Pathogenesis and Current Treatment of Osteosarcoma: Perspectives for Future Therapies
Abstract
:1. Introduction
2. Bone Biology
3. Bone Transformation to Osteosarcoma
3.1. Bone Cancer and Sarcoma
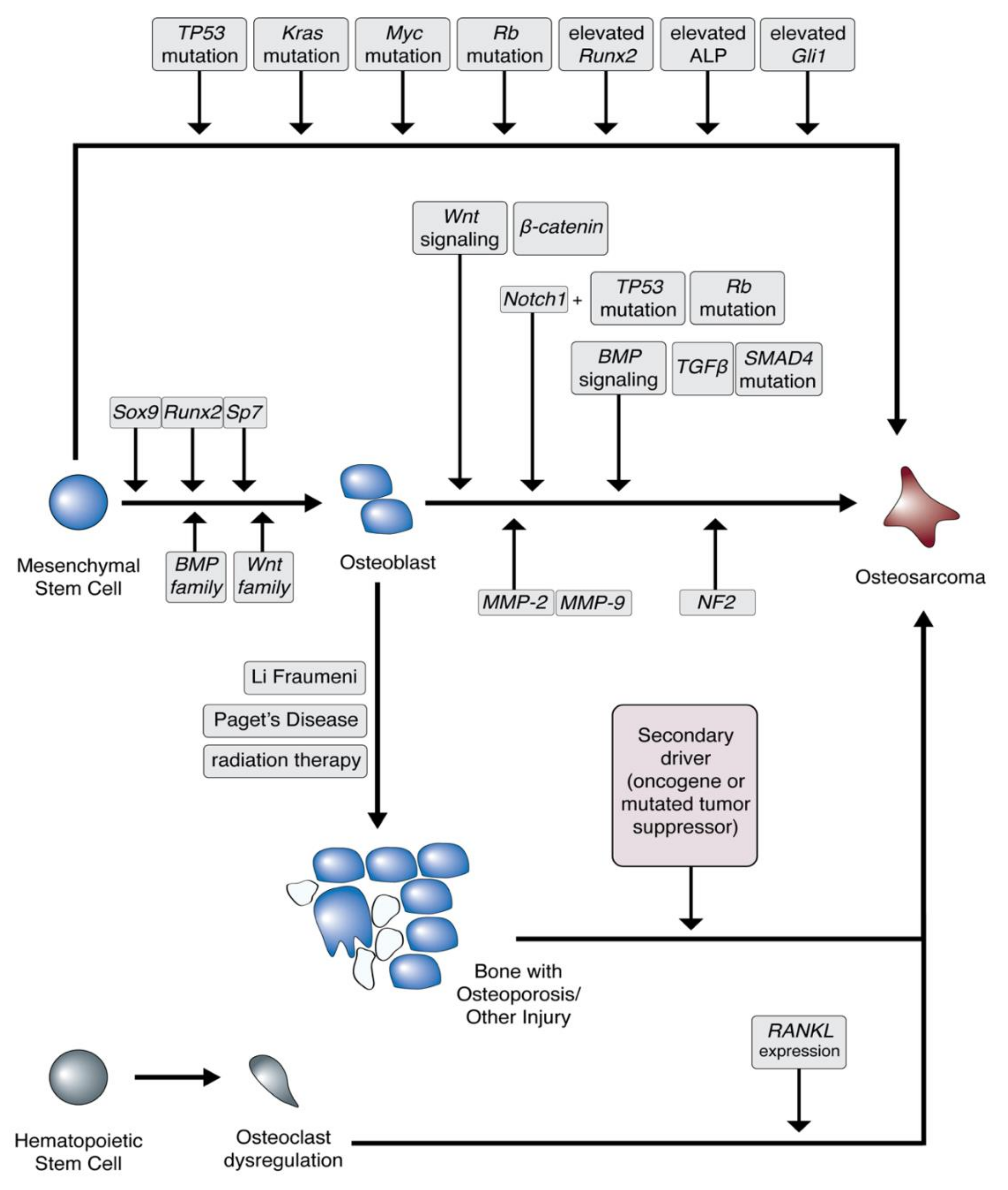
3.2. Osteosarcoma Cell of Origin
3.3. Bone Microenvironment
3.4. Osteosarcoma Predisposition
4. Osteosarcoma Epidemiology and Diagnosis
5. Treatment Strategies and Molecular Targets
5.1. Current Standard of Care
5.2. Clinical Trials: The Future of Osteosarcoma Treatment
5.3. Targeting p53 and RB
5.4. Gemcitabine and Docetaxel
5.5. Targeting ABCB1
5.6. RANK Ligand Antibodies
5.7. Tyrosine Kinase Inhibitors
5.8. Immune Checkpoint Inhibitors
5.9. mTOR Inhibitors
5.10. Combination Metabolic Therapies
5.11. HER2-Targeted Therapies
5.12. Engineered Mesenchymal Stem Cells
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Broadhead, M.L.; Sivaji, S.; Balogh, Z.; Choong, P.F.M. Osteosarcoma: From Molecular Biology to Mesenchymal Stem Cells. Osteosarcoma Biol. Behav. Mech. 2017. [Google Scholar] [CrossRef] [Green Version]
- Abarrategi, A.; Tornin, J.; Martinez-Cruzado, L.; Hamilton, A.; Martinez-Campos, E.; Rodrigo, J.P.; González, M.V.; Baldini, N.; Garcia-Castro, J.; Rodriguez, R.; et al. Osteosarcoma: Cells-of-Origin, Cancer Stem Cells, and Targeted Therapies. Stem Cells Int. 2016, 2016, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfranca, A.; Martinez-Cruzado, L.; Tornin, J.; Abarrategi, A.; Amaral, T.; De Alava, E.; Menendez, P.; Garcia-Castro, J.; Rodriguez, R. Bone microenvironment signals in osteosarcoma development. Cell. Mol. Life Sci. 2015, 72, 3097–3113. [Google Scholar] [CrossRef]
- Katagiri, T.; Takahashi, N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 2002, 8, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.F.; Kleine-Nulend, J.; Li, B. Bone Microenvironment, Stem Cells, and Bone Tissue Regeneration. Stem Cells Int. 2017, 2017, 2–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, N.; Song, W.X.; Luo, J.; Haydon, R.C.; He, T.C. Osteosarcoma development and stem cell differentiation. Clin. Orthop. Relat. Res. 2008, 466, 2114–2130. [Google Scholar] [CrossRef] [Green Version]
- Tam, W.L.; Luyten, F.P.; Roberts, S.J. From skeletal development to the creation of pluripotent stem cell-derived bone-forming progenitors. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Li, Z.; Hou, Y.; Fang, W. Potential mechanisms underlying the Runx2 induced osteogenesis of bone marrow mesenchymal stem cells. Am. J. Transl. Res. 2015, 7, 2527–2535. [Google Scholar]
- Hu, L.; Yin, C.; Zhao, F.; Ali, A.; Ma, J.; Qian, A. Mesenchymal stem cells: Cell fate decision to osteoblast or adipocyte and application in osteoporosis treatment. Int. J. Mol. Sci. 2018, 19, 360. [Google Scholar] [CrossRef] [Green Version]
- Kansara, M.; Thomas, D.M. Molecular pathogenesis of osteosarcoma. DNA Cell Biol. 2007, 26, 1–18. [Google Scholar] [CrossRef]
- Kim, E.-K.; Lim, S.; Park, J.-M.; Seo, J.K.; Kim, J.H.; Kim, K.T.; Ryu, S.H.; Suh, P.-G. Human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by AMP-activated protein kinase. J. Cell. Physiol. 2012, 227, 1680–1687. [Google Scholar] [CrossRef]
- Mohamed, A.M.F.S. An overview of bone cells and their regulating factors of differentiation. Malays. J. Med. Sci. 2008, 15, 4–12. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Dorfman, H.D.; Czerniak, B. Bone cancers. Cancer 1995, 75, 203–210. [Google Scholar] [CrossRef]
- Klein, M.J.; Siegal, G.P. Osteosarcoma. Am. J. Clin. Pathol. 2006, 125, 555–581. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Lengner, C.J.; Steinman, H.A.; Gagnon, J.; Smith, T.W.; Henderson, J.E.; Kream, B.E.; Stein, G.S.; Lian, J.B.; Jones, S.N. Osteoblast differentiation and skeletal development are regulated by Mdm2-p53 signaling. J. Cell Biol. 2006, 172, 909–921. [Google Scholar] [CrossRef]
- Martin, J.W.; Squire, J.A.; Zielenska, M. The Genetics of Osteosarcoma. Sarcoma 2012, 2012, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spangler, J.G. Bone biology and physiology: Implications for novel osteoblastic osteosarcoma treatments? Med. Hypotheses 2008, 70, 281–286. [Google Scholar] [CrossRef]
- Rainusso, N.; Wang, L.L.; Yustein, J.T. The adolescent and young adult with cancer: State of the art—Bone tumors. Curr. Oncol. Rep. 2013, 15, 296–307. [Google Scholar] [CrossRef]
- Chen, X.; Bahrami, A.; Pappo, A.; Easton, J.; Dalton, J.; Hedlund, E.; Ellison, D.; Shurtleff, S.; Wu, G.; Wei, L.; et al. Recurrent Somatic Structural Variations Contribute to Tumorigenesis in Pediatric Osteosarcoma. Cell Rep. 2014, 7, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Rubio, R.; Gutierrez-Aranda, I.; Sáez-Castillo, A.I.; Labarga, A.; Rosu-Myles, M.; Gonzalez-Garcia, S.; Toribio, M.L.; Menendez, P.; Rodriguez, R. The differentiation stage of p53-Rb-deficient bone marrow mesenchymal stem cells imposes the phenotype of in vivo sarcoma development. Oncogene 2013, 32, 4970–4980. [Google Scholar] [CrossRef] [Green Version]
- Adamopoulos, C.; Gargalionis, A.N.; Basdra, E.K.; Papavassiliou, A.G. Deciphering signaling networks in osteosarcoma pathobiology. Exp. Biol. Med. 2016, 241, 1296–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haydon, R.C.; Deyrup, A.; Ishikawa, A.; Heck, R.; Jiang, W.; Zhou, L.; Feng, T.; King, D.; Cheng, H.; Breyer, B.; et al. Cytoplasmic and/or Nuclear Accumulation of the β-Catenin Protein is a Frequent Event in Human Osteosarcoma. Int. J. Cancer 2002, 102, 338–342. [Google Scholar] [CrossRef] [Green Version]
- Alliston, T.; Choy, L.; Ducy, P.; Karsenty, G.; Derynck, R. TGF-β-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. EMBO J. 2001, 20, 2254–2272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Deng, C.; Li, Y.P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [Green Version]
- Lamora, A.; Talbot, J.; Mullard, M.; Brounais-Le Royer, B.; Redini, F.; Verrecchia, F. TGF-β Signaling in Bone Remodeling and Osteosarcoma Progression. J. Clin. Med. 2016, 5, 96. [Google Scholar] [CrossRef] [PubMed]
- Nathan, S.S.; Pereira, B.P.; Zhou, Y.F.; Gupta, A.; Dombrowski, C.; Soong, R.; Pho, R.W.H.; Stein, G.S.; Salto-Tellez, M.; Cool, S.M.; et al. Elevated expression of Runx2 as a key parameter in the etiology of osteosarcoma. Mol. Biol. Rep. 2009, 36, 153–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, H.Y.; Sun, L.L.; Li, H.Y.; Ye, Z.M. Prognostic significance of serum alkaline phosphatase level in osteosarcoma: A meta-analysis of published data. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.J.; Ye, F.; Cheng, L.J.; Shi, Y.J.; Bao, J.; Sun, H.Q.; Wang, W.; Zhang, P.; Bu, H. Osteogenic differentiation of mesenchymal stem cells promoted by overexpression of connective tissue growth factor. J. Zhejiang Univ. Sci. B 2009, 10, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Sadikovic, B.; Thorner, P.; Chilton-MacNeill, S.; Martin, J.W.; Cervigne, N.K.; Squire, J.; Zielenska, M. Expression analysis of genes associated with human osteosarcoma tumors shows correlation of RUNX2 overexpression with poor response to chemotherapy. BMC Cancer 2010, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; He, G.; Lee, W.C.; McKenzie, J.A.; Silva, M.J.; Long, F. Gli1 identifies osteogenic progenitors for bone formation and fracture repair. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Angulo, P.; Kaushik, G.; Subramaniam, D.; Dandawate, P.; Neville, K.; Chastain, K.; Anant, S. Natural compounds targeting major cell signaling pathways: A novel paradigm for osteosarcoma therapy. J. Hematol. Oncol. 2017, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beristain, A.G.; Narala, S.R.; Di Grappa, M.A.; Khokha, R. Homotypic RANK signaling differentially regulates proliferation, motility and cell survival in osteosarcoma and mammary epithelial cells. J. Cell Sci. 2012, 125, 943–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Z.; Feng, C.; Lu, Y.; Lin, Y.; Dong, C. PHGDH is an independent prognosis marker and contributes cell proliferation, migration and invasion in human pancreatic cancer. Gene 2018, 642, 43–50. [Google Scholar] [CrossRef]
- Rathore, R.; Caldwell, K.E.; Schutt, C.; Brashears, C.B.; Prudner, B.C.; Ehrhardt, W.R.; Leung, C.H.; Lin, H.; Daw, N.C.; Beird, H.C.; et al. Metabolic compensation activates pro-survival mTORC1 signaling upon 3-phosphoglycerate dehydrogenase inhibition in osteosarcoma. Cell Rep. 2021, 34, 108678. [Google Scholar] [CrossRef] [PubMed]
- Rubio, R.; Abarrategi, A.; Garcia-Castro, J.; Martinez-Cruzado, L.; Suarez, C.; Tornin, J.; Santos, L.; Astudillo, A.; Colmenero, I.; Mulero, F.; et al. Bone environment is essential for osteosarcoma development from transformed mesenchymal stem cells. Stem Cells 2014, 32, 1136–1148. [Google Scholar] [CrossRef]
- Wang, L.; Park, P.; La Marca, F.; Than, K.; Rahman, S.; Lin, C.Y. Bone formation induced by BMP-2 in human osteosarcoma cells. Int. J. Oncol. 2013, 43, 1095–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Azevedo, J.W.V.; de Medeiros Fernandes, T.A.A.; Fernandes, J.V.; de Azevedo, J.C.V.; Lanza, D.C.F.; Bezerra, C.M.; Andrade, V.S.; de Araújo, J.M.G.; Fernandes, J.V. Biology and pathogenesis of human osteosarcoma (Review). Oncol. Lett. 2020, 19, 1099–1116. [Google Scholar] [CrossRef]
- Huang, J.; Ni, J.; Liu, K.; Yu, Y.; Xie, M.; Kang, R.; Vernon, P.; Cao, L.; Tang, D. HMGB1 promotes drug resistance in osteosarcoma. Cancer Res. 2012, 72, 230–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, A.I.; Schwertschkow, A.H.; Nolta, J.A.; Wu, J. Involvement of Mesenchymal Stem Cells in Cancer Progression and Metastases. Curr. Cancer Drug Targets 2015, 15, 88–98. [Google Scholar] [CrossRef]
- Stamatopoulos, A.; Stamatopoulos, T.; Gamie, Z.; Kenanidis, E.; Ribeiro, R.D.C.; Rankin, K.S.; Gerrand, C.; Dalgarno, K.; Tsiridis, E. Mesenchymal stromal cells for bone sarcoma treatment: Roadmap to clinical practice. J. Bone Oncol. 2019, 16, 100231. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, G.; Chen, R.; Hua, Y.; Cai, Z. Mesenchymal stem cells in the osteosarcoma microenvironment: Their biological properties, influence on tumor growth, and therapeutic implications. Stem Cell Res. Ther. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weilbaecher, K.N.; Guise, T.A.; McCauley, L.K. Cancer to bone: A fatal attraction. Nat. Rev. Cancer 2011, 11, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Kozlow, W.; Guise, T.A. Breast cancer metastasis to bone: Mechanisms of osteolysis and implications for therapy. J. Mammary Gland Biol. Neoplasia 2005, 10, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Pollari, S.; Käkönen, S.M.; Edgren, H.; Wolf, M.; Kohonen, P.; Sara, H.; Guise, T.; Nees, M.; Kallioniemi, O. Enhanced serine production by bone metastatic breast cancer cells stimulates osteoclastogenesis. Breast Cancer Res. Treat. 2011, 125, 421–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivier, M.; Hollstein, M.; Hainaut, P. TP53 Mutations in Human Cancers: Origins, Consequences, and Clinical Use. Cold Spring Harb. Perspect. Biol. 2010, 2, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Iurlaro, R.; León-Annicchiarico, C.L.; Muñoz-Pinedo, C. Regulation of cancer metabolism by oncogenes and tumor suppressors. Methods Enzymol. 2014, 542, 59–80. [Google Scholar] [CrossRef]
- Hansen, M.F.; Seton, M.; Merchant, A. Osteosarcoma in Paget’s Disease of Bone. J. Bone Miner. Res. 2006, 21, P58–P63. [Google Scholar] [CrossRef] [PubMed]
- Nellissery, M.J.; Padalecki, S.S.; Brkanac, Z.; Singer, F.R.; Roodman, G.D.; Unni, K.K.; Leach, R.J.; Hansen, M.F. Evidence for a novel osteosarcoma tumor-suppressor gene in the chromosome 18 region genetically linked with Paget disease of bone. Am. J. Hum. Genet. 1998, 63, 817–824. [Google Scholar] [CrossRef] [Green Version]
- Ricafort, R.; Gorlick, R. Molecularly Targeted Therapy for Osteosarcoma: Where Do We Go from Here? Mol. Target. Ther. Child. Cancer 2010, 459–498. [Google Scholar] [CrossRef]
- Fuchs, B.; Pritchard, D.J. Etiology of osteosarcoma. Clin. Orthop. Relat. Res. 2002, 40–52. [Google Scholar] [CrossRef]
- Mendoza, S.M.; Konishi, T.; Miller, C.W. Integration of SV40 in human osteosarcoma DNA. Oncogene 1998, 17, 2457–2462. [Google Scholar] [CrossRef] [Green Version]
- Geller, D.S.; Gorlick, R. Osteosarcoma: A Review of Diagnosis, Management, and Treatment Strategies. Clin. Adv. Hematol. Oncol. 2010, 8, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Heare, T.; Hensley, M.A.; DellʼOrfano, S. Bone tumors: Osteosarcoma and Ewingʼs sarcoma. Curr. Opin. Pediatr. 2009, 21, 365–372. [Google Scholar] [CrossRef]
- Choi, J.H.; Ro, J.Y. The 2020 WHO Classification of Tumors of Bone: An Updated Review. Adv. Anat. Pathol. 2021, 1–20. [Google Scholar] [CrossRef]
- Isakoff, M.S.; Bielack, S.S.; Meltzer, P.; Gorlick, R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J. Clin. Oncol. 2015, 33, 3029–3035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whelan, J.S.; Davis, L.E. Osteosarcoma, chondrosarcoma, and chordoma. J. Clin. Oncol. 2018, 36, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Bielack, S.S.; Werner, M.; Tunn, P.U.; Helmke, K.; Jürgens, H.; Calaminus, G.; Gerss, J.; Butterfass-Bahloul, T.; Reichardt, P.; Smeland, S.; et al. Methotrexate, doxorubicin, and cisplatin (MAP) plus maintenance pegylated interferon alfa-2b versus MAP alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative MAP: First results of the EURAMOS-1 good respons. J. Clin. Oncol. 2015, 33, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, Q.; Li, Y. Prediction and evaluation of neoadjuvant chemotherapy using the dual mechanisms of 99m-Tc-MIBI scintigraphy in patients with osteosarcoma. J. Bone Oncol. 2019, 17. [Google Scholar] [CrossRef]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meredith, A.M.; Dass, C.R. Increasing role of the cancer chemotherapeutic doxorubicin in cellular metabolism. J. Pharm. Pharmacol. 2016, 68, 729–741. [Google Scholar] [CrossRef] [Green Version]
- Dechant, K.L.; Brogden, R.N.; Pilkington, T.; Faulds, D. Ifosfamide/Mesna: A Review of its Antineoplastic Activity, Pharmacokinetic Properties and Therapeutic Efficacy in Cancer. Drugs 1991, 42, 428–467. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, N.; Puri, A.; Gelderblom, H. Osteosarcoma: Evolution of Treatment Paradigms. Sarcoma 2013, 203531. [Google Scholar] [CrossRef] [Green Version]
- Balamuth, N.J.; Womer, R.B. Ewing’s sarcoma. Lancet Oncol. 2010, 11, 184–192. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y.; Lin, N.; Jiang, S.; Wang, Y.; Ye, Z. High-Dose Methotrexate (HD-MTX) Used as an Adjunct with Other Chemotherapeutics for the Treatment of Osteosarcoma. Cell Biochem. Biophys. 2015, 71, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Patiño-García, A.; Zalacaín, M.; Marrodán, L.; San-Julián, M.; Sierrasesúmaga, L. Methotrexate in Pediatric Osteosarcoma: Response and Toxicity in Relation to Genetic Polymorphisms and Dihydrofolate Reductase and Reduced Folate Carrier 1 Expression. J. Pediatr. 2009, 154, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, P.T.R.; Zhang, Z.; McCourt, L.; Dwyer, M.; Benkovic, S.J.; Hammes, G.G. Interaction of dihydrofolate reductase with methotrexate: Ensemble and single-molecule kinetics. Proc. Natl. Acad. Sci. USA 2002, 99, 13481–13486. [Google Scholar] [CrossRef] [Green Version]
- Serra, M.; Reverter-Branchat, G.; Maurici, D.; Benini, S.; Shen, J.N.; Chano, T.; Hattinger, C.M.; Manara, M.C.; Pasello, M.; Scotlandi, K.; et al. Analysis of dihydrofolate reductase and reduced folate carrier gene status in relation to methotrexate resistance in osteosarcoma cells. Ann. Oncol. 2004, 15, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Holmboe, L.; Andersen, A.M.; Mørkrid, L.; Slørdal, L.; Hall, K.S. High dose methotrexate chemotherapy: Pharmacokinetics, folate and toxicity in osteosarcoma patients. Br. J. Clin. Pharmacol. 2011, 73, 106–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaffe, N.; Gorlick, R. High-Dose Methotrexate in Osteosarcoma: Let the Questions Surcease—Time for Final Acceptance. J. Clin. Oncol. 2008, 26, 4365–4366. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.; Swinden Michael, V.; Tanjong Ghogomu, E.; Ortiz, Z.; Katchamart, W.; Rader, T.; Bombardier, C.; Wells George, A.; Tugwell, P. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Cochrane Database Syst. Rev. 2013, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Tishler, M.; Caspi, D.; Fishel, B.; Yaron, M. The effects of leucovorin (folinic acid) on methotrexate therapy in rheumatoid arthritis patients. Arthritis Rheum. 1988, 31, 906–908. [Google Scholar] [CrossRef] [PubMed]
- Visentin, M.; Zhao, R.; Goldman, I.D. The Antifolates. Hematol. Oncol. Clin. N. Am. 2012, 26, 629. [Google Scholar] [CrossRef] [Green Version]
- Wilmanns, W.; Sauer, H.; Schalhorn, A. Biochemical Control of High-Dose Methotrexate/Leucovorin Rescue Therapy. In Cancer Chemo-and Immunopharmacology; Springer: Berlin/Heidelberg, Germany, 1980; pp. 42–49. [Google Scholar] [CrossRef]
- Grem, J.L.; King, S.A.; Wittes, R.E.; Leyland-Jones, B. The role of methotrexate in osteosarcoma. J. Natl. Cancer Inst. 1988, 80, 626–655. [Google Scholar] [CrossRef] [PubMed]
- Freed-Pastor, W.A.; Prives, C. Mutant p53: One name, many proteins. Genes Dev. 2012, 26, 1268–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Kua, H.Y.; Hu, Y.; Guo, K.; Zeng, Q.; Wu, Q.; Ng, H.H.; Karsenty, G.; De Crombrugghe, B.; Yeh, J.; et al. P53 Functions As a Negative Regulator of Osteoblastogenesis, Osteoblast-Dependent Osteoclastogenesis, and Bone Remodeling. J. Cell Biol. 2006, 172, 115–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladanyi, M.; Cha, C.; Lewis, R.; Jhanwar, S.C.; Huvos, A.G.; Healey, J.H. MDM2 Gene Amplification in Metastatic Osteosarcoma. Cancer Res. 1993, 53, 16–18. [Google Scholar]
- Lonardo, F.; Ueda, T.; Huvos, A.G.; Healey, J.; Ladanyi, M. p53 and MDM2 Alterations in Osteosarcomas. Cancer 1997, 79, 1541–1547. [Google Scholar] [CrossRef]
- Manfredi, J.J. The Mdm2–p53 relationship evolves: Mdm2 swings both ways as an oncogene and a tumor suppressor. Genes Dev. 2010, 24, 1580–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, A.; Ushiku, T.; Motoi, T.; Beppu, Y.; Fukayama, M.; Tsuda, H.; Shibata, T. MDM2 and CDK4 Immunohistochemical Coexpression in High-grade Osteosarcoma: Correlation With a Dedifferentiated Subtype. Am. J. Surg. Pathol. 2012, 36, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Wang, S.J.; Jiang, L.; Zheng, B.; Gu, W. p53 Protein-mediated regulation of phosphoglycerate dehydrogenase (PHGDH) is crucial for the apoptotic response upon serine starvation. J. Biol. Chem. 2015, 290, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Bykov, V.J.N.; Eriksson, S.E.; Bianchi, J.; Wiman, K.G. Targeting mutant p53 for efficient cancer therapy. Nat. Rev. Cancer 2018, 18, 89–102. [Google Scholar] [CrossRef]
- Maki, R.G. Gemcitabine and Docetaxel in Metastatic Sarcoma: Past, Present, and Future. Oncologist 2007, 12, 999–1006. [Google Scholar] [CrossRef] [Green Version]
- Zakeri-Milani, P.; Farkhani, S.M.; Shirani, A.; Mohammadi, S.; Mojarrad, J.S.; Akbari, J.; Valizadeh, H. Cellular Uptake and Anti-Tumor Activity of Gemcitabine Conjugated with New Amphiphilic Cell Penetrating Peptides. EXCLI J. 2017, 16, 650–662. [Google Scholar] [PubMed]
- Prudner, B.C.; Rathore, R.; Robinson, A.M.; Godec, A.; Chang, S.F.; Hawkins, W.G.; Hirbe, A.C.; Van Tine, B.A. Arginine starvation and docetaxel induce c-Myc–driven HENT1 surface expression to overcome gemcitabine resistance in ASS1-negative tumors. Clin. Cancer Res. 2019, 25, 5122–5134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bush, J.A.; Li, G. Cancer chemoresistance: The relationship between p53 and multidrug transporters. Int. J. Cancer 2002, 98, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Zawadzka, I.; Jeleń, A.; Pietrzak, J.; Żebrowska-Nawrocka, M.; Michalska, K.; Szmajda-Krygier, D.; Mirowski, M.; Łochowski, M.; Kozak, J.; Balcerczak, E. The impact of ABCB1 gene polymorphism and its expression on non-small-cell lung cancer development, progression and therapy—Preliminary report. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Li, Z.; Zhang, Q.; Bernstein, K.D.A.; Lozano-Calderon, S.; Choy, E.; Hornicek, F.J.; Duan, Z. Targeting ABCB1 (MDR1) in multi-drug resistant osteosarcoma cells using the CRISPR-Cas9 system to reverse drug resistance. Oncotarget 2016, 7, 83502–83513. [Google Scholar] [CrossRef] [Green Version]
- Xiaohui, S.; Aiguo, L.; Xiaolin, G.; Ying, L.; Hongxing, Z.; Yilei, Z. Effect of ABCB1 polymorphism on the clinical outcome of osteosarcoma patients after receiving chemotherapy. Pakistan J. Med. Sci. 2014, 30, 886–890. [Google Scholar] [CrossRef]
- Cathomas, R.; Rothermundt, C.; Bode, B.; Fuchs, B.; Von Moos, R.; Schwitter, M. RANK ligand blockade with denosumab in combination with sorafenib in chemorefractory osteosarcoma: A possible step forward? Oncology 2015, 88, 257–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, M.K.; Mukhopadhyay, A.K. Tyrosine kinase—Role and significance in Cancer. Int. J. Med. Sci. 2004, 1, 101–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schöffski, P.; Blay, J.Y.; Ray-Coquard, I. Cabozantinib as an emerging treatment for sarcoma. Curr. Opin. Oncol. 2020, 32, 321–331. [Google Scholar] [CrossRef]
- Italiano, A.; Mir, O.; Mathoulin-Pelissier, S.; Penel, N.; Piperno-Neumann, S.; Bompas, E.; Chevreau, C.; Duffaud, F.; Entz-Werlé, N.; Saada, E.; et al. Cabozantinib in patients with advanced Ewing sarcoma or osteosarcoma (CABONE): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 446–455. [Google Scholar] [CrossRef]
- Grignani, G.; Palmerini, E.; Ferraresi, V.; D’Ambrosio, L.; Bertulli, R.; Asaftei, S.D.; Tamburini, A.; Pignochino, Y.; Sangiolo, D.; Marchesi, E.; et al. Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: A non-randomised phase 2 clinical trial. Lancet Oncol. 2015, 16, 98–107. [Google Scholar] [CrossRef]
- Raciborska, A.; Bilska, K. Sorafenib in patients with progressed and refractory bone tumors. Med. Oncol. 2018, 35, 1–5. [Google Scholar] [CrossRef]
- Arora, A.; Scholar, E.M. Role of tyrosine kinase inhibitors in cancer therapy. J. Pharmacol. Exp. Ther. 2005, 315, 971–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Du, Y.; Ma, H.; Liang, Q.; Zhu, X.; Tian, J. Preclinical comparison of regorafenib and sorafenib efficacy for hepatocellular carcinoma using multimodality molecular imaging. Cancer Lett. 2019, 453, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Bolejack, V.; Ryan, C.W.; Ganjoo, K.N.; Loggers, E.T.; Chawla, S.; Agulnik, M.; Livingston, M.B.; Reed, D.; Keedy, V.; et al. Randomized double-blind phase II study of regorafenib in patients with metastatic osteosarcoma. J. Clin. Oncol. 2019, 37, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Thanindratarn, P.; Dean, D.C.; Nelson, S.D.; Hornicek, F.J.; Duan, Z. Advances in immune checkpoint inhibitors for bone sarcoma therapy. J. Bone Oncol. 2019, 15, 100221. [Google Scholar] [CrossRef]
- Shaikh, A.B.; Li, F.; Li, M.; He, B.; He, X.; Chen, G.; Guo, B.; Li, D.; Jiang, F.; Dang, L.; et al. Present advances and future perspectives of molecular targeted therapy for osteosarcoma. Int. J. Mol. Sci. 2016, 17, 506. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.M.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and Activity of Anti-PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- Xie, L.; Xu, J.; Sun, X.; Guo, W.; Gu, J.; Liu, K.; Zheng, B.; Ren, T.; Huang, Y.; Tang, X.; et al. Apatinib plus camrelizumab (anti-PD1 therapy, SHR-1210) for advanced osteosarcoma (APFAO) progressing after chemotherapy: A single-arm, open-label, phase 2 trial. J. Immunother. Cancer 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Perry, J.A.; Kiezun, A.; Tonzi, P.; Van Allen, E.M.; Carter, S.L.; Baca, S.C.; Cowley, G.S.; Bhatt, A.S.; Rheinbay, E.; Pedamallu, C.S.; et al. Complementary genomic approaches highlight the PI3K/mTOR pathway as a common vulnerability in osteosarcoma. Proc. Natl. Acad. Sci. USA 2014, 111, E5564–E5573. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Wang, X.; Proud, C.G. mTOR inhibitors in cancer therapy [version 1; referees: 3 approved]. F1000Research 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Leone, M.; Crowell, K.J.; Chen, J.; Jung, D.; Chiang, G.G.; Sareth, S.; Abraham, R.T.; Pellecchia, M. The FRB domain of mTOR: NMR solution structure and inhibitor design. Biochemistry 2006, 45, 10294–10302. [Google Scholar] [CrossRef] [PubMed]
- Rodrik-Outmezguine, V.S.; Chandarlapaty, S.; Pagano, N.C.; Poulikakos, P.I.; Scaltriti, M.; Moskatel, E.; Baselga, J.; Guichard, S.; Rosen, N. mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT signaling. Cancer Discov. 2011, 1, 248–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mita, M.M.; Tolcher, A.W. The role of mTOR inhibitors for treatment of sarcomas. Curr. Oncol. Rep. 2007, 9, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Congwei, L.; Bei, Q.; Tao, Y.; Ruiguo, W.; Heze, Y.; Bo, D.; Zhihong, L. mTOR: An attractive therapeutic target for osteosarcoma? Oncotarget 2016, 7, 50805–50813. [Google Scholar] [CrossRef] [Green Version]
- Charest, M.; Hickeson, M.; Lisbona, R.; Novales-Diaz, J.A.; Derbekyan, V.; Turcotte, R.E. FDG PET/CT imaging in primary osseous and soft tissue sarcomas: A retrospective review of 212 cases. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1944–1951. [Google Scholar] [CrossRef]
- Tsiambas, E.; Fotiades, P.P.; Sioka, C.; Kotrotsios, D.; Gkika, E.; Fotopoulos, A.; Mastronikolis, S.N.; Armata, I.E.; Giotakis, E.; Ragos, V. Novel molecular and metabolic aspects in osteosarcoma. J. BUON 2017, 22, 1595–1598. [Google Scholar]
- Yang, J.; Cheng, D.; Zhou, S.; Zhu, B.; Hu, T.; Yang, Q. Overexpression of X-Box binding protein 1 (XBP1) correlates to poor prognosis and up-regulation of PI3K/mTOR in human osteosarcoma. Int. J. Mol. Sci. 2015, 16, 28635–28646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Sun, X.; Huo, C.; Sun, C.; Zhu, J. Monocarboxylate Transporter 4 (MCT4) Overexpression Is Correlated with Poor Prognosis of Osteosarcoma. Med. Sci. Monit. 2019, 25, 4278–4284. [Google Scholar] [CrossRef] [PubMed]
- Amorim, R.; Pinheiro, C.; Miranda-Gonçalves, V.; Pereira, H.; Moyer, M.P.; Preto, A.; Baltazar, F. Monocarboxylate transport inhibition potentiates the cytotoxic effect of 5-fluorouracil in colorectal cancer cells. Cancer Lett. 2015, 365, 68–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacold, M.E.; Brimacombe, K.R.; Chan, S.H.; Rohde, J.M.; Lewis, C.A.; Swier, L.J.Y.M.; Possemato, R.; Chen, W.W.; Sullivan, L.B.; Fiske, B.P.; et al. A PHGDH inhibitor reveals coordination of serine synthesis and one-carbon unit fate. Nat. Chem. Biol. 2016, 12, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Issaq, S.H.; Mendoza, A.; Kidner, R.; Rosales, T.; Duveau, D.Y.; Heske, C.M.; Rohde, J.M.; Boxer, M.B.; Thomas, C.J.; DeBerardinis, R.J.; et al. EWS-FLI1-regulated serine synthesis and exogenous serine are necessary for Ewing sarcoma cellular proliferation and tumor growth. Mol. Cancer Ther. 2020. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.C.; Maddocks, O.D.K. Serine and Functional Metabolites in Cancer. Trends Cell Biol. 2017, 27, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.P.M.; Thomas, D.G.; Giordano, T.J.; Baker, L.H.; McDonagh, K.T. Cell Surface Expression of Epidermal Growth Factor Receptor and Her-2 with Nuclear Expression of Her-4 in Primary Osteosarcoma. Cancer Res. 2004, 64, 2047–2053. [Google Scholar] [CrossRef] [Green Version]
- Gorlick, R.; Huvos, A.G.; Heller, G.; Aledo, A.; Beardsley, G.P.; Healey, J.H.; Meyers, P.A. Expression of HER2/erbB-2 Correlates With Survival in Osteosarcoma. J. Clin. Oncol. 1999, 17, 2781–2788. [Google Scholar] [CrossRef]
- Ebb, D.; Meyers, P.; Grier, H.; Bernstein, M.; Gorlick, R.; Lipshultz, S.E.; Krailo, M.; Devidas, M.; Barkauskas, D.A.; Siegal, G.P.; et al. Phase II trial of trastuzumab in combination with cytotoxic chemotherapy for treatment of metastatic osteosarcoma with human epidermal growth factor receptor 2 overexpression: A report from the children’s oncology group. J. Clin. Oncol. 2012, 30, 2545–2551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Castro, J.; Trigueros, C.; Madrenas, J.; Pérez-Simón, J.A.; Rodriguez, R.; Menendez, P. Mesenchymal stem cells and their use as cell replacement therapy and disease modelling tool. J. Cell. Mol. Med. 2008, 12, 2552–2565. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Identifier | Study Title | Status |
|---|---|---|
| NCT00470223 | Combined chemotherapy with or without zoledronic acid for patients with osteosarcoma | Active, not recruiting |
| NCT00788125 | Dasatinib, ifosfamide, carboplatin, and etoposide in treating young patients with metastatic or recurrent malignant solid tumors | Active, not recruiting |
| NCT01459484 | ABCB1/P-glycoprotein expression as biologic stratification factor for patients with non metastatic osteosarcoma (ISG/OS-2) | Active, not recruiting |
| NCT01661400 | Anti-angiogenic therapy post transplant (ASCR) for pediatric solid tumors | Recruiting |
| NCT01669369 | Clinical trial of lithium carbonate combined with neo-adjuvant chemotherapy to treat osteosarcoma (Li2CO3) | Recruiting |
| NCT01833520 | Phase I dose escalation of monthly intravenous Ra-223 dichloride in osteosarcoma | Active, not recruiting |
| NCT01953900 | iC9-GD2-CAR-VZV-CTLs/refractory or metastatic GD2-positive sarcoma and neuroblastoma | Active, not recruiting |
| NCT02013336 | Phase 1 study of MM-398 plus cyclophosphamide in pediatric solid tumors | Recruiting |
| NCT02173093 | Activated T cells armed with GD2 bispecific antibody in children and young adults with neuroblastoma and osteosarcoma | Recruiting |
| NCT02243605 | Cabozantinib S-malate in treating patients with relapsed osteosarcoma or ewing sarcoma | Active, not recruiting |
| NCT02357810 | Pazopanib hydrochloride and topotecan hydrochloride in treating patients with metastatic soft tissue and bone sarcomas | Active, not recruiting |
| NCT02389244 | A Phase II study evaluating efficacy and safety of regorafenib in patients with metastatic bone sarcomas | Recruiting |
| NCT02406781 | Combination of MK3475 and metronomic cyclophosphamide in patients with advanced sarcomas: multicentre phase II trial | Recruiting |
| NCT02432274 | Study of lenvatinib in children and adolescents with refractory or relapsed solid malignancies and young adults with osteosarcoma | Active, not recruiting |
| NCT02470091 | Denosumab in treating patients with recurrent or refractory osteosarcoma | Active, not recruiting |
| NCT02484443 | Dinutuximab in combination with sargramostim in treating patients with recurrent osteosarcoma | Active, not recruiting |
| NCT02502786 | Humanized monoclonal antibody 3F8 (Hu3F8) with granulocyte-macrophage Colony stimulating factor (GM-CSF) in the treatment of recurrent osteosarcoma | Recruiting |
| NCT02517918 | Metronomic chemotherapy in patients with advanced solid tumor with bone metastasis and advanced pretreated osteosarcoma | Recruiting |
| NCT02811523 | In vivo lung perfusion for pulmonary metastases of sarcoma | Recruiting |
| NCT02867592 | Cabozantinib-S-Malate in treating younger patients with recurrent, refractory, or newly diagnosed sarcomas, wilms tumor, or other rare tumors | Active, not recruiting |
| NCT02945800 | Nab-paclitaxel and gemcitabine for recurrent/refractory sarcoma | Recruiting |
| NCT03006848 | A phase II trial of avelumab in patients with recurrent or progressive osteosarcoma | Active, not recruiting |
| NCT03063983 | Clinical trial evaluating metronomic chemotherapy in patients with metastatic osteosarcoma (GLATO2017) | Recruiting |
| NCT03277924 | Trial of sunitinib plus nivolumab after standard treatment in advanced soft tissue and bone sarcomas | Recruiting |
| NCT03449108 | LN-145 or LN-145-S1 in treating patients with relapsed or refractory ovarian cancer, anaplastic thyroid cancer, osteosarcoma, or other bone and soft tissue sarcomas | Recruiting |
| NCT03449901 | ADI-PEG 20 in combination with gemcitabine and docetaxel for the treatment of soft tissue sarcoma, osteosarcoma, ewing’s sarcoma, and small cell lung cancer | Recruiting |
| NCT03478462 | Dose escalation study of CLR 131 in children and adolescents with relapsed or refractory malignant tumors including but not limited to neuroblastoma, rhabdomyosarcoma, ewings sarcoma, and osteosarcoma | Recruiting |
| NCT03598595 | Gemcitabine, docetaxel, and hydroxychloroquine in treating participants with recurrent or refractory osteosarcoma | Recruiting |
| NCT03618381 | EGFR806 CAR T cell immunotherapy for recurrent/refractory solid tumors in children and young adults | Recruiting |
| NCT03628209 | Nivolumab or nivolumab and azacitidine in patients with recurrent, resectable osteosarcoma | Recruiting |
| NCT03643133 | Mifamurtide combined with post-operative chemotherapy for newly diagnosed high risk osteosarcoma patients (SARCOME13) | Recruiting |
| NCT03676985 | A clinical study of PD-L1 antibody ZKAB001(Drug Code) in limited stage of high-grade osteosarcoma | Recruiting |
| NCT03718091 | M6620 (VX-970) in selected solid tumors | Recruiting |
| NCT03742193 | Pulmonary resectable metastases of osteosarcoma with apatinib and chemotherapy | Recruiting |
| NCT03860207 | Study of the safety and efficacy of humanized 3F8 bispecific antibody (Hu3F8-BsAb) in patients with relapsed/refractory neuroblastoma, osteosarcoma and other solid tumor cancers | Recruiting |
| NCT03900793 | Losartan + sunitinib in treatment of osteosarcoma | Recruiting |
| NCT03932071 | Zoledronic acid in decrease the lung metastatic rate of osteosarcoma | Recruiting |
| NCT03960177 | Glucarpidase after high-dose methotrexate in patients with osteosarcoma | Recruiting |
| NCT04040205 | Abemaciclib for bone and soft tissue sarcoma with cyclin-dependent kinase (CDK) pathway alteration | Recruiting |
| NCT04055220 | Efficacy and safety of regorafenib as maintenance therapy after first-line treatment in patients with bone sarcomas | Recruiting |
| NCT04154189 | A Study to compare the efficacy and safety of ifosfamide and etoposide with or without lenvatinib in children, adolescents and young adults with relapsed and refractory osteosarcoma | Recruiting |
| NCT04183062 | BIO-11006 for osteosarcoma and ewing’s sarcoma lung metastases | Recruiting |
| NCT04294511 | Study of camrelizumab in combination with neoadjuvant chemotherapy in the treatment of osteosarcoma | Recruiting |
| NCT04351308 | Comparison of MAPI + camrelizumab versus API + apatinib versus MAPI in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma | Recruiting |
| NCT04383288 | ABCB1/P-glycoprotein expression influence on non-metastatic osteosarcoma of the extremities | Recruiting |
| NCT04433221 | Combination immunotherapy targeting sarcomas | Recruiting |
| NCT04483778 | B7H3 CAR T cell immunotherapy for recurrent/refractory solid tumors in children and young adults | Recruiting |
| NCT04595994 | Selinexor plus gemcitabine in selected advanced soft-tissue sarcoma and osteosarcoma | Recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rathore, R.; Van Tine, B.A. Pathogenesis and Current Treatment of Osteosarcoma: Perspectives for Future Therapies. J. Clin. Med. 2021, 10, 1182. https://doi.org/10.3390/jcm10061182
Rathore R, Van Tine BA. Pathogenesis and Current Treatment of Osteosarcoma: Perspectives for Future Therapies. Journal of Clinical Medicine. 2021; 10(6):1182. https://doi.org/10.3390/jcm10061182
Chicago/Turabian StyleRathore, Richa, and Brian A. Van Tine. 2021. "Pathogenesis and Current Treatment of Osteosarcoma: Perspectives for Future Therapies" Journal of Clinical Medicine 10, no. 6: 1182. https://doi.org/10.3390/jcm10061182
APA StyleRathore, R., & Van Tine, B. A. (2021). Pathogenesis and Current Treatment of Osteosarcoma: Perspectives for Future Therapies. Journal of Clinical Medicine, 10(6), 1182. https://doi.org/10.3390/jcm10061182







