Head-to-Head Comparison of Two Nomograms Predicting Probability of Lymph Node Invasion in Prostate Cancer and the Therapeutic Impact of Higher Nomogram Threshold
Abstract
:1. Introduction
2. Patients and Methods
3. Statistical Aanalysis
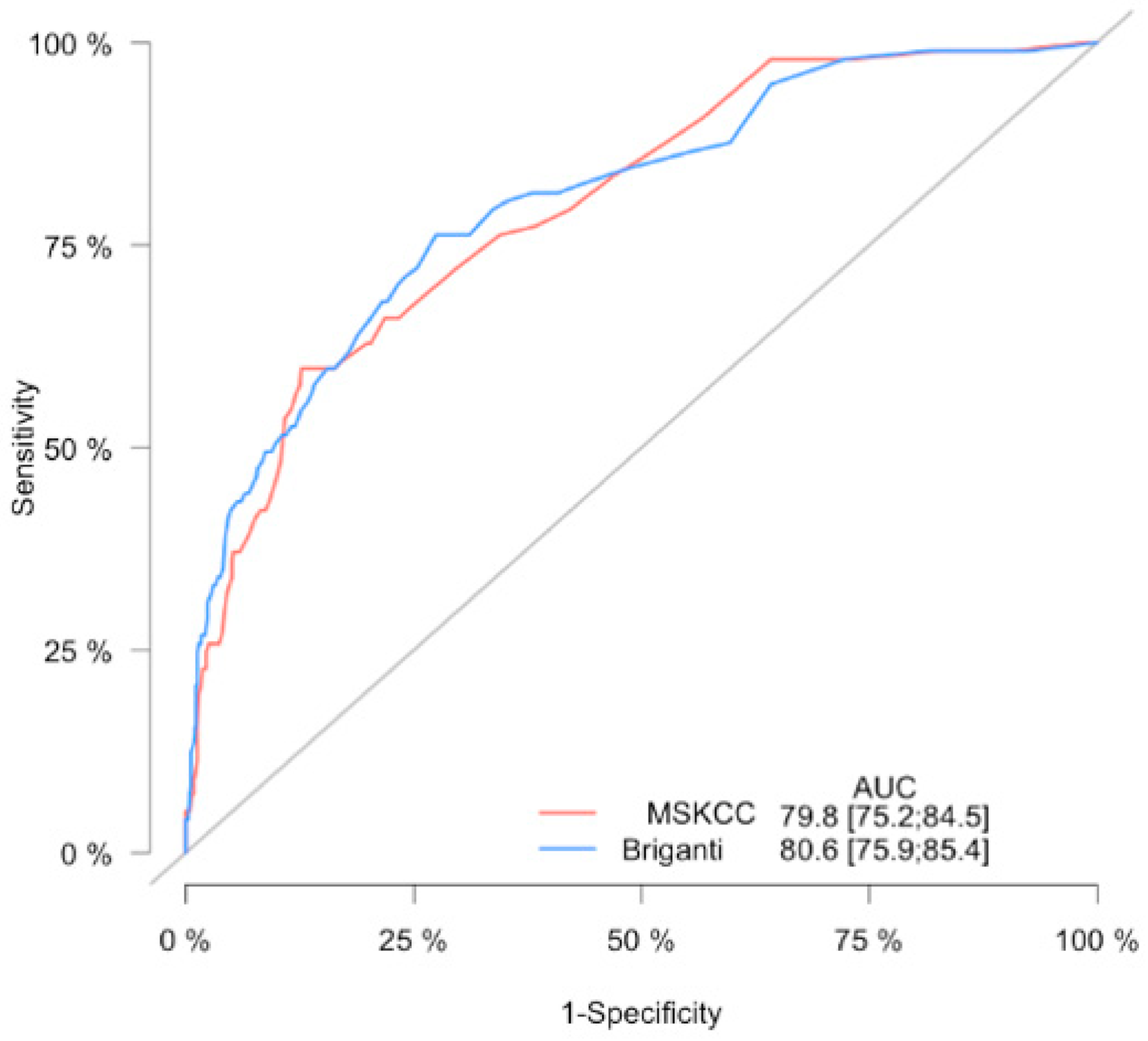
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Abdollah, F.; Sun, M.; Briganti, A.; Thuret, R.; Schmitges, J.; Gallina, A.; Suardi, N.; Capitanio, U.; Salonia, A.; Shariat, S.F.; et al. Critical assessment of the European Association of Urology guideline indications for pelvic lymph node dissection at radical prostatectomy. BJU Int. 2011, 108, 1769–1775. [Google Scholar] [CrossRef]
- Hoshi, S.; Hayashi, N.; Kurota, Y.; Hoshi, K.; Muto, A.; Sugano, O.; Numahata, K.; Bilim, V.; Sasagawa, I.; Ohta, S. Comparison of semi-extended and standard lymph node dissection in radical prostatectomy: A single-institute experience. Mol. Clin. Oncol. 2015, 3, 1085–1087. [Google Scholar] [CrossRef] [Green Version]
- Fossati, N.; Willemse, P.-P.M.; Broeck, T.V.D.; Bergh, R.C.V.D.; Yuan, C.Y.; Briers, E.; Bellmunt, J.; Bolla, M.; Cornford, P.; De Santis, M.; et al. The Benefits and Harms of Different Extents of Lymph Node Dissection During Radical Prostatectomy for Prostate Cancer: A Systematic Review. Eur. Urol. 2017, 72, 84–109. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, L.; Lerut, E.; Haustermans, K.; Deroose, C.M.; Oyen, R.; Isebaert, S.; Budiharto, T.; Ameye, F.; Mottaghy, F.M.; Bogaerts, K.; et al. Final analysis of a prospective trial on functional imaging for nodal staging in patients with prostate cancer at high risk for lymph node involvement. Urol. Oncol. 2015, 33, 109.e23-31. [Google Scholar] [CrossRef]
- Wu, H.; Xu, T.; Wang, X.; Yu, Y.-B.; Fan, Z.-Y.; Li, D.-X.; Luo, L.; Yang, X.-C.; Jiao, W.; Niu, H.-T. Diagnostic Performance of 68Gallium Labelled Prostate-Specific Membrane Antigen Positron Emission Tomography/Computed Tomography and Magnetic Resonance Imaging for Staging the Prostate Cancer with Intermediate or High Risk Prior to Radical Prostatectomy: A Systematic Review and Meta-analysis. World J. Men’s Health 2020, 38, 208. [Google Scholar]
- Heidenreich, A. Updated Nomogram Predicting Lymph Node Invasion in Patients with Prostate Cancer Undergoing Extended Pelvic Lymphadenectomy: Optimizing a Risk-Adapted Surgical Approach. Eur. Urol. 2012, 61, 488–490. [Google Scholar] [CrossRef] [PubMed]
- Makarov, D.V.; Trock, B.J.; Humphreys, E.B.; Mangold, L.A.; Walsh, P.C.; Epstein, J.I.; Partin, A.W. Updated Nomogram to Predict Pathologic Stage of Prostate Cancer Given Prostate-Specific Antigen Level, Clinical Stage, and Biopsy Gleason Score (Partin Tables) Based on Cases from 2000 to 2005. Urology 2007, 69, 1095–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandaglia, G.; Fossati, N.; Zaffuto, E.; Bandini, M.; Dell’Oglio, P.; Bravi, C.A.; Fallara, G.; Pellegrino, F.; Nocera, L.; Karakiewicz, P.I.; et al. Development and Internal Validation of a Novel Model to Identify the Candidates for Extended Pelvic Lymph Node Dissection in Prostate Cancer. Eur. Urol. 2017, 72, 632–640. [Google Scholar] [CrossRef]
- Hansen, J.; Rink, M.; Bianchi, M.; Kluth, L.A.; Tian, Z.; Ahyai, S.A.; Shariat, S.F.; Briganti, A.; Steuber, T.; Fisch, M.; et al. External validation of the updated briganti nomogram to predict lymph node invasion in prostate cancer patients undergoing extended lymph node dissection. Prostate 2012, 73, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Cagiannos, I.; Karakiewicz, P.; Eastham, J.A.; Ohori, M.; Rabbani, F.; Gerigk, C.; Reuter, V.; Graefen, M.; Hammerer, P.G.; Erbersdobler, A.; et al. A Preoperative Nomogram Identifying Decreased Risk of Positive Pelvic Lymph Nodes in Patients With Prostate Cancer. J. Urol. 2003, 170, 1798–1803. [Google Scholar] [CrossRef] [PubMed]
- Partin, A.W.; A Mangold, L.; Lamm, D.M.; Walsh, P.C.; I Epstein, J.; Pearson, J.D. Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology 2001, 58, 843–848. [Google Scholar] [CrossRef]
- Godoy, G.; Chong, K.T.; Cronin, A.; Vickers, A.; Laudone, V.; Touijer, K.; Guillonneau, B.; Eastham, J.A.; Scardino, P.T.; Coleman, J.A. Extent of Pelvic Lymph Node Dissection and the Impact of Standard Template Dissection on Nomogram Prediction of Lymph Node Involvement. Eur. Urol. 2011, 60, 195–201. [Google Scholar] [CrossRef]
- Briganti, A.; Larcher, A.; Abdollah, F.; Capitanio, U.; Gallina, A.; Suardi, N.; Bianchi, M.; Sun, M.; Freschi, M.; Salonia, A.; et al. Updated Nomogram Predicting Lymph Node Invasion in Patients with Prostate Cancer Undergoing Extended Pelvic Lymph Node Dissection: The Essential Importance of Percentage of Positive Cores. Eur. Urol. 2012, 61, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Milonas, D.; Venclovas, Z.; Muilwijk, T.; Jievaltas, M.; Joniau, S. External validation of Memorial Sloan Kettering Cancer Center nomogram and prediction of optimal candidate for lymph node dissection in clinically localized prostate cancer. Central Eur. J. Urol. 2020, 73, 19–25. [Google Scholar]
- Schmitges, J.; Karakiewicz, P.; Sun, M.; Abdollah, F.; Budäus, L.; Isbarn, H.; Bianchi, M.; Trinh, Q.-D.; Schlomm, T.; Chun, F.; et al. Predicting the risk of lymph node invasion during radical prostatectomy using the European association of urology guideline nomogram: A validation study. Eur. J. Surg. Oncol. (EJSO) 2012, 38, 624–629. [Google Scholar] [CrossRef]
- Epstein, J.I.; Allsbrook, W.C.; Amin, M.B.; Egevad, L.L. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2005, 29, 1228–1242. [Google Scholar] [CrossRef] [Green Version]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Briganti 2012 Nomogram: “Prediction of Lymph Node Involvement in patients w—Evidencio”. Available online: https://www.evidencio.com/models/show/670 (accessed on 21 October 2020).
- Prostate Cancer Nomograms: Pre-Radical Prostatectomy | Memorial Sloan Kettering Cancer Center. Available online: https://www.mskcc.org/nomograms/prostate/pre_op (accessed on 21 October 2020).
- Mattei, A.; Fuechsel, F.G.; Dhar, N.B.; Warncke, S.H.; Thalmann, G.N.; Krause, T.; Studer, U.E. The Template of the Primary Lymphatic Landing Sites of the Prostate Should Be Revisited: Results of a Multimodality Mapping Study. Eur. Urol. 2008, 53, 118–125. [Google Scholar] [CrossRef]
- Bivalacqua, T.J.; Pierorazio, P.M.; Gorin, M.A.; Allaf, M.E.; Carter, H.B.; Walsh, P.C. Anatomic Extent of Pelvic Lymph Node Dissection: Impact on Long-term Cancer-specific Outcomes in Men With Positive Lymph Nodes at Time of Radical Prostatectomy. Urology 2013, 82, 653–659. [Google Scholar] [CrossRef] [Green Version]
- Schiavina, R.; Bertaccini, A.; Franceschelli, A.; Manferrari, F.; Vagnoni, V.; Borghesi, M.; Morselli-Labate, A.M.; Martorana, G. The impact of the extent of lymph-node dissection on biochemical relapse after radical prostatectomy in node-negative patients. Anticancer Res. 2010, 30, 2297. [Google Scholar]
- Mohler, J.L.; Antonarakis, E.S.; Armstrong, A.J.; D’Amico, A.V.; Davis, B.J.; Dorff, T.; Eastham, J.A.; Enke, C.A.; Farrington, T.A.; Higano, C.S.; et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 479–505. [Google Scholar] [CrossRef] [Green Version]
- Bandini, M.; Marchioni, M.; Pompe, R.S.; Tian, Z.; Gandaglia, G.; Fossati, N.; Abdollah, F.; Graefen, M.; Montorsi, F.; Saad, F.; et al. First North American validation and head-to-head comparison of four preoperative nomograms for prediction of lymph node invasion before radical prostatectomy. BJU Int. 2017, 121, 592–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turo, R.; Forster, J.A.; West, R.M.; Prescott, S.; Paul, A.B.; Cross, W.R. Do prostate cancer nomograms give accurate information when applied to European patients? Scand. J. Urol. 2015, 49, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Hueting, T.A.; Cornel, E.B.; Somford, D.M.; Jansen, H.; Van Basten, J.-P.A.; Pleijhuis, R.G.; Korthorst, R.A.; Van Der Palen, J.A.; Koffijberg, H. External Validation of Models Predicting the Probability of Lymph Node Involvement in Prostate Cancer Patients. Eur. Urol. Oncol. 2018, 1, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Hinev, A.I.; Anakievski, D.; Kolev, N.H.; Hadjiev, V.I. Validation of Nomograms Predicting Lymph Node Involvement in Patients with Prostate Cancer Undergoing Extended Pelvic Lymph Node Dissection. Urol. Int. 2014, 92, 300–305. [Google Scholar] [CrossRef]
- De Reijke, T.M.; Coenen, J.L.L.M.; Gietema, J.A. Dutch Guideline on Prostate Cancer; Dutch Association of Urology: Rotterdam, The Netherlands, 2016. [Google Scholar]
- Venclovas, Z.; Jievaltas, M.; Milonas, D. Significance of Time Until PSA Recurrence After Radical Prostatectomy Without Neo- or Adjuvant Treatment to Clinical Progression and Cancer-Related Death in High-Risk Prostate Cancer Patients. Front. Oncol. 2019, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Sagalovich, D.; Calaway, A.; Srivastava, A.; Sooriakumaran, P.; Tewari, A.K. Assessment of required nodal yield in a high risk cohort undergoing extended pelvic lymphadenectomy in robotic-assisted radical prostatectomy and its impact on functional outcomes. BJU Int. 2012, 111, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Parameter | pN0 (n = 710) | pN1 (n = 97) | p Value | All (n = 807) |
|---|---|---|---|---|
| Age (yr): median, (IQR) | 65 (60–69) | 64 (57–67.5) | 0.034 | 65 (60–69) |
| PSA (ng/mL): median, (IQR) | 10.1 (6.28–14.23) | 12.4 (8.27–19.85) | <0.001 | 10.3 (6.5–14.7) |
| Clinical stage: n, (%) | <0.001 | |||
| cT1 | 101 (14.2) | 3 (3.1) | 104 (12.9) | |
| cT2 | 444 (62.5) | 34 (35.1) | 478 (59.2) | |
| cT3 | 165 (23.3) | 60 (61.8) | 225 (27.9) | |
| Biopsy Gleason Score: n, (%) | <0.001 | |||
| 6 | 286 (40.3) | 11 (11.3) | 297 (36.8) | |
| 3 + 4 | 258 (36.3) | 25 (25.8) | 283 (35.1) | |
| 4 + 3 | 57 (8.0) | 20 (20.6) | 77 (9.5) | |
| 8 | 76 (10.7) | 22 (22.7) | 98 (12.1) | |
| 9–10 | 33 (4.6) | 19 (19.6) | 52 (6.4) | |
| % of positive cores: median, (IQR) | 40 (25–62) | 62 (38–88) | <0.001 | 50 (25–64.5) |
| Pathological Gleason Score: n, (%) | <0.001 | |||
| 6 | 136 (19.2) | 1 (1.0) | 137 (16.9) | |
| 3 + 4 | 334 (47.0) | 13 (13.4) | 347 (43) | |
| 4 + 3 | 112 (15.8) | 19 (19.6) | 131 (16.2) | |
| 8 | 60 (8.5) | 15 (15.5) | 75 (9.3) | |
| 9–10 | 68 (9.6) | 49 (50.5) | 117 (14.5) | |
| Pathologic stage: n, (%) | <0.001 | |||
| pT2 | 350 (49.3) | 6 (6.2) | 356 (44.3) | |
| pT3a | 278 (39.2) | 29 (29.9) | 307 (38) | |
| pT3b | 82 (11.5) | 60 (61.9) | 142 (17.6) | |
| pT4 | 0 | 2 (2.1) | 2 (0.2) | |
| No, of LN removed: median, (IQR) | 6 (4–10) | 11 (8–18) | <0.001 | 7 (4–11) |
| Positive surgical margin: n, (%) | 267 (37.6) | 60 (61.9) | <0.001 | 327 (40.5) |
| MSKCC: median, (IQR) | 8 (4–16) | 32 (12–48.5) | <0.001 | 9 (5–20) |
| Briganti: median, (IQR) | 7 (3–17) | 37 (16–67.5) | <0.001 | 8 (4–22) |
| Cut-off | Patients in Whom PLND is Not Recommended According to the Cut-Off (below Cut-Off) | Missing % | Patients below Cut-Off without Histologic LNI | Patients below Cut-Off with Histologic LNI | Patients above Cut-Off without Histologic LNI | Patients above Cut-Off with Histologic LNI | NPV |
|---|---|---|---|---|---|---|---|
| 1 | 54 (6.7) | 0 | 54 (7.61) | 0 (0) | 656 (92.39) | 97 (100) | 100 |
| 2 | 132 (16.4) | 0.76 | 131 (18.45) | 1 (1.03) | 579 (81.55) | 96 (98.97) | 99.24 |
| 3 | 199 (24.7) | 1 | 197 (27.75) | 2 (2.06) | 513 (72.25) | 95 (97.94) | 99 |
| 4 | 259 (32.1) | 1.93 | 254 (35.77) | 5 (5.15) | 456 (64.23) | 92 (94.85) | 98.07 |
| 5 | 298 (36.9) | 4.03 | 286 (40.28) | 12 (12.37) | 424 (59.72) | 85 (87.63) | 95.97 |
| 6 | 328 (40.6) | 3.96 | 315 (44.37) | 13 (13.4) | 395 (55.63) | 84 (86.6) | 96.04 |
| 7 | 379 (47) | 3.96 | 364 (51.27) | 15 (15.46) | 346 (48.73) | 82 (84.54) | 96.04 |
| 8 | 420 (52) | 4.05 | 403 (56.76) | 17 (17.53) | 307 (43.24) | 80 (82.47) | 95.95 |
| 9 | 438 (54.3) | 4.11 | 420 (59.15) | 18 (18.56) | 290 (40.85) | 79 (81.44) | 95.89 |
| 10 | 458 (56.8) | 3.93 | 440 (61.97) | 18 (18.56) | 270 (38.03) | 79 (81.44) | 96.07 |
| 15 | 538 (66.7) | 4.28 | 515 (72.54) | 23 (23.71) | 195 (27.46) | 74 (76.29) | 95.72 |
| Cut-off | Patients in whom PLND Is not Recommended According to the cut-off (below cut-off) | Missing % | Patients below Cut-off without Histologic LNI | Patients below Cut-off with Histologic LNI | Patients above Cut-off without Histologic LNI | Patients above Cut-off with Histologic LNI | NPV |
|---|---|---|---|---|---|---|---|
| 1 | 10 (1.2) | 0 | 10 (1.4) | 0 (0) | 700 (98.6) | 97 (100) | 100 |
| 2 | 69 (8.6) | 1.4 | 68 (9.6) | 1 (1.03) | 642 (90.4) | 96 (98.97) | 98.6 |
| 3 | 123 (15.2) | 0.8 | 122 (17.2) | 1 (1.03) | 588 (82.8) | 96 (98.97) | 99.2 |
| 4 | 190 (23.5) | 1.05 | 188 (24.48) | 2 (2.06) | 522 (73.52) | 95 (97.94) | 98.95 |
| 5 | 256 (31.7) | 0.78 | 254 (35.77) | 2 (2.06) | 456 (64.22) | 95 (97.94) | 99.22 |
| 6 | 316 (39.2) | 2.85 | 307 (43.24) | 9 (9.28) | 403 (56.76) | 88 (90.72) | 97.15 |
| 7 | 359 (44.5) | 3.62 | 346 (48.73) | 13 (13.4) | 364 (51.27) | 84 (86.6) | 96.38 |
| 8 | 393 (48.7) | 4.07 | 377 (53.1) | 16 (16.49) | 333 (46.9) | 81 (83.51) | 95.93 |
| 9 | 431 (53.4) | 4.64 | 411 (57.89) | 20 (20.62) | 299 (42.11) | 77 (79.38) | 95.36 |
| 10 | 460 (57) | 4.78 | 438 (61.69) | 22 (22.68) | 272 (38.31) | 75 (77.32) | 95.22 |
| 15 | 561 (69.5) | 5.53 | 530 (74.65) | 31 (31.96) | 180 (25.35) | 66 (68.04) | 94.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venclovas, Z.; Muilwijk, T.; Matjosaitis, A.J.; Jievaltas, M.; Joniau, S.; Milonas, D. Head-to-Head Comparison of Two Nomograms Predicting Probability of Lymph Node Invasion in Prostate Cancer and the Therapeutic Impact of Higher Nomogram Threshold. J. Clin. Med. 2021, 10, 999. https://doi.org/10.3390/jcm10050999
Venclovas Z, Muilwijk T, Matjosaitis AJ, Jievaltas M, Joniau S, Milonas D. Head-to-Head Comparison of Two Nomograms Predicting Probability of Lymph Node Invasion in Prostate Cancer and the Therapeutic Impact of Higher Nomogram Threshold. Journal of Clinical Medicine. 2021; 10(5):999. https://doi.org/10.3390/jcm10050999
Chicago/Turabian StyleVenclovas, Zilvinas, Tim Muilwijk, Aivaras J. Matjosaitis, Mindaugas Jievaltas, Steven Joniau, and Daimantas Milonas. 2021. "Head-to-Head Comparison of Two Nomograms Predicting Probability of Lymph Node Invasion in Prostate Cancer and the Therapeutic Impact of Higher Nomogram Threshold" Journal of Clinical Medicine 10, no. 5: 999. https://doi.org/10.3390/jcm10050999
APA StyleVenclovas, Z., Muilwijk, T., Matjosaitis, A. J., Jievaltas, M., Joniau, S., & Milonas, D. (2021). Head-to-Head Comparison of Two Nomograms Predicting Probability of Lymph Node Invasion in Prostate Cancer and the Therapeutic Impact of Higher Nomogram Threshold. Journal of Clinical Medicine, 10(5), 999. https://doi.org/10.3390/jcm10050999






