1871–1879.e3. [Google Scholar] [CrossRef]
- Komatsu, T.; Chen-Yoshikawa, T.F.; Oshima, A.; Harashima, S.-I.; Aoyama, A.; Inagaki, N.; Date, H. Severe underweight decreases the survival rate in adult lung transplantation. Surg. Today 2017, 47, 1243–1248. [Google Scholar] [CrossRef]
- Enomoto, Y.; Nakamura, Y.; Satake, Y.; Sumikawa, H.; Johkoh, T.; Colby, T.V.; Yasui, H.; Hozumi, H.; Karayama, M.; Suzuki, Y.; et al. Clinical diagnosis of idiopathic pleuroparenchymal fibroelastosis: A retrospective multicenter study. Respir. Med. 2017, 133, 1–5. [Google Scholar] [CrossRef]
- Shiiya, H.; Nakajima, J.; Date, H.; Chen-Yoshikawa, T.F.; Tanizawa, K.; Handa, T.; Oto, T.; Otani, S.; Shiotani, T.; Okada, Y.; et al. Outcomes of lung transplantation for idiopathic pleuroparenchymal fibroelastosis. Surg. Today 2021. [Google Scholar] [CrossRef] [PubMed]
- Weill, D.; Benden, C.; Corris, P.A.; Dark, J.H.; Davis, R.D.; Keshavjee, S.; Lederer, D.J.; Mulligan, M.J.; Patterson, G.A.; Singer, L.G.; et al. A consensus document for the selection of lung transplant candidates: 2014—An update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2015, 34, 1–15. [Google Scholar] [CrossRef]
- Staufer, K.; Halilbasic, E.; Hillebrand, P.; Harm, S.; Schwarz, S.; Jaksch, P.; Kivaranovic, D.; Klepetko, W.; Trauner, M.; Kazemi-Shirazi, L. Impact of nutritional status on pulmonary function after lung transplantation for cystic fibrosis. United Eur. Gastroenterol. J. 2018, 6, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Hollander, F.M.; van Pierre, D.D.; de Roos, N.M.; van de Graaf, E.A.; Iestra, J.A. Effects of nutritional status and dietetic interventions on survival in Cystic Fibrosis patients before and after lung transplantation. J. Cyst. Fibros. 2014, 13, 212–218. [Google Scholar] [CrossRef]
- Kashani, K.; Sarvottam, K.; Pereira, N.L.; Barreto, E.F.; Kennedy, C.C. The sarcopenia index: A novel measure of muscle mass in lung transplant candidates. Clin. Transplant. 2018, 32, e13182. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yoshimura, K.; Enomoto, Y.; Yasui, H.; Hozumi, H.; Karayama, M.; Furuhashi, K.; Enomoto, N.; Fujisawa, T.; Nakamura, Y.; et al. Distinct profile and prognostic impact of body composition changes in idiopathic pulmonary fibrosis and idiopathic pleuroparenchymal fibroelastosis. Sci. Rep. 2018, 8, 14074. [Google Scholar] [CrossRef] [PubMed]
- Chohan, K.; Park, J.; Dales, S.; Varughese, R.; Wickerson, L.; Singer, L.G.; Stewart, B.; Rozenberg, D. Evaluation of Malnutrition Risk in Lung Transplant Candidates Using the Nutritional Risk Index. Transplant. Direct 2020, 6, e574. [Google Scholar] [CrossRef]
- Miyoshi, R.; Chen-Yoshikawa, T.F.; Takahagi, A.; Oshima, Y.; Hijiya, K.; Motoyama, H.; Aoyama, A.; Date, H. Pulmonary Function and Exercise Capacity in Patients with Flat Chests After Lung Transplantation. Ann. Thorac. Surg. 2017, 104, 1695–1701. [Google Scholar] [CrossRef][Green Version]
- Miyahara, S.; Chen-Yoshikawa, T.F.; Motoyama, H.; Nakajima, D.; Hamaji, M.; Aoyama, A.; Date, H. Impact of flat chest on cadaveric lung transplantation: Postoperative pulmonary function and survival. Eur. J. Cardio Thorac. Surg. 2019, 55, 316–322. [Google Scholar] [CrossRef]
- Yanagiya, M.; Sato, M.; Kawashima, S.; Kuwano, H.; Nagayama, K.; Nitadori, J.-I.; Anraku, M.; Nakajima, J. Flat Chest of Pleuroparenchymal Fibroelastosis Reversed by Lung Transplantation. Ann. Thorac. Surg. 2016, 102, e347–e349. [Google Scholar] [CrossRef]
- Von Der Thüsen, J.H.; Hansell, D.M.; Tominaga, M.; Veys, P.A.; Ashworth, M.T.; Owens, C.M.; Nicholson, A.G. Pleuroparenchymal fibroelastosis in patients with pulmonary disease secondary to bone marrow transplantation. Mod. Pathol. 2011, 24, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Maeda, T.; Kida, T.; Fujita, J.; Tsuji, H.; Morii, E.; Kanakura, Y. Pleuroparenchymal fibroelastosis after allogenic hematopoietic stem cell transplantation: Important histological component of late-onset noninfectious pulmonary complication accompanied with recurrent pneumothorax. Int. J. Hematol. 2016, 104, 525–530. [Google Scholar] [CrossRef]
- Liu, Y.C.; Chou, Y.H.; Ko, P.S.; Wang, H.Y.; Fan, N.W.; Liu, C.J.; Hsiao, L.T.; Chien, S.H.; Chiou, T.J.; Liu, J.H.; et al. Risk factors and clinical features for post-transplant thoracic air-leak syndrome in adult patients receiving allogeneic haematopoietic stem cell transplantation. Sci. Rep. 2019, 9, 11795. [Google Scholar] [CrossRef]
- Kunou, H.; Kanzaki, R.; Kawamura, T.; Kanou, T.; Ose, N.; Funaki, S.; Shintani, Y.; Minami, M.; Okumura, M. Two cases of air leak syndrome after bone marrow transplantation successfully treated by the pleural covering technique. Gen. Thorac. Cardiovasc. Surg. 2019, 67, 987–990. [Google Scholar] [CrossRef]
- Yoshida, Y.; Nagata, N.; Tsuruta, N.; Kitasato, Y.; Wakamatsu, K.; Yoshimi, M.; Ishii, H.; Hirota, T.; Hamada, N.; Fujita, M.; et al. Heterogeneous clinical features in patients with pulmonary fibrosis showing histology of pleuroparenchymal fibroelastosis. Respir. Investig. 2016, 54, 162–169. [Google Scholar] [CrossRef]
- Suzuki, Y.; Fujisawa, T.; Sumikawa, H.; Tanaka, T.; Sugimoto, C.; Kono, M.; Hozumi, H.; Karayama, M.; Furuhashi, K.; Enomoto, N.; et al. Disease course and prognosis of pleuroparenchymal fibroelastosis compared with idiopathic pulmonary fibrosis. Respir. Med. 2020, 171, 106078. [Google Scholar] [CrossRef] [PubMed]
- Sumikawa, H.; Johkoh, T.; Egashira, R.; Sugiura, H.; Yamano, Y.; Kataoka, K.; Kondoh, Y.; Arakawa, H.; Nakamura, M.; Kuriu, A.; et al. Pleuroparenchymal fibroelastosis-like lesions in patients with interstitial pneumonia diagnosed by multidisciplinary discussion with surgical lung biopsy. Eur. J. Radiol. Open 2020, 7, 100298. [Google Scholar] [CrossRef]
- Kono, M.; Fujita, Y.; Takeda, K.; Miyashita, K.; Tsutsumi, A.; Kobayashi, T.; Miki, Y.; Hashimoto, D.; Enomoto, N.; Nakamura, Y.; et al. Clinical significance of lower-lobe interstitial lung disease on high-resolution computed tomography in patients with idiopathic pleuroparenchymal fibroelastosis. Respir. Med. 2019, 154, 122–126. [Google Scholar] [CrossRef]
- Kato, M.; Sasaki, S.; Kurokawa, K.; Nakamura, T.; Yamada, T.; Sasano, H.; Arano, N.; Komura, M.; Ihara, H.; Nagashima, O.; et al. Usual Interstitial Pneumonia Pattern in the Lower Lung Lobes as a Prognostic Factor in Idiopathic Pleuroparenchymal Fibroelastosis. Respiration 2019, 97, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Odink, A.; Brun, A.L.; Macaluso, C.; de Lauretis, A.; Kokosi, M.; Devaraj, A.; Desai, S.; Renzoni, E.; Wells, A.U. Functional associations of pleuroparenchymal fibroelastosis and emphysema with hypersensitivity pneumonitis. Respir. Med. 2018, 138, 95–101. [Google Scholar] [CrossRef]
- Chen, F.; Matsubara, K.; Miyagawa-Hayashino, A.; Tada, K.; Handa, T.; Yamada, T.; Sato, M.; Aoyama, A.; Date, H. Lung transplantation for pleuroparenchymal fibroelastosis after chemotherapy. Ann. Thorac. Surg. 2014, 98, e115–e117. [Google Scholar] [CrossRef]
- Hata, A.; Nakajima, T.; Yoshida, S.; Kinoshita, T.; Terada, J.; Tatsumi, K.; Matsumiya, G.; Date, H.; Yoshino, I. Living Donor Lung Transplantation for Pleuroparenchymal Fibroelastosis. Ann. Thorac. Surg. 2016, 101, 1970–1972. [Google Scholar] [CrossRef] [PubMed]
- Rasciti, E.; Cancellieri, A.; Romagnoli, M.; Dell’Amore, A.; Zompatori, M. Suspected pleuroparenchymal fibroelastosis relapse after lung transplantation: A case report and literature review. BJR Case Rep. 2019, 5, 20190040. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A.; Terada, J.; Tsushima, K.; Tateishi, Y.; Abe, R.; Oda, S.; Kobayashi, M.; Yamane, M.; Oto, T.; Tatsumi, K. Veno-venous extracorporeal membrane oxygenation bridged living-donor lung transplantation for rapid progressive respiratory failure with pleuroparenchymal fibroelastosis after allogeneic hematopoietic stem cell transplantation. Respir. Investig. 2018, 56, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Righi, I.; Morlacchi, L.; Rossetti, V.; Mendogni, P.; Palleschi, A.; Tosi, D.; Pieropan, S.; Gobbo, A.D.; Nosotti, M. Lung Transplantation as Successful Treatment of End-stage Idiopathic Pleuroparenchymal Fibroelastosis: A Case Report. Transplant. Proc. 2019, 51, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Shiiya, H.; Sato, M.; Shinozaki-Ushiku, A.; Konoeda, C.; Kitano, K.; Nakajima, J. Exacerbation of Secondary Pulmonary Hypertension by Flat Chest after Lung Transplantation. Ann. Thorac. Cardiovasc. Surg. 2020, 1–4. [Google Scholar] [CrossRef]
- Ali, M.S.; Ramalingam, V.S.; Haasler, G.; Presberg, K. Pleuroparenchymal fibroelastosis (PPFE) treated with lung transplantation and review of the literature. BMJ Case Rep. 2019, 12, e229402. [Google Scholar] [CrossRef]
- Aljefri, N.A.; Abothenain, F.F.; Hussein, A.M.; Saleh, W.; Alkattan, K.; Mohammed, S.F.; Alhajji, M. Idiopathic pleuroparenchymal fibroelastosis: The first case to be managed with a successful lung transplant at King Faisal Specialist Hospital and Research Center, Riyadh. Ann. Thorac. Med. 2019, 14, 94–98. [Google Scholar] [CrossRef]
- Sekine, A.; Seo, K.; Matsukura, S.; Sato, M.; Shinozaki-Ushiku, A.; Ogura, T.; Kitami, A.; Kadokura, M.; Dohi, S.; Ichizuka, K.; et al. A case report of idiopathic pleuroparenchymal fibroelastosis with severe respiratory failure in pregnancy. BMC Pulm. Med. 2020, 20, 264. [Google Scholar] [CrossRef]
- Portillo, K.; Guasch, I.; Becker, C.; Andreo, F.; Fernández-Figueras, M.T.; Ramirez Ruz, J.; Martinez-Barenys, C.; García-Reina, S.; Lopez de Castro, P.; Sansano, I.; et al. Pleuroparenchymal Fibroelastosis: A New Entity within the Spectrum of Rare Idiopathic Interstitial Pneumonias. Case Rep. Pulmonol. 2015, 2015, 810515. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Feng, R.; Li, S.; Wu, B.; Xu, K.; Xu, Z.; Chen, J. A CARE-compliant case report: Lung transplantation for a Chinese young man with idiopathic pleuroparenchymal fibroelastosis. Medicine 2017, 96, e6900. [Google Scholar] [CrossRef] [PubMed]
- Tsubosaka, A.; Matsushima, J.; Ota, M.; Suzuki, M.; Yonemori, Y.; Ota, S.; Yoshino, I.; Tsushima, K.; Tatsumi, K.; Nakatani, Y. Whole-lung pathology of pleuroparenchymal fibroelastosis (PPFE) in an explanted lung: Significance of elastic fiber-rich, non-specific interstitial pneumonia-like change in chemotherapy-related PPFE. Pathol. Int. 2019, 69, 547–555. [Google Scholar] [CrossRef]

| Author | Sex | Age | Underlying Condition | Procedure | Posttransplant Course | Outcome |
|---|---|---|---|---|---|---|
| Chen et al. (2014) | Male | 14 | Chemotherapy | Left single | uneventful | Alive |
| Portillo et al. (2015) | Male | 25 | Castleman’s disease | Bilateral | NA | Alive |
| Hata et al. (2016) | Male | 19 | Chemotherapy | LDLLT | uneventful | Alive |
| Yanagiya et al. (2016) | Female | 27 | Idiopathic | LDLLT | complicated | Alive |
| Huan et al. (2017) | Male | 34 | Idiopathic | Bilateral | NA | NA |
| Shimada et al. (2018) | Female | 21 | HSCT | LDLLT | complicated | Alive |
| Tsubosaka et al. (2018) | Male | 19 | Chemotherapy | Living-donor | NA | NA |
| Righi et al. (2018) | Male | 42 | Idiopathic | Bilateral | complicated | Alive |
| Ali et al. (2019) | Female | 26 | Idiopathic | Bilateral | complicated | Alive |
| Aljefri et al. (2019) | Male | 27 | Idiopathic | Bilateral | complicated | Alive |
| Sekine et al. (2020) | Female | 29 | Idiopathic | LDLLT | complicated | Alive |
| Rasciti et al. (2020) | Male | 48 | Idiopathic | Bilateral | possible PPFE relapse | Re-transplant |
| Shiiya et al. (2020) | Female | 40 | Idiopathic | Left single | complicated | Dead |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiiya, H.; Sato, M. Lung Transplantation for Pleuroparenchymal Fibroelastosis. J. Clin. Med. 2021, 10, 957. https://doi.org/10.3390/jcm10050957
Shiiya H, Sato M. Lung Transplantation for Pleuroparenchymal Fibroelastosis. Journal of Clinical Medicine. 2021; 10(5):957. https://doi.org/10.3390/jcm10050957
Chicago/Turabian StyleShiiya, Haruhiko, and Masaaki Sato. 2021. "Lung Transplantation for Pleuroparenchymal Fibroelastosis" Journal of Clinical Medicine 10, no. 5: 957. https://doi.org/10.3390/jcm10050957
APA StyleShiiya, H., & Sato, M. (2021). Lung Transplantation for Pleuroparenchymal Fibroelastosis. Journal of Clinical Medicine, 10(5), 957. https://doi.org/10.3390/jcm10050957






