High Plasma Cystine Levels Are Associated with Blood Pressure and Reversed by CPAP in Patients with Obstructive Sleep Apnea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Inpatient Polysomnography
2.3. Blood Parameters
2.4. Statistics
3. Results
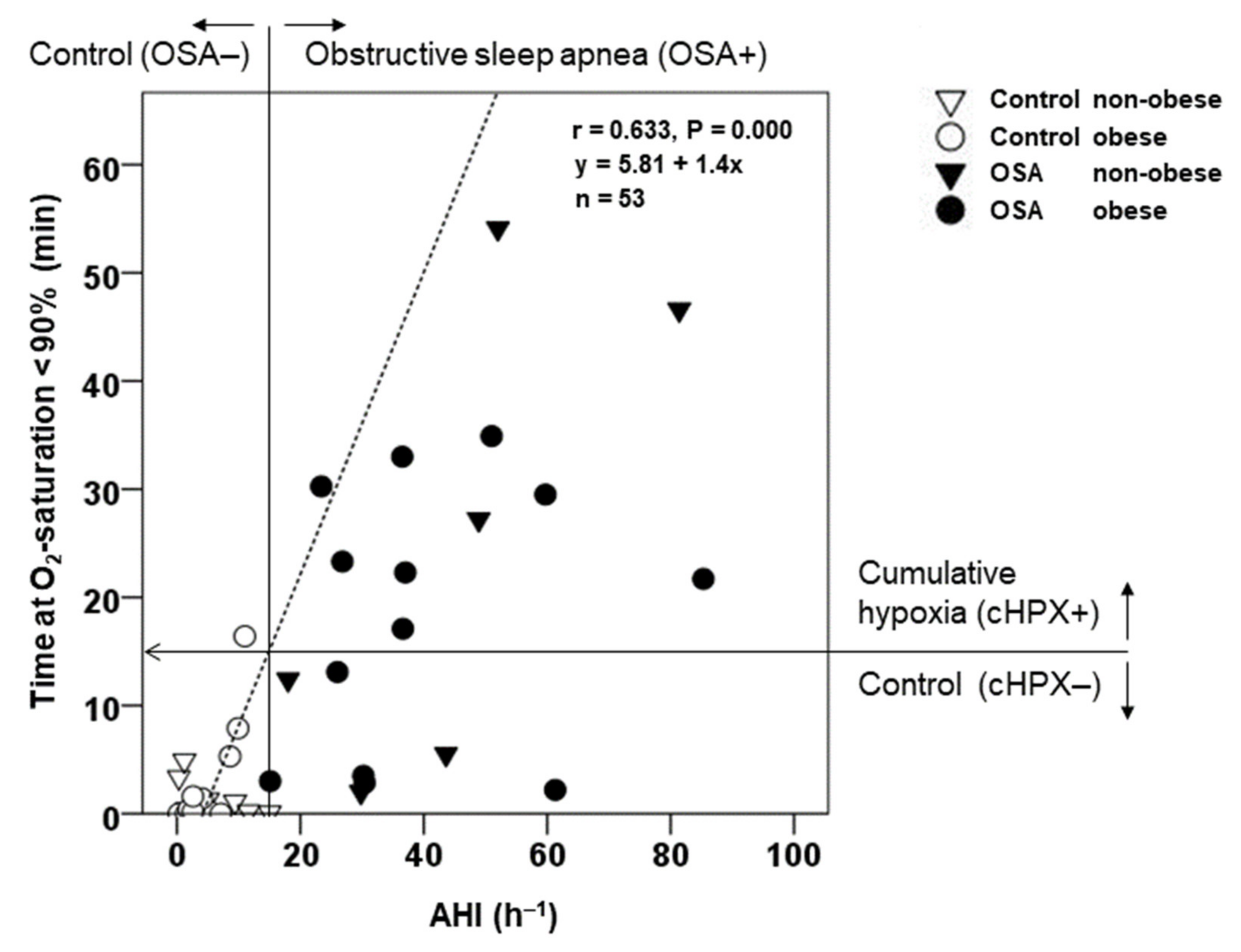
3.1. Epidemiology, Polysomnography, and Cardiovascular Risk Profile at Baseline
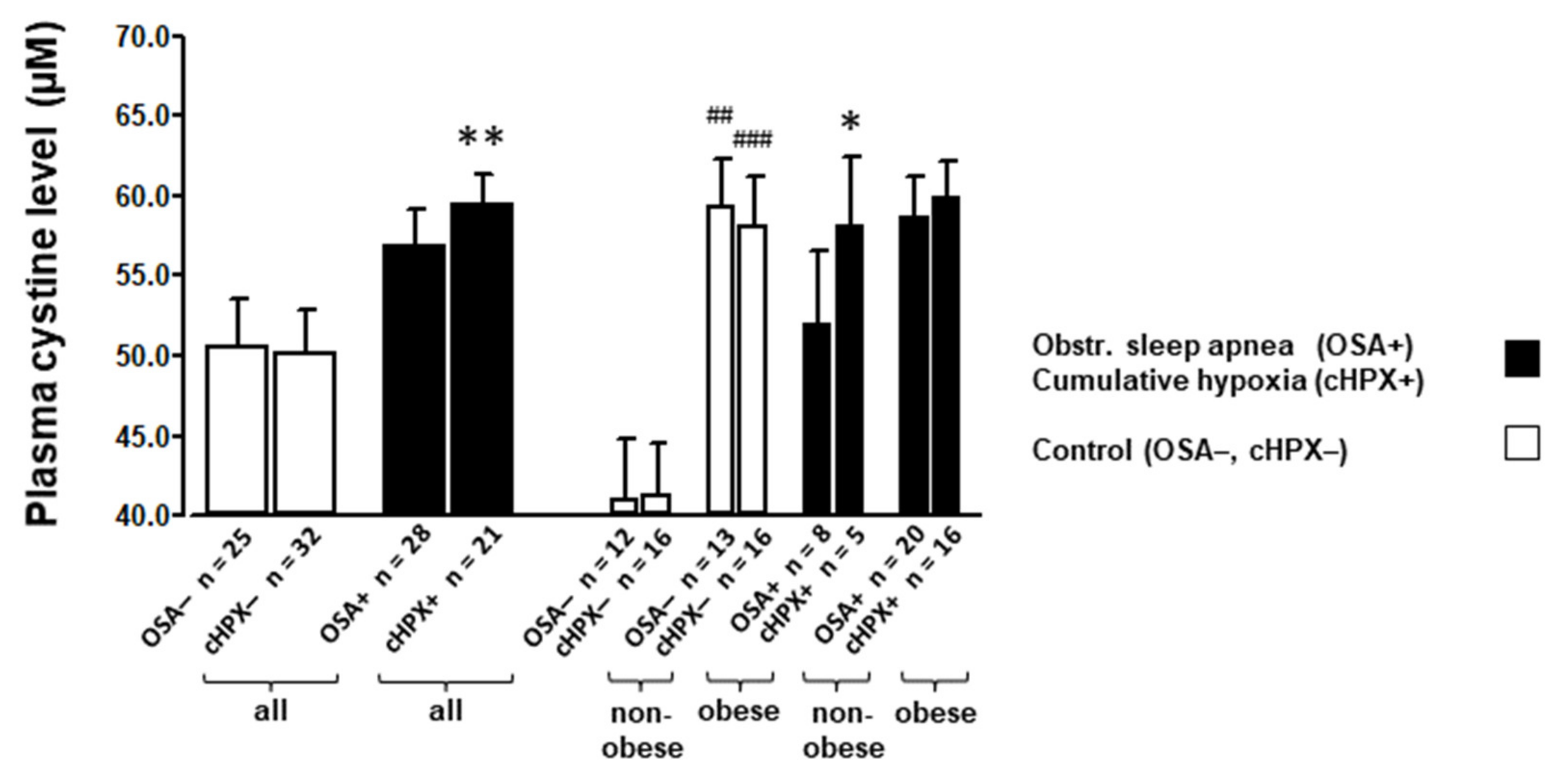
3.2. Amino Acids and Glutathione Levels at Baseline
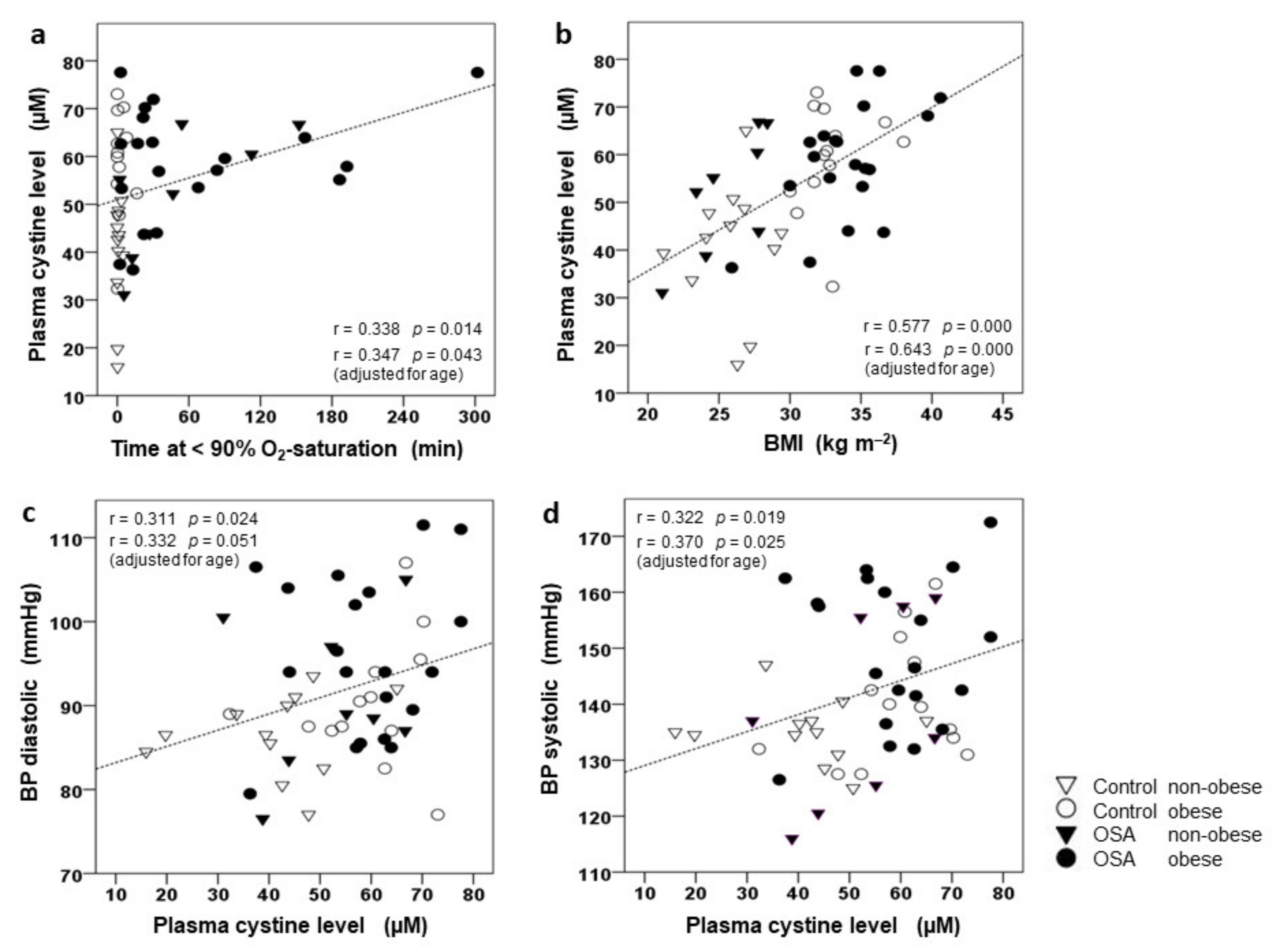
3.3. Correlation of CySS and Glutamate to BMI, AHI, and BP
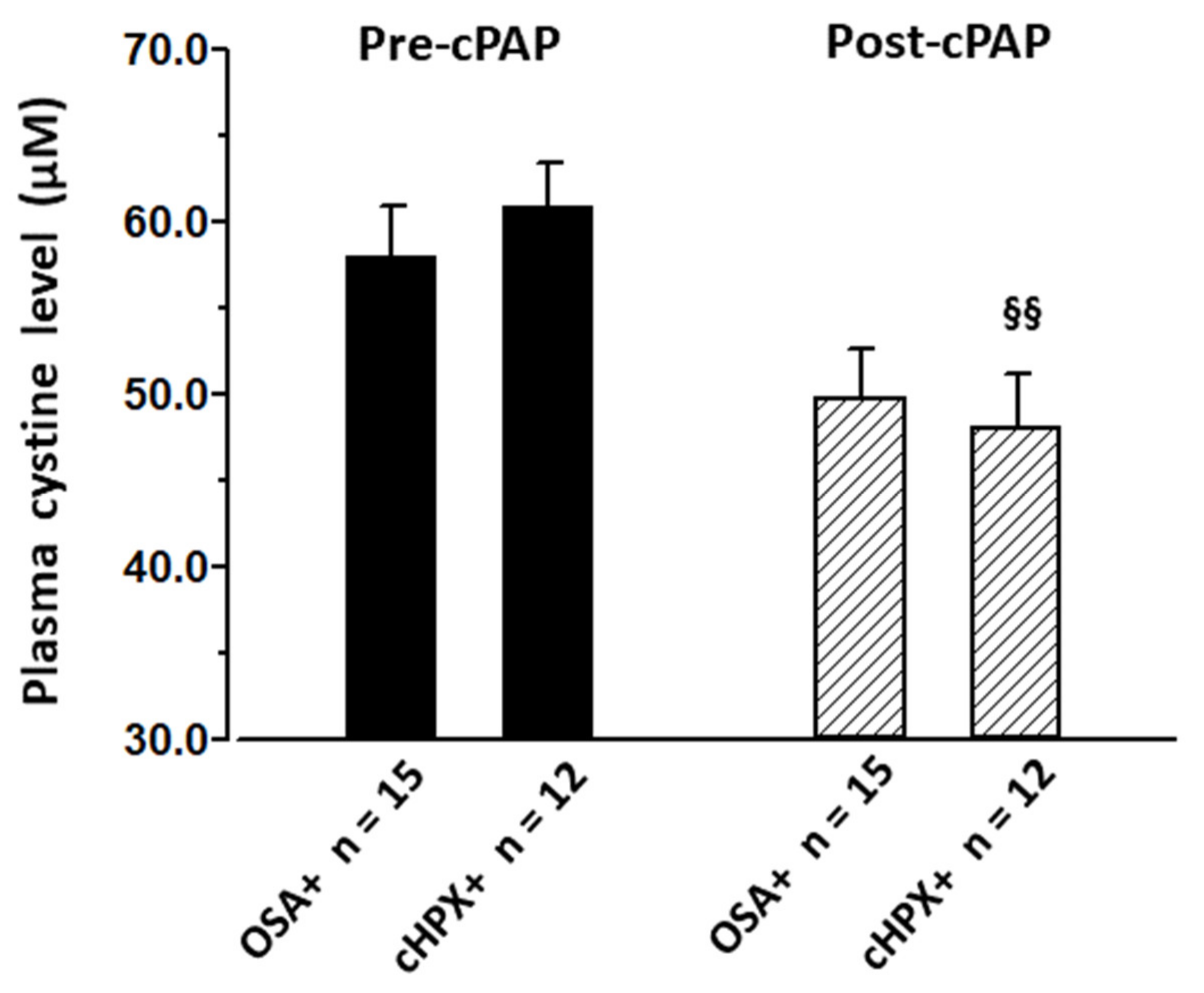
3.4. Effect of CPAP on CySS and Cardiovascular Risk Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lévy, P.; Kohler, M.; McNicholas, W.T.; Barbé, F.; McEvoy, R.D.; Somers, V.K.; Lavie, L.; Pépin, J.-L. Obstructive sleep apnoea syndrome. Nat. Rev. Dis. Primers 2015, 1, 15015. [Google Scholar] [CrossRef]
- Bauters, F.; Rietzschel, E.R.; Hertegonne, K.B.C.; Chirinos, J.A. The link between obstructive sleep apnea and cardiovascular disease. Curr. Atheroscler. Rep. 2016, 18, 1. [Google Scholar] [CrossRef]
- Marshall, N.S.; Wong, K.K.; Cullen, S.R.; Knuiman, M.W.; Grunstein, R.R. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J. Clin. Sleep Med. 2014, 10, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Pamidi, S.; Wroblewski, K.; Stepien, M.; Sharif-Sidi, K.; Kilkus, J.; Whitmore, H.; Tasali, E. Eight hours of nightly continuous positive airway pressure treatment of obstructive sleep apnea improves glucose metabolism in patients with prediabetes. A randomized controlled trial. Am. J. Respir Crit. Care Med. 2015, 192, 96–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of sleep apnea. Physiol. Rev. 2010, 90, 47–112. [Google Scholar] [CrossRef] [PubMed]
- Jelic, S.; Lederer, D.J.; Adams, T.; Padeletti, M.; Colombo, P.C.; Factor, P.H.; Le Jemtel, T.H. Vascular inflammation in obesity and sleep apnea. Circulation 2010, 121, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, D.J.; Punjabi, N.M.; Mehra, R.; Patel, S.R.; Quan, S.F.; Babineau, D.C.; Tracy, R.P.; Rueschman, M.; Blumenthal, R.S.; Lewis, E.F.; et al. CPAP versus oxygen in obstructive sleep apnea. N. Engl. J. Med. 2014, 370, 2276–2285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parra, O.; Sánchez-Armengol, Á.; Capote, F.; Bonnin, M.; Arboix, A.; Campos-Rodríguez, F.; Pérez-Ronchel, J.; Durán-Cantolla, J.; Martínez-Null, C.; De La Peña, M.; et al. Efficacy of continuous positive airway pressure treatment on 5-year survival in patients with ischaemic stroke and obstructive sleep apnea: A randomized controlled trial. J. Sleep Res. 2015, 24, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Kendzerska, T.; Gershon, A.S.; Hawker, G.; Leung, R.S.; Tomlinson, G. Obstructive sleep apnea and risk of cardiovascular events and all-cause mortality: A decade-long historical cohort study. PLoS Med. 2014, 11, e1001599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marin, J.M.; Carrizo, S.J.; Vicente, E.; Agusti, A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005, 365, 1046–1053. [Google Scholar] [CrossRef]
- Yaggi, H.K.; Concato, J.; Kernan, W.N.; Lichtman, J.H.; Brass, L.M.; Mohsenin, V. Obstructive sleep apnea as a risk factor for stroke and death. N. Engl. J. Med. 2005, 353, 2034–2041. [Google Scholar] [CrossRef] [Green Version]
- Chirinos, J.A.; Gurubhagavatula, I.; Teff, K.; Rader, D.J.; Wadden, T.A.; Townsend, R.; Foster, G.D.; Maislin, G.; Saif, H.; Broderick, P.; et al. CPAP, weight loss, or both for obstructive sleep apnea. N. Engl. J. Med. 2014, 370, 2265–2275. [Google Scholar] [CrossRef] [Green Version]
- Peppard, P.E.; Young, T.; Palta, M.; Skatrud, J. Prospective study of the association between sleep-disordered breathing and hypertension. N. Engl. J. Med. 2000, 342, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Tamisier, R.; Pepin, J.L.; Remy, J.; Baguet, J.P.; Taylor, J.A.; Weiss, J.W.; Levy, P. 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur. Respir. J. 2011, 37, 119–128. [Google Scholar] [CrossRef]
- Kim, Y.; Koo, Y.S.; Lee, H.Y.; Lee, S.-Y. Can continuous positive airway pressure reduce the risk of stroke in obstructive sleep apnea patients? A systematic review and meta-analysis. PLoS ONE 2016, 11, e0146317. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, D.J.; Yenokyan, G.; Newman, A.B.; O’Connor, G.T.; Punjabi, N.M.; Quan, S.F.; Redline, S.; Resnick, H.E.; Tong, E.K.; Diener-West, M.; et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The sleep heart health study. Circulation 2010, 122, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Hla, K.M.; Young, T.; Hagen, E.W.; Stein, J.H.; Finn, L.A.; Nieto, F.J.; Peppard, P.E. Coronary heart disease incidence in sleep disordered breathing: The Wisconsin Sleep Cohort Study. Sleep 2015, 38, 677–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunnarsson, S.I.; Peppard, P.E.; Korcarz, C.E.; Barnet, J.H.; Aeschlimann, S.E.; Hagen, E.W.; Young, T.; Hla, K.M.; Stein, J.H. Obstructive sleep apnea is associated with future subclinical carotid artery disease: Thirteen-year follow-up from the Wisconsin sleep cohort. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2338–2342. [Google Scholar] [CrossRef] [Green Version]
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z.; et al. SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef]
- Barbé, F.; Durán-Cantolla, J.; Capote, F.; De La Peña, M.; Chiner, E.; Masa, J.F.; Gonzalez, M.; Marín, J.M.; Garcia-Rio, F.; De Atauri, J.D.; et al. Spanish Sleep and Breathing Group. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am. J. Respir. Crit. Care Med. 2008, 181, 718–726. [Google Scholar] [CrossRef] [Green Version]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-isordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, T.; Peppard, P.E.; Taheri, S. Excess weight and sleep disordered breathing. J. Appl. Physiol. 2005, 99, 1592–1599. [Google Scholar] [CrossRef]
- Bozkurt, N.C.; Beysel, S.; Karbek, B.; Unsal, I.O.; Cakir, E.; Delibasi, T. Visceral obesity mediates the association between metabolic syndrome and obstructive sleep apnea syndrome. Metab. Syndr. Relat. Disord. 2016, 14, 217–221. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, X. Tumor necrosis factor alpha is a promising circulating biomarker for the development of obstructive sleep apnea syndrome: A meta-analysis. Oncotarget 2017, 8, 27616–27626. [Google Scholar] [CrossRef] [Green Version]
- Polotsky, V.Y.; Bevans-Fonti, S.; Grigoryev, D.N.; Punjabi, N.M. Intermittent hypoxia alters gene expression in peripheral blood mononuclear cells of healthy volunteers. PLoS ONE 2015, 10, e0144725. [Google Scholar] [CrossRef] [Green Version]
- Baessler, A.; Nadeem, R.; Harvey, M.; Madbouly, E.; Younus, A.; Sajid, H.; Naseem, J.; Asif, A.; Bawaadam, H.J. Treatment for sleep apnea by continuous positive airway pressure improves levels of inflammatory markers—A meta-analysis. J. Inflamm. 2013, 10, 13. [Google Scholar] [CrossRef] [Green Version]
- Quintero, M.; Gonzalez-Martin, M.D.C.; Vega-Agapito, V.; Gonzalez, C.; Obeso, A.; Farré, R.; Agapito, T.; Yubero, S. The effects of intermittent hypoxia on redox status NF-κB activation, and plasma lipid levels are dependent on the lowest oxygen saturation. Free Radic. Biol. Med. 2013, 65, 1143–1154. [Google Scholar] [CrossRef]
- Hermsdorff, H.H.M.; Puchau, B.; Zulet, M.Á.; Martínez, J.A. Association of body fat distribution with proinflammatory gene expression in peripheral blood mononuclear cells from young adult subjects. OMICS 2010, 14, 297–307. [Google Scholar] [CrossRef]
- Ghanim, H.; Aljada, A.; Daoud, N.; Deopurkar, R.; Chaudhuri, A.; Dandona, P. Role of inflammatory mediators in the suppression of insulin receptor phosphorylation in circulating mononuclear cells of obese subjects. Diabetologia 2007, 50, 278–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyugovskaya, L.; Polyakov, A.; Ginsberg, D.; Lavie, P.; Lavie, L. Molecular pathways of spontaneous and TNF-alpha-mediated neutrophil apoptosis under intermittent hypoxia. Am. J. Respir. Cell Mol. Biol. 2011, 45, 154–162. [Google Scholar] [CrossRef]
- Dyugovskaya, L.; Lavie, P.; Lavie, L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am. J. Respir. Crit. Care Med. 2002, 165, 934–939. [Google Scholar] [CrossRef]
- Li, R.; Wang, W.-Q.; Zhang, H.; Yang, X.; Fan, Q.; Christopher, T.A.; Lopez, B.L.; Tao, L.; Goldstein, B.J.; Gao, F.; et al. Adiponectin improves endothelial function in hyperlipidemic rats by reducing oxidative/nitrative stress and differential regulation of eNOS/iNOS activity. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1703–E1708. [Google Scholar] [CrossRef] [Green Version]
- Murzabekova, G.; Eisele, H.-J.; Egemnazarov, B.; Schermuly, R.T.; Ghofrani, H.A.; Reichenberger, F.; Mayer, K.; Seeger, W.; Grimminger, F.; Weissmann, N.; et al. Arterial hypertension in a murine model of sleep apnea: Role of NADPH oxidase 2. J. Hypertens. 2014, 32, 300–305. [Google Scholar]
- Schulz, R.; Mahmoudi, S.; Hattar, K.; Sibelius, U.; Olschewski, H.; Mayer, K.; Seeger, W.; Grimminger, F. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive apnea. Am. J. Respir. Crit. Care Med. 2000, 162, 566–570. [Google Scholar] [CrossRef]
- Ohga, E.; Nagase, T.; Tomita, T.; Teramoto, S.; Matsuse, T.; Katayama, H.; Ouchi, Y. Increased levels of circulating ICAM-1, VCAM-1, and L-selectin in obstructive sleep apnea syndrome. J. Appl. Physiol. 1999, 87, 10–14. [Google Scholar] [CrossRef]
- Takahashi, S.; Nakamura, Y.; Nishijima, T.; Sakurai, S.; Inoue, H. Essential roles of angiotensin II in vascular endothelial growth factor expression in sleep apnea syndrome. Respir. Med. 2005, 99, 1125–1131. [Google Scholar] [CrossRef] [Green Version]
- Schulz, R.; Hummel, C.; Heinemann, S.; Seeger, W.; Grimminger, F. Serum levels of vascular endothelial growth factor (VEGF) are elevated in patients with obstructive sleep apnea and severe nighttime hypoxia. Am. J. Respir. Crit. Care Med. 2002, 165, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Asker, S.; Asker, M.; Sarikaya, E.; Sunnetcioglu, A.; Aslan, M.; Demir, H. Oxidative stress parameters and their correlation with clinical, metabolic and polysomnographic parameters in severe obstructive sleep apnea syndrome. Int. J. Clin. Exp. Med. 2015, 8, 11449–11455. [Google Scholar]
- Guasti, L.; Marino, F.; Cosentino, M.; Maroni, L.; Maresca, A.M.; Colombo, F.; Maio, R.C.; Castiglioni, L.; Saporiti, F.; Loraschi, A.; et al. Cytokine production from peripheral blood mononuclear cells and polymorphonuclear leukocytes in patients studied for suspected obstructive sleep apnea. Sleep Breath. 2011, 15, 3–11. [Google Scholar] [CrossRef]
- Ashfaq, S.; Abramson, J.L.; Jones, D.P.; Rhodes, S.D.; Weintraub, W.S.; Hooper, W.C.; Vaccarino, V.; Alexander, R.W.; Harrison, D.G.; Quyyumi, A.A. Endothelial function and aminothiol biomarkers of oxidative stress in healthy adults. Hypertension 2008, 52, 80–85. [Google Scholar] [CrossRef]
- Patel, R.S.; Al Mheid, I. Oxidative stress is associated with impaired arterial elasticity. Atherosclerosis 2011, 218, 90–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, R.S.; Ghasemzadeh, N.; Eapen, D.J.; Sher, S.; Arshad, S.; Ko, Y.A.; Veledar, E.; Samady, H.; Zafari, A.M.; Sperling, L.; et al. Novel biomarker of oxidative stress is associated with risk of death in patients with coronary artery disease. Circulation 2016, 133, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.M.; Jones, D.P. Cysteine/cystine redox signaling in cardiovascular disease. Free Radic. Biol. Med. 2011, 50, 495–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Go, Y.M.; Jones, D.P. Intracellular proatherogenic events and cell adhesion modulated by extracellular thiol/disulfide redox state. Circulation 2005, 111, 2973–2980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildebrandt, W.; Hamann, A.; Krakowski-Roosen, H.; Kinscherf, R.; Dugi, K.; Sauer, R.; Lacher, S.; Bodens, A.; Bellou, V.; Edler, L.; et al. Effect of thiol antioxidant on body fat and insulin reactivity. J. Mol. Med. 2004, 82, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Cintra, F.; Tufik, S.; D’Almeida, V.; Calegare, B.F.A.; de Paola, A.; Oliveira, W.; Rizzi, C.; Roizenblatt, S.; Poyares, D. Cysteine: A potential biomarker for obstructive sleep apnea. Chest 2011, 139, 246–252. [Google Scholar] [CrossRef]
- Kinscherf, R.; Cafaltzis, K.; Röder, F.; Hildebrandt, W.; Edler, L.; Deigner, H.P.; Breitkreutz, R.; Feussner, G.; Kreuzer, J.; Werle, E.; et al. Cholesterol levels linked to abnormal plasma thiol concentrations and thiol/disulfide redox status in hyperlipidemic subjects. Free Radic. Biol. Med. 2003, 35, 1286–1292. [Google Scholar] [CrossRef]
- Hildebrandt, W.; Kinscherf, R.; Hauer, K.; Holm, E.; Dröge, W. Plasma cystine concentration and redox state in aging and physical exercise. Mech. Ageing Dev. 2002, 123, 1269–1281. [Google Scholar] [CrossRef]
- Hildebrandt, W.; Sauer, R.; Bonaterra, G.; A Dugi, K.; Edler, L.; Kinscherf, R. Oral N-acetylcysteine reduces plasma homocysteine concentrations regardless of lipid or smoking status. Am. J. Clin. Nutr. 2015, 102, 1014–1024. [Google Scholar] [CrossRef] [Green Version]
- Bonso, E.; Dorigatti, F.; Saladini, F.; Palatini, P. Validation of the Visomat Comfort form device for home blood pressure measurement according to the International Protocol. Blood Press. Monit. 2010, 15, 63–66. [Google Scholar] [CrossRef]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Bonaterra, G.; Hildebrandt, W.; Bodens, A.; Sauer, R.; Dugi, K.; Deigner, H.; Dröge, W.; Metz, J.; Kinscherf, R. Increased cyclooxygenase-2 expression in peripheral blood mononuclear cells of smokers and hyperlipidemic subjects. Free Radic. Biol. Med. 2005, 38, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Mar, H.L.P.Y.; Hazen, S.L.; Tracy, R.P.; Strohl, K.P.; Auckley, D.; Bena, J.; Wang, L.; Walia, H.K.; Patel, S.R.; Mehra, R. Effect of continuous positive airway pressure on cardiovascular biomarkers: The sleep apnea stress randomized controlled trial. Chest 2016, 150, 80–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohler, M.; Ayers, L.; Pepperell, J.C.T.; Packwood, K.L.; Ferry, B.; Crosthwaite, N.; Craig, S.; Siccoli, M.M.; O’Davies, R.J.; Stradling, J.R. Effects of continuous positive airway pressure on systemic inflammation in patients with moderate to severe obstructive sleep apnoea: A randomised controlled trial. Thorax 2009, 64, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, K.; Kato, M.; Kato, Y.; Yanagihara, K.; Kinugasa, Y.; Kotani, K.; Igawa, O.; Hisatome, I.; Shigemasa, C.; Somers, V.K. Appropriate use of nasal continuous positive airway pressure decreases elevated C-reactive protein in patients with obstructive sleep apnea. Chest 2009, 136, 125–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, W.; Wang, L.; He, S.; Yan, L.; Li, T.; Wang, J.; Kong, A.-N.T.; Yu, S.; Zhang, Y. Effects of acute hypoxia exposure with different durations on activation of Nrf2-ARE pathway in mouse skeletal muscle. PLoS ONE 2018, 13, e0208474. [Google Scholar] [CrossRef]
- Syu, J.P.; Chi, J.T.; Kung, H.N. Nrf2 is the key to chemotherapy resistance in MCF7 breast cancer cells under hypoxia. Oncotarget 2016, 7, 14659–14672. [Google Scholar] [CrossRef] [Green Version]
- Kolamunne, R.T.; Dias, I.H.K.; Vernallis, A.B.; Grant, M.M.; Griffiths, H.R. Nrf2 activation supports cell survival during hypoxia and hypoxia/reoxygenation in cardiomyoblasts; the roles of reactive oxygen and nitrogen species. Redox. Biol. 2013, 22, 418–426. [Google Scholar] [CrossRef] [Green Version]
- Ibbotson, K.; Yell, J.; Ronaldson, P.T. Nrf2 signaling increases expression of ATP-binding cassette subfamily C mRNA transcripts at the blood-brain barrier following hypoxia-reoxygenation stress. Fluids Barriers CNS 2017, 14, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mysona, B.; Dun, Y.; Duplantier, J.; Ganapathy, V.; Smith, S.B. Effects of hyperglycemia and oxidative stress on the glutamate transporters GLAST and system xc- in mouse retinal Müller glial cells. Cell Tissue Res. 2009, 335, 477–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasini, A.M.F.; Ferrari, M.; Stranieri, C.; Vallerio, P.; Mozzini, C.; Garbin, U.; Zambon, G.; Cominacini, L. Nrf2 expression is increased in peripheral blood mononuclear cells derived from mild-moderate ex-smoker COPD patients with persistent oxidative stress. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 1733–1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantin, A.M. Cellular response to cigarette smoke and oxidants: Adapting to survive. Proc. Am. Thorac. Soc. 2010, 7, 368–375. [Google Scholar] [CrossRef]
- Zhu, J.; Li, S.; Marshall, Z.M.; Whorton, A.R. A cystine-cysteine shuttle mediated by xCT facilitates cellular responses to S-nitrosoalbumin. Am. J. Physiol. Cell Physiol. 2008, 294, C1012–C1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banjac, A.; Perisic, T.; Sato, H.; Seiler, A.; Bannai, S.; Weiss, N.; Kölle, P.; Tschoep, K.; Issels, R.D.; Daniel, P.T.; et al. The cystine/cysteine cycle: A redox cycle regulating susceptibility versus resistance to cell death. Oncogene 2008, 27, 1618–1628. [Google Scholar] [CrossRef] [Green Version]
| Control | OSA | Control | OSA | p (ANOVA) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Total | Non-Obese | Obese | Non-Obese | Obese | OSA | OBS | Interact. | ||
| n | 25 | 28 | 12 | 13 | 8 | 20 | 53 | 53 | 53 | |
| Age | (years) | 44.5 ± 1.2 | 48.8 ± 1.3 * | 43.5 ± 1.82 | 45.4 ± 1.6 | 52.6 ± 1.9 ** | 47.3 ± 1.5 # | 0.004 | 0.378 | 0.055 |
| BMI | (kg m−2) | 29.5 ± 1.2 | 31.6 ± 0.9 | 25.8 ± 0.7 | 32.8 ± 0.6 ### | 25.6 ± 1.0 | 34.0 ± 0.7 ###* | 0.333 | <0.001 | 0.216 |
| AHI | (h−1) | 5.1 ± 0.8 | 48.8 ± 4.5 *** | 5.3 ± 1.3 | 4.8 ± 1.0 | 43.8 ± 7.3 *** | 50.8 ± 5.6 *** | <0.001 | 0.335 | 0.290 |
| Tu90% | (min) | 1.9 ± 0.86 | 61.8 ± 14.0 *** | 1.02 ± 0.45 | 2.82 ± 1.43 | 51.6 ± 19.2 * | 65.8 ± 18.3 ** | 0.001 | 0.470 | 0.542 |
| Mean SaO2 | (%) | 94.3 ± 0.2 | 92.5 ± 0.36 *** | 94.3 ± 0.2 | 94.3 ± 0.3 | 92.7 ± 0.5 * | 92.3 ± 0.5 ** | <0.001 | 0.465 | 0.443 |
| Heart rate (at rest) | (min−1) | 66.2 ± 2.1 | 70.9 ± 2.2 | 69.8 ± 3.3 | 63.0 ± 2.4 | 71.5 ± 5.2 | 70.7 ± 2.3 ** | 0.163 | 0.341 | 0.239 |
| BP systolic | (mmHg) | 137.9 ± 1.8 | 146.3 ± 2.8 * | 135.1 ± 1.6 | 140.5 ± 3.1 | 138.1 ± 6.1 | 149.5 ± 2.9 | 0.081 | 0.006 | 0.210 |
| BP diastolic | (mmHg) | 88.6 ± 1.3 | 94.5 ± 1.8 * | 86.5 ± 1.4 | 90.4 ± 2.1 | 91.0 ± 3.3 | 95.9 ± 2.1 | 0.041 | 0.018 | 0.470 |
| carotid IMT | (mm) | 0.62 ± 0.02 | 0.70 ± 0.03 * | 0.60 ± 0.03 | 0.63 ± 0.03 | 0.72 ± 0.06 | 0.69 ± 0.03 | 0.024 | 0.719 | 0.559 |
| HbA1c | (%) | 5.51 ± 0.05 | 5.74 ± 0.08 * | 5.49 ± 0.06 | 5.52 ± 0.09 | 5.73 ± 0.06 * | 5.74 ± 0.11 | 0.037 | 0.720 | 0.951 |
| Glucose (fasted) | (mg 100 mL−1) | 92.6 ± 1.4 | 98.4 ± 2.2 * | 93.3 ± 1.8 | 91.9 ± 2.2 | 98.3 ± 4.9 ** | 98.5 ± 2.5 | 0.049 | 0.891 | 0.718 |
| Insulin (fasted) | (µU mL−1) | 8.93 ± 1.91 | 14.16 ± 2.05 | 9.90 ± 4.10 | 9.24 ± 1.91 | 18.81 ± 7.09 | 13.98 ± 1.81 | 0.094 | 0.800 | 0.975 |
| HOMA-IR | (---) | 1.68 ± 0.28 | 3.57 ± 0.59 ** | 1.18 ± 0.21 | 2.15 ± 0.47 | 3.97 ± 1.79 | 3.42 ± 0.45 | 0.008 | 0.558 | 0.437 |
| HDL | (mg 100 mL−1) | 48.1 ± 1.8 | 45.5 ± 2.0 | 51.2 ± 2.9 | 45.3 ± 2.1 | 42.4 ± 2.8 | 46.8 ± 2.6 | 0.235 | 0.735 | 0.087 |
| LDL | (mg 100 mL−1) | 137.8 ± 6.0 | 143.6 ± 7.1 | 152.0 ± 6.1 | 124.8 ± 8.9 # | 144.5 ± 19.3 | 143.2 ± 6.5 | 0.524 | 0.093 | 0.247 |
| Total cholesterol | (mg 100 mL−1) | 203.4 ± 7.2 | 213.6 ± 7.0 | 217.8 ± 8.4 | 190.2 ± 10.4 | 223.6 ± 16.1 | 209.6 ± 7.6 | 0.178 | 0.015 | 0.810 |
| Triglycerides | (mg 100 mL−1) | 113.2 ± 12.1 | 156.9 ± 19.8 | 96.2 ± 11.6 | 128.9 ± 20.3 | 203.3 ± 44.5 * | 138.4 ± 20.6 | 0.014 | 0.270 | 0.016 |
| Homocysteine | (µmol L−1) | 11.15 ± 1.22 | 8.92 ± 0.45 | 12.37 ± 2.46 | 10.04 ± 0.75 | 7.84 ± 0.70 | 9.37 ± 0.55 | 0.042 | 0.749 | 0.126 |
| TNF | (pg mL−1) | 8.88 ± 1.06 | 7.03 ± 0.29 | 8.67 ± 2.07 | 9.09 ± 0.67 | 7.44 ± 0.73 | 6.86 ± 0.27 ** | 0.111 | 0.863 | 0.572 |
| cystine (plasma) | (µmol L−1) | 50.6 ± 3.0 | 56.7 ± 2.3 | 41.0 ± 3.8 | 59.3 ± 3.0 ## | 51.9 ± 4.6 | 58.6 ± 2.6 | 0.164 | <0.001 | 0.207 |
| cystine (PBMC) | (nmol mg−1) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |||
| glutamate (plasma) | (µmol L−1) | 51.7 ± 7.1 | 67.2 ± 5.1 | 29.0 ± 5.6 | 70.9 ± 9.4 ## | 68.1 ± 13.4 ** | 66.8 ± 5.1 | 0.043 | 0.007 | 0.020 |
| glutamate (PBMC) | (nmol mg−1) | 150.0 ± 9.4 | 162.6 ± 10.6 | 153.3 ± 12.3 | 146.7 ± 14.8 | 135.3 ± 26.0 | 172.6 ± 10.5 | 0.791 | 0.188 | 0.081 |
| glycine (plasma) | (µmol L−1) | 173.0 ± 11.2 | 163.6 ± 7.5 | 160.7 ± 13.3 | 184.3 ± 17.8 | 169.4 ± 19.7 | 161.2 ± 7.2 | 0.564 | 0.508 | 0.297 |
| glycine (PBMC) | (nmol mg−1) | 22.8 ± 2.0 | 19.8 ± 1.1 | 25.4 ± 3.0 | 20.1 ± 2.4 | 16.1 ± 3.2 | 21.2 ± 0.9 # | 0.088 | 0.986 | 0.025 |
| Total GSH | (nmol mg−1) | 59.01 | 75.28 | 39.42 | 77.10 | 77.47 | 74.45 | |||
| Reduced GSH | (nmol mg−1) | 48.87 | 67.43 | 34.20 | 64.42 | 69.04 | 66.81 | |||
| GSSG | (nmol mg−1) | 9.09 | 7.86 | 5.22 | 12.67 | 8.43 | 7.64 | |||
| Red. GSH/GSSG | (ratio) | 5.38 | 8.56 | 6.56 | 5.08 | 8.19 | 8.74 | |||
| Control | cHPX | Control | cHPX | p (ANOVA) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Total | Non-Obese | Obese | Non-Obese | Obese | cHPX | OBS | Interact. | ||
| n | 32 | 21 | 16 | 16 | 5 | 16 | 53 | 53 | 53 | |
| Age | (years) | 45.9 ± 1.1 | 48.1 ± 1.6 | 45.1 ± 1.6 | 46.7 ± 1.5 | 54.0 ± 2.9 * | 46.3 ± 1.7 # | 0.038 | 0.125 | 0.023 |
| BMI | (kg m−2) | 29.2 ± 0.8 | 32.7 ± 0.9 ** | 25.3 ± 0.6 | 33.1 ± 0.5 ### | 27.0 ± 0.9 | 34.5 ± 0.7 ### | 0.053 | <0.001 | 0.842 |
| AHI | (h−1) | 11.8 ± 2.6 | 53.4 ± 5.4 *** | 11.3 ± 3.1 | 12.3 ± 4.3 | 51.7 ± 9.6 *** | 54.0 ± 6.6 *** | <0.001 | 0.795 | 0.915 |
| Tu90% | (min) | 2.4 ± 0.6 | 81.0 ± 16.7 *** | 2.8 ± 1.1 | 1.9 ± 0.6 | 78.6 ± 23.3 * | 81.8 ± 21.1 ** | <0.001 | 0.941 | 0.896 |
| Mean SaO2 | (%) | 94.4 ± 0.1 | 91.8 ± 0.4 *** | 94.3 ± 0.2 | 94.5 ± 0.2 | 91.9 ± 0.5 *** | 91.7 ± 0.5 *** | <0.001 | 0.976 | 0.686 |
| Heart rate (at rest) | (min−1) | 67.2 ± 1.8 | 71.1 ± 2.8 | 70.0 ± 2.8 | 64.3 ± 2.1 | 70.2 ± 7.7 | 71.4 ± 2.9 * | 0.298 | 0.520 | 0.325 |
| BP systolic | (mmHg) | 138.6 ± 2.0 | 148.0 ± 3.0 * | 132.9 ± 1.8 | 144.4 ± 3.1 ## | 145.3 ± 7.7 | 148.8 ± 3.2 | 0.026 | 0.047 | 0.281 |
| BP diastolic | (mmHg) | 89.4 ± 1.4 | 95.1 ± 1.9 * | 86.5 ± 1.6 | 92.3 ± 2.1 # | 92.2 ± 3.9 | 91.7 ± 0.5 | 0.067 | 0.059 | 0.690 |
| carotid IMT | (mm) | 0.62 ± 0.02 | 0.72 ± 0.03 ** | 0.60 ± 0.03 | 0.64 ± 0.02 | 0.79 ± 0.08 ** | 0.70 ± 0.03 | 0.001 | 0.539 | 0.107 |
| HbA1c | (%) | 5.50 ± 0.05 | 5.82 ± 0.10 ** | 5.54 ± 0.05 | 5.48 ± 0.08 | 5.72 ± 0.07 | 5.85 ± 0.13 * | 0.015 | 0.770 | 0.363 |
| Glucose (fasted) | (mg 100 mL−1) | 92.1 ± 1.5 | 101.1 ± 2.2 *** | 94.1 ± 2.5 | 90.2 ± 1.6 | 99.4 ± 3.7 | 101.6 ± 2.7 *** | 0.005 | 0.773 | 0.289 |
| Insulin (fasted) | (µU mL−1) | 9.8 ± 2.1 | 14.6 ± 1.7 | 10.7 ± 3.9 | 8.9 ± 1.5 | 10.9 ± 2.9 | 15.8 ± 2.0 * | 0.272 | 0.640 | 0.312 |
| HOMA-IR | (---) | 2.04 ± 0.48 | 3.67 ± 0.43 * | 2.08 ± 0.93 | 2.00 ± 0.37 | 2.79 ± 0.81 | 3.94 ± 0.50 ** | 0.100 | 0.499 | 0.440 |
| HDL | (mg 100 mL−1) | 49.3 ± 1.7 | 42.8 ± 2.1 * | 50.7 ± 2.2 | 47.8 ± 2.7 | 37.8 ± 2.6 ** | 44.5 ± 2.5 | 0.008 | 0.524 | 0.113 |
| LDL | (mg 100 mL−1) | 139.9 ± 4.9 | 142.3 ± 9.4 | 149.4 ± 5.5 | 130.5 ± 7.5 | 151.4 ± 30.7 | 139.2 ± 8.3 | 0.612 | 0.145 | 0.752 |
| Total cholesterol | (mg 100 mL−1) | 208.8 ± 6.2 | 208.9 ± 8.7 | 220.1 ± 8.3 | 197.5 ± 8.5 | 230.6 ± 21.9 | 202.1 ± 8.9 | 0.500 | 0.026 | 0.789 |
| Triglycerides | (mg 100 mL−1) | 130.0 ± 13.8 | 145.8 ± 22.8* | 128.1 ± 22.0 | 131.9 ± 17.5 | 207.2 ± 59.4 | 126.6 ± 22.6 | 0.186 | 0.169 | 0.131 |
| Homocysteine | (µmol L−1) | 10.3 ± 1.0 | 9.3 ± 0.5 | 10.7 ± 1.9 | 9.9 ± 0.6 | 9.1 ± 0.8 | 9.3 ± 0.6 | 0.460 | 0.848 | 0.733 |
| TNF | (pg mL−1) | 8.18 ± 0.82 | 7.40 ± 0.41 | 8.16 ± 1.53 | 8.21 ± 0.58 | 8.20 ± 0.98 | 7.13 ± 0.43 | 0.661 | 0.665 | 0.637 |
| cystine (plasma) | (µmol L−1) | 50.1 ± 2.7 | 59.4 ± 2.0 ** | 40.9 ± 3.1 | 59.4 ± 3.1 ### | 58.0 ± 4.4 * | 59.8 ± 2.3 | 0.016 | 0.006 | 0.022 |
| cystine (PBMC) | (nmol mg−1) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |||
| glutamate (plasma) | (µmol L−1) | 52.4 ± 6.1 | 71.3 ± 5.2 * | 33.4 ± 6.3 | 70.2 ± 8.2 ### | 80.3 ± 14.2 ** | 68.5 ± 5.3 | 0.011 | 0.148 | 0.006 |
| glutamate (PBMC) | (nmol mg−1) | 145.2 ± 8.1 | 173.5 ± 12.3 | 143.9 ± 10.4 | 146.8 ± 13.2 | 146.8 ± 46.3 | 180.2 ± 10.9 | 0.276 | 0.276 | 0.359 |
| glycine (plasma) | (µmol L−1) | 167.1 ± 9.8 | 169.4 ± 7.4 | 154.8 ± 11.2 | 179.3 ± 15.9 | 191.1 ± 23.1 | 162.6 ± 6.3 | 0.512 | 0.894 | 0.080 |
| glycine (PBMC) | (nmol mg−1) | 20.9 ± 1.6 | 21.8 ± 1.5 | 22.7 ± 2.6 | 18.8 ± 1.7 | 18.1 ± 5.6 | 22.7 ± 1.3 | 0.901 | 0.873 | 0.115 |
| OSA (AHI > 15 h−1) | cHPX (Tu90% > 15.2 min) | ||||
|---|---|---|---|---|---|
| Pre-cPAP | Post-cPAP | Pre-cPAP | Post-cPAP | ||
| n | 15 | 15 | 12 | 12 | |
| BMI | (kg m−2) | 32.5 ± 1.3 | 32.7 ± 1.3 | 33.4 ± 1.4 | 33.7 ± 1.4 |
| CPAP | (cm H2O) | 7.80 ± 0.42 | 8.23 ± 0.41 | ||
| AHI | (h−1) | 47.9 ± 7.5 | 3.5 ± 1.4 §§§ | 52.1 ± 6.9 | 5.1 ± 1.6 §§§ |
| Tu90% | (min) | 73.0 ± 21.9 | 0.0 ± 0.0 §§ | 89.8 ± 25.1 | 0.0 ± 0.0 §§ |
| Heart rate (at rest) | (min−1) | 69.6 ± 2.5 | 65.4 ± 2.3 | 69.3 ± 3.0 | 63.8 ± 2.6 |
| BP systolic | (mmHg) | 148.0 ± 3.8 | 143.3 ± 2.6 | 150.8 ± 3.2 | 145.2 ± 3.0 § |
| BP diastolic | (mmHg) | 93.8 ± 2.5 | 89.9 ± 2.0 | 94.9 ± 2.4 | 91.3 ± 2.4 § |
| carotid IMT | (mm) | 0.68 ± 0.03 | 0.71 ± 0.04 | 0.69 ± 0.03 | 0.73 ± 0.04 |
| HbA1c | (%) | 5.78 ± 0.12 | 5.74 ± 0.12 | 5.79 ± 0.13 | 5.75 ± 0.12 |
| Glucose (fasted) | (mg 100 mL−1) | 102.3 ± 3.3 | 100.0 ± 3.3 | 103.4 ± 3.2 | 101.7 ± 4.0 |
| Insulin (fasted) | (µU mL−1) | 17.2 ± 3.2 | 13.1 ± 1.6 | 15.1 ± 2.3 | 13.0 ± 1.6 |
| HOMA-IR | (---) | 4.44 ± 0.93 | 3.21 ± 0.39 | 3.82 ± 0.54 | 3.22 ± 0.41 |
| HDL | (mg 100 mL−1) | 45.1 ± 2.6 | 47.4 ± 1.8 | 42.5 ± 2.4 | 45.2 ± 1.5 |
| LDL | (mg 100 mL−1) | 148.9 ± 12.2 | 148.6 ± 11.5 | 153.2 ± 14.7 | 151.3 ± 13.7 |
| Total cholesterol | (mg 100 mL−1) | 214.5 ± 11.5 | 216.5 ± 11.8 | 215.7 ± 13.9 | 215.0 ± 13.6 |
| Triglycerides | (mg 100 mL−1) | 148.0 ± 25.0 | 156.0 ± 27.2 | 143.1 ± 28.3 | 142.2 ± 23.8 |
| Homocysteine | (µmol L−1) | 8.50 ± 0.53 | 8.77 ± 0.45 | 8.65 ± 0.68 | 8.78 ± 0.52 |
| TNF | (pg mL−1) | 6.94 ± 0.35 | 7.12 ± 0.35 | 7.01 ± 0.44 | 7.24 ± 0.41 |
| cystine (plasma) | (µmol L−1) | 57.9 ± 3.0 | 49.8 ± 2.8 | 60.8 ± 2.7 | 48.1 ± 3.1 §§ |
| cystine (PBMC) | (nmol mg−1) | n.d. | n.d. | n.d. | n.d. |
| glutamate (plasma) | (µmol L−1) | 65.1 ± 5.1 | 50.1 ± 4.6 § | 69.3 ± 5.8 | 49.3 ± 5.1 §§ |
| glutamate (PBMC) | (nmol mg−1) | 161.1 ± 13.6 | 144.7 ± 11.9 | 166.2 ± 16.3 | 137.9 ± 12.7 |
| glycine (plasma) | (µmol L−1) | 154.0 ± 9.1 | 134.1 ± 7.0 § | 159.7 ± 5.9 | 138.8 ± 7.6 § |
| glycine (PBMC) | (nmol mg−1) | 19.7 ± 1.6 | 17.2 ± 1.3 | 20.3 ± 2.0 | 16.5 ± 1.3 |
| Total GSH | (nmol mg−1) | 75.28 | 58.05 | ||
| Reduced GSH | (nmol mg−1) | 67.43 | 51.81 | ||
| GSSG | (nmol mg−1) | 7.86 | 6.25 | ||
| Red. GSH/GSSG | (ratio) | 8.56 | 8.37 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boneberg, R.; Pardun, A.; Hannemann, L.; Hildebrandt, O.; Koehler, U.; Kinscherf, R.; Hildebrandt, W. High Plasma Cystine Levels Are Associated with Blood Pressure and Reversed by CPAP in Patients with Obstructive Sleep Apnea. J. Clin. Med. 2021, 10, 1387. https://doi.org/10.3390/jcm10071387
Boneberg R, Pardun A, Hannemann L, Hildebrandt O, Koehler U, Kinscherf R, Hildebrandt W. High Plasma Cystine Levels Are Associated with Blood Pressure and Reversed by CPAP in Patients with Obstructive Sleep Apnea. Journal of Clinical Medicine. 2021; 10(7):1387. https://doi.org/10.3390/jcm10071387
Chicago/Turabian StyleBoneberg, Raphael, Anita Pardun, Lena Hannemann, Olaf Hildebrandt, Ulrich Koehler, Ralf Kinscherf, and Wulf Hildebrandt. 2021. "High Plasma Cystine Levels Are Associated with Blood Pressure and Reversed by CPAP in Patients with Obstructive Sleep Apnea" Journal of Clinical Medicine 10, no. 7: 1387. https://doi.org/10.3390/jcm10071387
APA StyleBoneberg, R., Pardun, A., Hannemann, L., Hildebrandt, O., Koehler, U., Kinscherf, R., & Hildebrandt, W. (2021). High Plasma Cystine Levels Are Associated with Blood Pressure and Reversed by CPAP in Patients with Obstructive Sleep Apnea. Journal of Clinical Medicine, 10(7), 1387. https://doi.org/10.3390/jcm10071387





