The GERtality Score: The Development of a Simple Tool to Help Predict in-Hospital Mortality in Geriatric Trauma Patients
Abstract
:1. Introduction
2. Experimental Section
2.1. The TraumaRegister DGU®
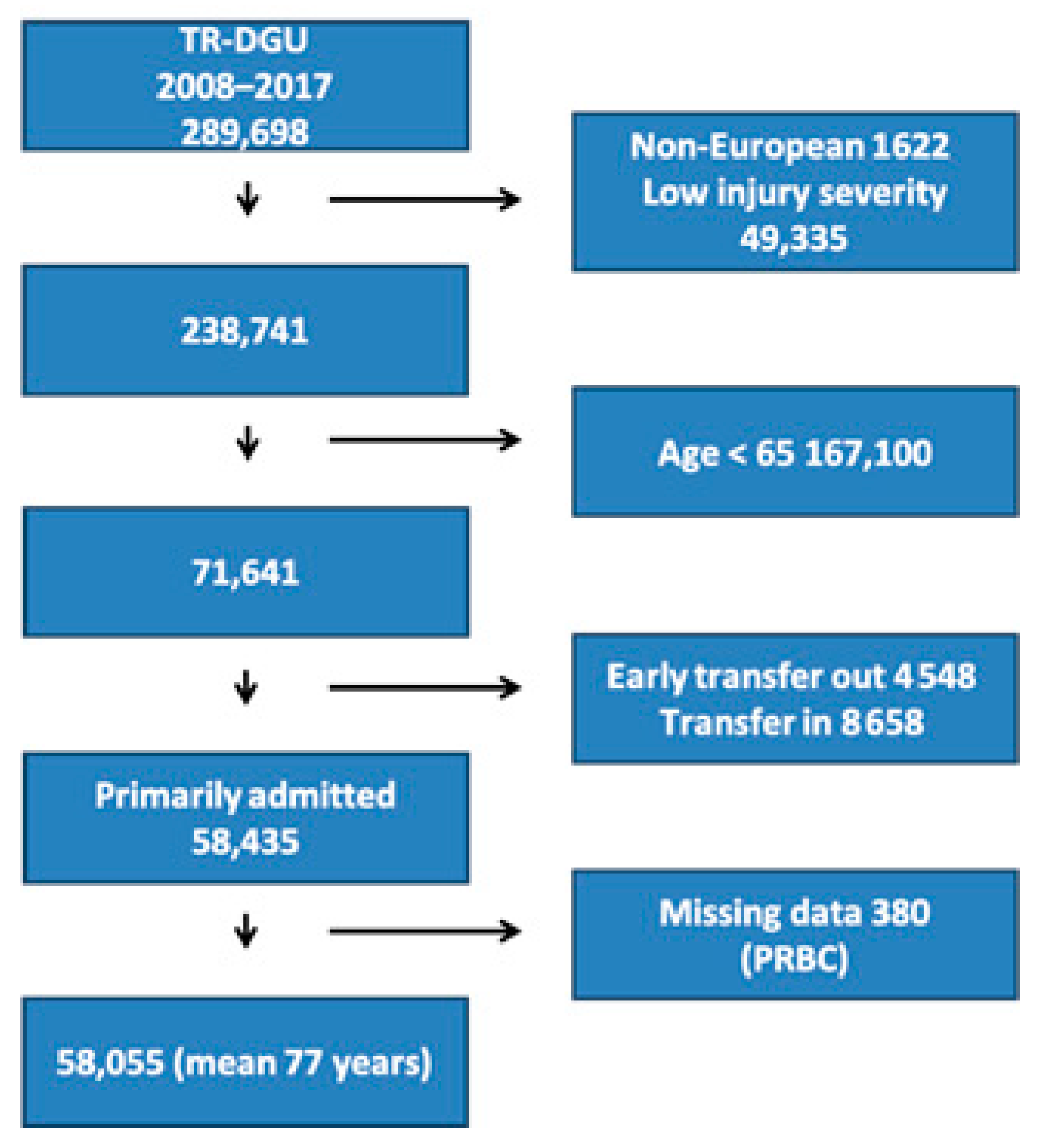
2.2. Inclusion and Exclusion Criteria
2.3. Statistical Analysis
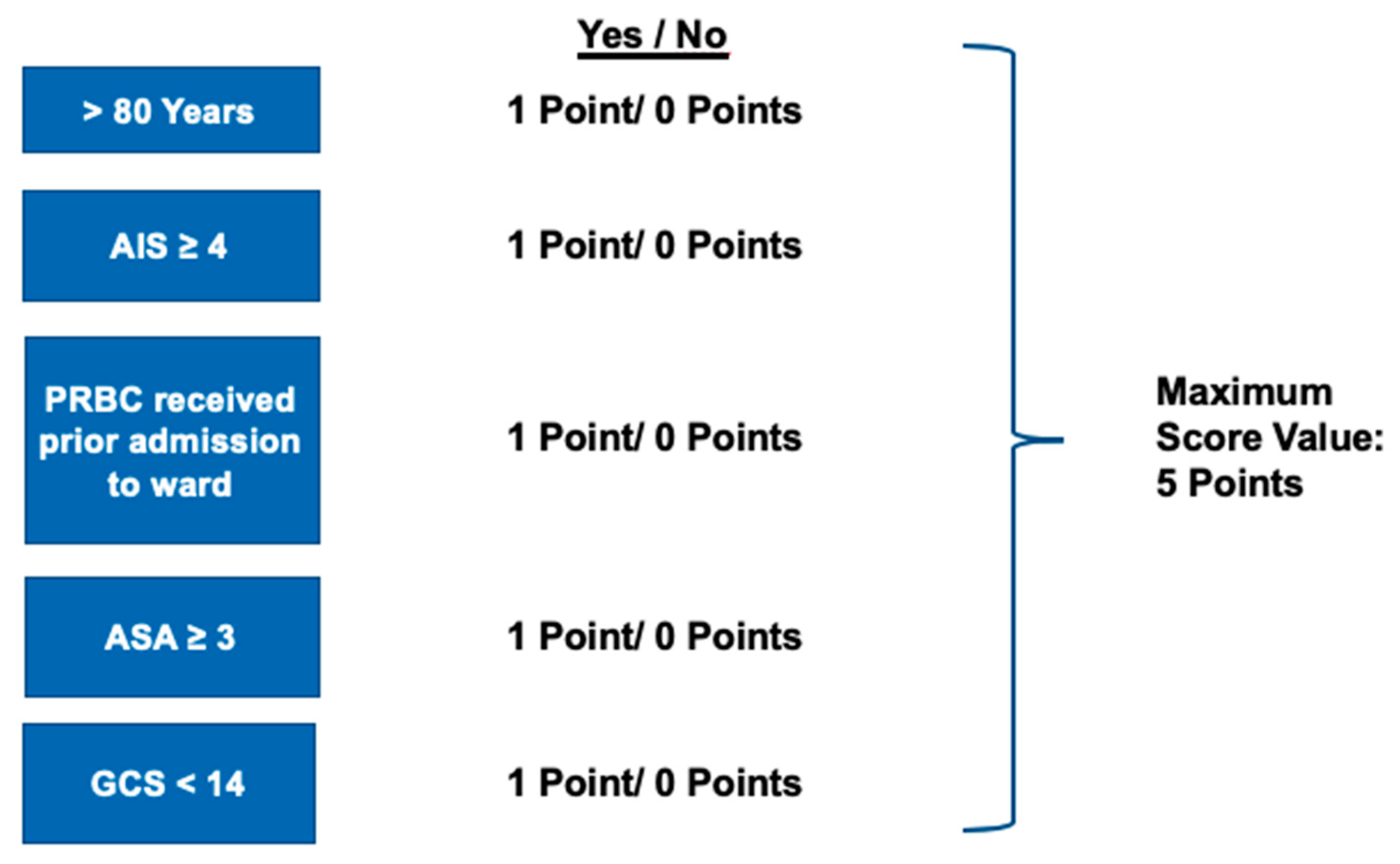
3. Results
- Age ≥ 80 years
- Maximum AIS in any body region ≥4
- PRBCs received prior to admission to the ICU
- ASA ≥ 3
- GCS < 14
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Ageing and Health; 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 16 December 2020).
- Hukkelhoven, C.W.P.M.; Steyerberg, E.W.; Rampen, A.J.J.; Farace, E.; Habbema, J.D.F.; Marshall, L.F.; Murray, G.D.; Maas, A.I.R. Patient age and Outcome FOLLOWING Severe Traumatic brain Injury: An Analysis of 5600 patients. J. Neurosurg. 2003, 99, 666–673. [Google Scholar] [CrossRef]
- Demetriades, D.; Sava, J.; Alo, K.; Newton, E.; Velmahos, G.C.; Murray, J.A.; Belzberg, H.; Asensio, J.A.; Berne, T.V. Old age as a criterion for trauma team activation. J. Trauma 2001, 51, 754–756; discussion 756–757. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Zangbar, B.; Pandit, V.; Kulvatunyou, N.; Haider, A.; O’Keeffe, T.; Khalil, M.; Tang, A.; Vercruysse, G.; Gries, L.; et al. Mortality after trauma laparotomy in geriatric patients. J. Surg. Res. 2014, 190, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Con, J.; Friese, R.S.; Long, D.M.; Zangbar, B.; O’Keeffe, T.; Joseph, B.; Rhee, P.; Tang, A.L. Falls from ladders: Age matters more than height. J. Surg. Res. 2014, 191, 262–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterer, L.; Ossendorf, C.; Jensen, K.O.; Osterhoff, G.; Mica, L.; Seifert, B.; Werner, C.M.L.; Simmen, H.-P.; Pape, H.-C.; Sprengel, K. Implementation of new standard operating procedures for geriatric trauma patients with multiple injuries: A single level I trauma centre study. BMC Geriatr. 2019, 19, 359. [Google Scholar] [CrossRef] [PubMed]
- Champion, H.R.; Copes, W.S.; Sacco, W.J.; Lawnick, M.M.; Keast, S.L.; Bain, L.W.; E Flanagan, M.; Frey, C.F. The Major Trauma Outcome Study: Establishing national norms for trauma care. J. Trauma 1990, 30, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Maduz, R.; Kugelmeier, P.; Meili, S.; Döring, R.; Meier, C.; Wahl, P. Major influence of interobserver reliability on polytrauma identification with the Injury Severity Score (ISS): Time for a centralised coding in trauma registries? Injury 2017, 48, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Pothmann, C.E.M.; Baumann, S.; Jensen, K.O.; Mica, L.; Osterhoff, G.; Simmen, H.-P.; Sprengel, K. Assessment of polytraumatized patients according to the Berlin Definition: Does the addition of physiological data really improve interobserver reliability? PLoS ONE 2018, 13, e0201818. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.Z.; Wolf, S.E.; Nakonezny, P.A.; Minhajuddin, A.; Rhodes, R.L.; Paulk, M.E.; Phelan, H.A. Estimating Geriatric Mortality after Injury Using Age, Injury Severity, and Performance of a Transfusion: The Geriatric Trauma Outcome Score. J. Palliat. Med. 2015, 18, 677–681. [Google Scholar] [CrossRef]
- Lefering, R.; Huber-Wagner, S.; Nienaber, U.; Maegele, M.; Bouillon, B. Update of the trauma risk adjustment model of the TraumaRegister DGU: The Revised Injury Severity Classification, version II. Crit Care 2014, 18, 476. [Google Scholar] [CrossRef] [Green Version]
- Deutsche Gesellschaft für Orthopädie und Unfallchirurgie. Traumaregister DGU®. 2019. Available online: www.traumaregister-dgu.de (accessed on 30 November 2020).
- Haasper, C.; Junge, M.; Ernstberger, A.; Brehme, H.; Hannawald, L.; Langer, C.; Nehmzow, J.; Otte, D.; Sander, U.; Krettek, C.; et al. The Abbreviated Injury Scale (AIS). Options and problems in application. Unfallchirurg 2010, 113, 366–372. [Google Scholar] [PubMed]
- Doyle, D.J.; Garmon, E.H. American Society of Anesthesiologists Classification (ASA Class); StatPearls: Treasure Island, FL, USA, 2019. [Google Scholar]
- Abu-Hanna, A.; Lucas, P.J. Prognostic models in medicine. AI and statistical approaches. Methods Inf. Med. 2001, 40, 1–5. [Google Scholar]
- Laun, R.A.; Schröder, O.; Schoppnies, M.; Röher, H.D.; Ekkernkamp, A.; Schulte, K.M. Transforming growth factor-beta1 and major trauma: Time-dependent association with hepatic and renal insufficiency. Shock 2003, 19, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.P.; Draper, E.A. Acute physiology and chronic health evaluation (APACHE II) and Medicare reimbursement. Health Care Financ. Rev. 1984, Suppl, 91–105. [Google Scholar]
- Boyd, C.R.; Tolson, M.A.; Copes, W.S. Evaluating trauma care: The TRISS method. Trauma Score and the Injury Severity Score. J. Trauma 1987, 27, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Champion, H.R.; Sacco, W.J.; Copes, W.S.; Gann, D.S.; Gennarelli, T.A.; Flanagan, M.E. A revision of the Trauma Score. J. Trauma 1989, 29, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Lefering, R. Development and validation of the revised injury severity classification score for severely injured patients. Eur. J. Trauma Emerg. Surg. 2009, 35, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.S.; Milia, D.; Glover, J.; Napolitano, L.M.; Chen, B.; Lindemann, E.; Hemmila, M.R.; Stein, D.; Kummerfeld, E.; Chipman, J.; et al. Predictors of elderly mortality after trauma: A novel outcome score. J. Trauma Acute Care Surg. 2020, 88, 416–424. [Google Scholar] [CrossRef]
- O’Reilly, D.; Mahendran, K.; West, A.; Shirley, P.; Walsh, M.; Tai, N. Opportunities for improvement in the management of patients who die from haemorrhage after trauma. Br. J. Surg. 2013, 100, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Lustenberger, T.; Talving, P. Focus on challenges and advances in the treatment of patients with penetrating injuries. Eur. J. Trauma Emerg. Surg. 2016, 42, 661–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, B.M.; Kornblith, L.Z.; Conroy, A.S.; Burlew, C.C.; Wagenaar, A.E.; Chouliaras, K.; Hill, J.R.; Carrick, M.M.; Mallory, G.R.; Watkins, J.R.; et al. The found down patient: A Western Trauma Association multicenter study. J. Trauma Acute Care Surg. 2015, 79, 976–982. [Google Scholar] [CrossRef] [Green Version]
- Randolph, A.G.; Guyatt, G.H.; Richardson, W.S. Prognosis in the intensive care unit: Finding accurate and useful estimates for counseling patients. Crit. Care Med. 1998, 26, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Matthes, G.; Seifert, J.; Bogatzki, S.; Steinhage, K.; Ekkernkamp, A.; Stengel, D. Age and survival likelihood of polytrauma patients. “Local tailoring” of the DGU prognosis model. Unfallchirurg 2005, 108, 288–292. [Google Scholar]
- Di Saverio, S.; Gambale, G.; Coccolini, F.; Catena, F.; Giorgini, E.; Ansaloni, L.; Amadori, N.; Coniglio, C.; Giugni, A.; Biscardi, A.; et al. Changes in the outcomes of severe trauma patients from 15-year experience in a Western European trauma ICU of Emilia Romagna region (1996–2010). A population cross-sectional survey study. Langenbecks Arch. Surg. 2014, 399, 109–126. [Google Scholar] [CrossRef]
- Broos, P.L.O.; D’Hoore, A.; Vanderschot, P.; Rommens, P.M.; Stappaerts, K.H. Multiple trauma in elderly patients. Factors influencing outcome: Importance of aggressive care. Injury 1993, 24, 365–368. [Google Scholar] [PubMed]
- Broos, P.L.; D’Hoore, A.; Vanderschot, P.; Rommens, P.M.; Stappaerts, K.H. Multiple trauma in patients of 65 and over. Injury patterns. Factors influencing outcome. The importance of an aggressive care. Acta Chir. Belg. 1993, 93, 126–130. [Google Scholar] [PubMed]
- Dzankic, S.; Pastor, D.; Gonzalez, C.; Leung, J.M. The prevalence and predictive value of abnormal preoperative laboratory tests in elderly surgical patients. Anesth Analg. 2001, 93, 301–308. [Google Scholar]
- Frankenfield, D.; Cooney, R.N.; Smith, J.S.; Rowe, W.A. Age-related differences in the metabolic response to injury. J. Trauma 2000, 48, 49–56; discussion 56–57. [Google Scholar] [CrossRef]
- Knies, R.C., Jr. Assessment in geriatric trauma: What you need to know. Int. J. Trauma Nurs. 1996, 2, 85–91. [Google Scholar] [CrossRef]
- Leung, J.M.; Dzankic, S. Relative importance of preoperative health status versus intraoperative factors in predicting postoperative adverse outcomes in geriatric surgical patients. J. Am. Geriatr. Soc. 2001, 49, 1080–1085. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, G.; Leng, S.X. Frailty syndrome: An overview. Clin. Interv. Aging 2014, 9, 433–441. [Google Scholar] [PubMed] [Green Version]
- Di Saverio, S.; Birindelli, A.; Podda, M.; Segalini, E.; Piccinini, A.; Coniglio, C.; Frattini, C.; Tugnoli, G. Trauma laparoscopy and the six w’s: Why, where, who, when, what, and how? J. Trauma Acute Care Surg. 2019, 86, 344–367. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Hadley, E.C.; Walston, J.D.; Newman, A.B.; Guralnik, J.M.; Studenski, S.; Harris, T.B.; Ershler, W.B.; Ferrucci, L. From bedside to bench: Research agenda for frailty. Sci. Aging Knowl. Environ. 2005, 2005, pe24. [Google Scholar] [CrossRef] [PubMed]
- Romero-Ortuno, R.; Kenny, R.A. The frailty index in Europeans: Association with age and mortality. Age Ageing 2012, 41, 684–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ang, D.; Kurek, S.; McKenney, M.; Norwood, S.; Kimbrell, B.; Barquist, E.; Liu, H.; O’Dell, A.; Ziglar, M.; Hurst, J. Outcomes of Geriatric Trauma Patients on Preinjury Anticoagulation: A Multicenter Study. Am. Surg. 2017, 83, 527–535. [Google Scholar] [CrossRef] [PubMed]


| Measurement | Unit | Value |
|---|---|---|
| Age | years | 77.2/77 (7.6) |
| Male sex | n (%) | 33,483 (57.8%) |
| Injury Severity Score (ISS) | points | 19.2/17 (11.8) |
| Number of injuries | n | 4.2/4 (2.6) |
| Penetrating trauma | n (%) | 1426 (2.6%) |
| Mechanism: traffic | n (%) | 19,910 (35.1%) |
| Mechanism: low fall (<3 m) | n (%) | 25,218 (45.0%) |
| Mechanism: high fall | n (%) | 7727 (13.6%) |
| Severe head injury (AIS ≥ 3) | n (%) | 26,504 (45.7%) |
| Treated on intensive care unit | n (%) | 51,166 (88.1%) |
| Ventilated on ICU | n (%) | 22,486 (38.7%) |
| Length of stay in hospital | days | 16.6/12 (16.7) |
| Hospital mortality | n (%) | 12,969 (22.3%) |
| Variable | Subgroups | No. of Patients | Mortality Rate | Odds Ratio (OR) | 95% CI of OR |
|---|---|---|---|---|---|
| Age | ≥80 years <80 years | 21,810 (38%) 36,245 | 31.5% 16.8% | 2.27 | 2.18–2.36 |
| Max. AIS | 4 or more 2–3 | 25,924 (45%) 32,131 | 38.7% 9.2% | 6.25 | 5.97–6.54 |
| Blood transfusion | yes no | 4813 (8%) 53,242 | 43.4% 20.4% | 2.99 | 2.81–3.17 |
| ASA | 3/4 1/2 | 20,235 (41%) 28,269 | 29.1% 16.4% | 2.09 | 2.00–2.18 |
| GCS | 3–13 14–15 | 22,559 (41%) 32,719 | 42.2% 8.8% | 7.61 | 7.27–7.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scherer, J.; Kalbas, Y.; Ziegenhain, F.; Neuhaus, V.; Lefering, R.; Teuben, M.; Sprengel, K.; Pape, H.-C.; Jensen, K.O. The GERtality Score: The Development of a Simple Tool to Help Predict in-Hospital Mortality in Geriatric Trauma Patients. J. Clin. Med. 2021, 10, 1362. https://doi.org/10.3390/jcm10071362
Scherer J, Kalbas Y, Ziegenhain F, Neuhaus V, Lefering R, Teuben M, Sprengel K, Pape H-C, Jensen KO. The GERtality Score: The Development of a Simple Tool to Help Predict in-Hospital Mortality in Geriatric Trauma Patients. Journal of Clinical Medicine. 2021; 10(7):1362. https://doi.org/10.3390/jcm10071362
Chicago/Turabian StyleScherer, Julian, Yannik Kalbas, Franziska Ziegenhain, Valentin Neuhaus, Rolf Lefering, Michel Teuben, Kai Sprengel, Hans-Christoph Pape, and Kai Oliver Jensen. 2021. "The GERtality Score: The Development of a Simple Tool to Help Predict in-Hospital Mortality in Geriatric Trauma Patients" Journal of Clinical Medicine 10, no. 7: 1362. https://doi.org/10.3390/jcm10071362
APA StyleScherer, J., Kalbas, Y., Ziegenhain, F., Neuhaus, V., Lefering, R., Teuben, M., Sprengel, K., Pape, H.-C., & Jensen, K. O. (2021). The GERtality Score: The Development of a Simple Tool to Help Predict in-Hospital Mortality in Geriatric Trauma Patients. Journal of Clinical Medicine, 10(7), 1362. https://doi.org/10.3390/jcm10071362







