Efficacy of Non-Invasive Radiofrequency-Based Diathermy in the Postoperative Phase of Knee Arthroplasty: A Double-Blind Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Sample Size Calculation
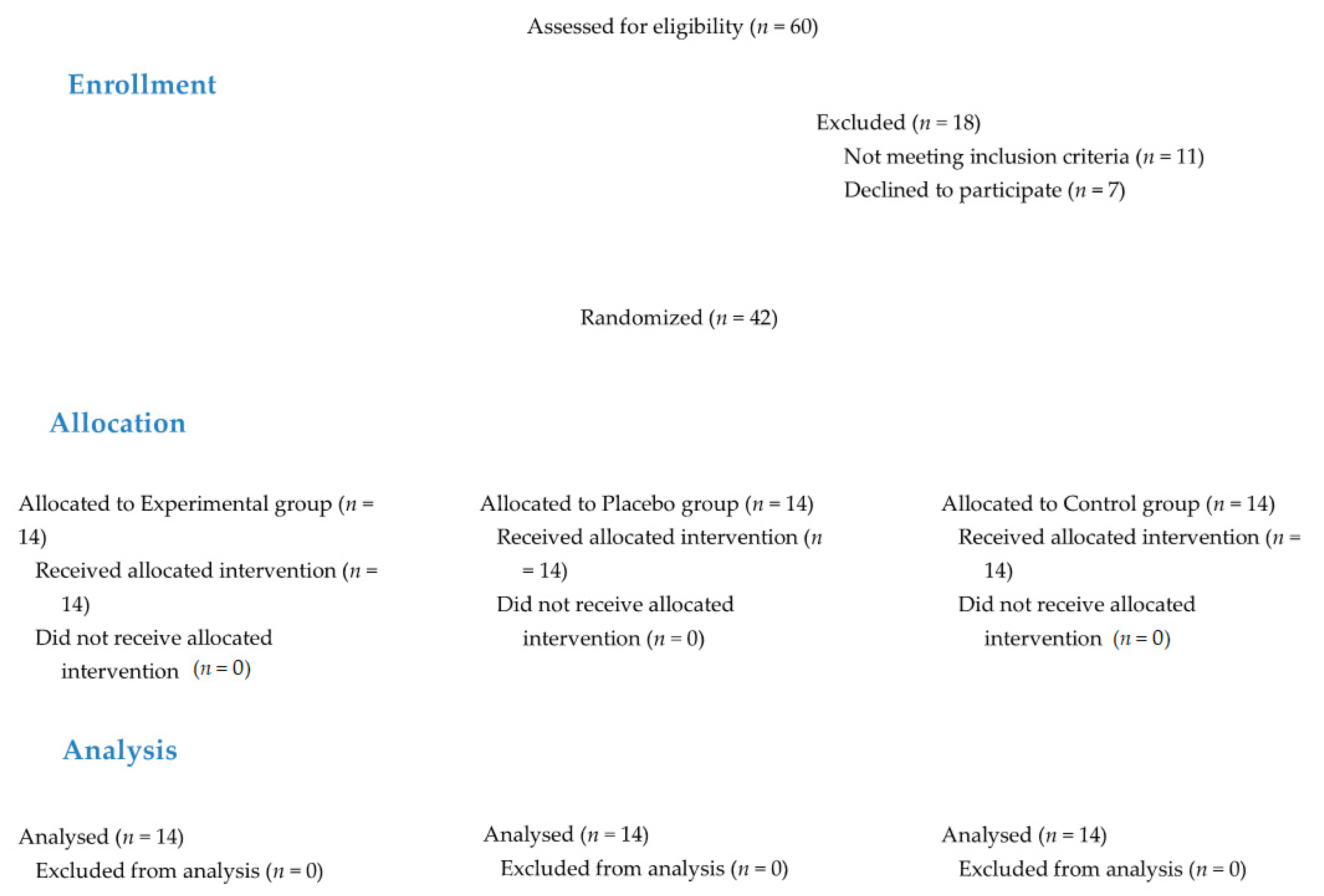
2.4. Randomization and Blinding
2.5. Measurements
2.6. Interventions
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hochman, M.G.; Melenevsky, Y.V.; Metter, D.F.; Roberts, C.C.; Bencardino, J.T.; Cassidy, R.C.; Fox, M.G.; Kransdorf, M.J.; Mintz, D.N.; Shah, N.A.; et al. ACR Appropriateness Criteria® Imaging After Total Knee Arthroplasty. J. Am. Coll. Radiol. 2017, 14, S421–S448. [Google Scholar] [CrossRef]
- Ethgen, O.; Bruyerè, O.; Richy, F.; Dardennes, C.; Reginster, J.Y. Health-Related Quality of Life in Total Hip and Total Knee Arthroplasty: A Qualitative and Systematic Review of the Literature. J. Bone Jt. Surg. Ser. A 2004, 86, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Von Keudell, A.; Sodha, S.; Collins, J.; Minas, T.; Fitz, W.; Gomoll, A.H. Patient satisfaction after primary total and unicompartmental knee arthroplasty: An age-dependent analysis. Knee 2014, 21, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Pua, Y.H.; Poon, C.L.L.; Seah, F.J.T.; Thumboo, J.; Clark, R.A.; Tan, M.H.; Chong, H.C.; Tan, J.W.M.; Chew, E.S.X.; Yeo, S.J. Predicting individual knee range of motion, knee pain, and walking limitation outcomes following total knee arthroplasty. Acta Orthop. 2019, 90, 179–186. [Google Scholar] [CrossRef] [PubMed]
- De Vroey, H.; Claeys, K.; Shariatmadar, K.; Weygers, I.; Vereecke, E.; Van Damme, G.; Hallez, H.; Staes, F. High Levels of Kinesiophobia at Discharge from the Hospital May Negatively Affect the Short-Term Functional Outcome of Patients Who Have Undergone Knee Replacement Surgery. J. Clin. Med. 2020, 9, 738. [Google Scholar] [CrossRef]
- Karlsen, A.P.H.; Wetterslev, M.; Hansen, S.E.; Hansen, M.S.; Mathiesen, O.; Dahl, J.B. Postoperative pain treatment after total knee arthroplasty: A systematic review. PLoS ONE 2017, 12, e0173107. [Google Scholar] [CrossRef]
- Tedesco, D.; Gori, D.; Desai, K.R.; Asch, S.; Carroll, I.R.; Curtin, C.; McDonald, K.M.; Fantini, M.P.; Hernandez-Boussard, T. Drug-free interventions to reduce pain OR opioid consumption after total knee arthroplasty a systematic review and meta-analysis. JAMA Surg. 2017, 152, e172872. [Google Scholar] [CrossRef]
- Henderson, K.G.; Wallis, J.A.; Snowdon, D.A. Active physiotherapy interventions following total knee arthroplasty in the hospital and inpatient rehabilitation settings: A systematic review and meta-analysis. Physiotherapy 2018, 104, 25–35. [Google Scholar] [CrossRef]
- Iles, R.; Davidson, M. Status of Physiotherapy rehabilitation after total knee replacement in Australia. Physiother. Res. Int. 2006, 11, 35–47. [Google Scholar]
- Zech, A.; Hendrich, S.; Pfeifer, K. Association Between Exercise Therapy Dose and Functional Improvements in the Early Postoperative Phase After Hip and Knee Arthroplasty: An Observational Study. PM R 2015, 7, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, B.; Watson, T. Thermal build-up, decay and retention responses to local therapeutic application of 448 kHz capacitive resistive monopolar radiofrequency: A prospective randomised crossover study in healthy adults. Int. J. Hyperth. 2015, 31, 883–895. [Google Scholar] [CrossRef]
- Albornoz-Cabello, M.; Ibáñez-Vera, A.J.; Aguilar-Ferrándiz, M.E.; Espejo-Antúnez, L. Monopolar dielectric diathermy by emission of radiofrequency in Patellofemoral pain. A single-blind-randomized clinical trial. Electromagn. Biol. Med. 2020, 39, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, B.; Watson, T. Radiofrequency-based treatment in therapy-related clinical practice–a narrative review. Part II: Chronic conditions. Phys. Ther. Rev. 2015, 20, 325–343. [Google Scholar] [CrossRef][Green Version]
- Albornoz-Cabello, M.; Ibáñez-Vera, A.J.; de la Cruz-Torres, B. Efficacy of monopolar dielectric transmission radio frequency in panniculus adiposus and cellulite reduction Efficacy of monopolar dielectric transmission radio frequency in panniculus adiposus and cellulite reduction. J. Cosmet. Laser Ther. 2017, 19, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Vera, A.J. Radiofrequency in aesthetics skin treatment: Classification and modalities. J. Dermatol. Res. Skin Care 2017, 1, 8–10. [Google Scholar]
- Coccetta, C.A.; Sale, P.; Ferrara, P.E.; Specchia, A.; Maccauro, G.; Ferriero, G.; Ronconi, G. Effects of capacitive and resistive electric transfer therapy in patients with knee osteoarthritis: A randomized controlled trial. Int. J. Rehabil. Res. 2019, 42, 106–111. [Google Scholar] [CrossRef]
- Carr, A.J.; Robertsson, O.; Graves, S.; Price, A.J.; Arden, N.K.; Judge, A.; Beard, D.J. Knee replacement. Lancet 2012, 379, 1331–1340. [Google Scholar] [CrossRef]
- Kumaran, B.; Watson, T. Treatment using 448 kHz capacitive resistive monopolar radiofrequency improves pain and function in patients with osteoarthritis of the knee joint: A randomised controlled trial. Physiotherapy 2019, 105, 98–107. [Google Scholar] [CrossRef]
- Alghadir, A.; Anwer, S.; Iqbal, A.; Iqbal, Z. Test-retest reliability, validity, and minimum detectable change of visual analog, numerical rating, and verbal rating scales for measurement of osteoarthritic knee pain. J. Pain Res. 2018, 11, 851. [Google Scholar] [CrossRef]
- Medina-Mirapeix, F.; Vivo-Fernández, I.; López-Cañizares, J.; García-Vidal, J.A.; Benítez-Martínez, J.C.; del Baño-Aledo, M.E. Five times sit-to-stand test in subjects with total knee replacement: Reliability and relationship with functional mobility tests. Gait Posture 2018, 59, 258–260. [Google Scholar] [CrossRef]
- Piva, S.R.; Fitzgerald, G.K.; Irrgang, J.J.; Bouzubar, F.; Starz, T.W. Get Up and Go Test in Patients with Knee Osteoarthritis. Arch. Phys. Med. Rehabil. 2004, 85, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Escobar, A.; Quintana, J.M.; Bilbao, A.; Azka, J. Validation of the Spanish Version of the WOMAC Questionnaire for Patients with Hip of Knee Osteoarthritis. Clin. Rheumatol. 2002, 21, 466–471. [Google Scholar] [CrossRef]
- Vilagut, G.; Valderas, J.M.; Ferrer, M.; Garin, O.; López-García, E.; Alonso, J. Interpretation of SF-36 and SF-12 questionnaires in Spain: Physical and mental components. Med. Clin. 2008, 130, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Vera, A.J.; García-Romero, J.C.; Alvero-Cruz, J.R.; Lomas-Vega, R. Effects of Monopolar Dielectric Radiofrequency Signals on the Symptoms of Fibromyalgia: A Single-Blind Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 2465. [Google Scholar] [CrossRef]
- Laigaard, J.; Pedersen, C.; Rønsbo, T.N.; Mathiesen, O.; Karlsen, A.P.H. Minimal clinically important differences in randomised clinical trials on pain management after total hip and knee arthroplasty: A systematic review. Br. J. Anaesth. 2021. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, F.; White, D.K.; Snyder-Mackler, L.; Zeni, J.A. Restoring physical function after knee replacement: A cross sectional comparison of progressive strengthening vs standard physical therapy. Physiother. Theory Pract. 2020, 36, 122–133. [Google Scholar] [CrossRef]
- Hamilton, D.F.; Loth, F.C.; MacDonald, D.J.; MacFarlane, G.J.; Beard, D.J.; Simpson, A.H.R.W.; Patton, J.T.; Howie, C.R. Exploring variation in patient access of post-discharge physiotherapy following total hip and knee arthroplasty under a choice based system in the UK: An observational cohort study. BMJ Open 2019, 9, e021614. [Google Scholar] [CrossRef] [PubMed]
| All Participants | Control | Placebo | Experimental | p-Value ** | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | Male | 15 | 35.7% | 5 | 35.7% | 4 | 28.6% | 6 | 42.9% | 0.733 |
| Female | 27 | 64.3% | 9 | 64.3% | 10 | 71.4% | 8 | 57.1% | ||
| TKR Side | Left | 20 | 47.6% | 3 | 21.4% | 8 | 57.1% | 9 | 64.3% | 0.052 |
| Right | 22 | 52.4% | 11 | 78.6% | 6 | 42.9% | 5 | 35.7% | ||
| M | SD | M | SD | M | SD | M | SD | |||
| Age | (years) | 69.36 | 7.16 | 69.71 | 6.89 | 69.29 | 8.33 | 69.07 | 6.68 | 0.973 |
| Weight | (kg) | 82.68 | 15.79 | 90.18 | 15.13 | 83.21 | 18.87 | 74.64 | 8.40 | 0.029 * |
| Height | (m) | 1.58 | 0.09 | 1.60 | 0.07 | 1.57 | 0.10 | 1.57 | 0.10 | 0.611 |
| BMI | 33.07 | 4.76 | 35.17 | 4.40 | 33.46 | 5.09 | 30.58 | 3.84 | 0.032 * | |
| Days since surgery | 20.24 | 13.86 | 20.50 | 14.55 | 23.50 | 16.92 | 16.71 | 9.11 | 0.441 | |
| VAS | 5.67 | 2.04 | 5.18 | 2.04 | 5.29 | 1.77 | 6.54 | 2.15 | 0.149 | |
| TUG | 16.98 | 10.39 | 13.89 | 3.73 | 15.90 | 11.02 | 21.17 | 13.21 | 0.161 | |
| FSST | 16.35 | 5.84 | 17.54 | 5.17 | 14.67 | 5.44 | 16.83 | 6.82 | 0.411 | |
| WOMAC | 27.73 | 16.73 | 26.29 | 9.90 | 26.15 | 14.49 | 30.85 | 24.04 | 0.726 | |
| PCS | 42.50 | 4.90 | 41.95 | 4.57 | 44.72 | 4.28 | 40.15 | 5.37 | 0.082 | |
| MCS | 37.21 | 8.52 | 39.08 | 8.32 | 37.39 | 9.87 | 34.05 | 6.47 | 0.396 | |
| CONTROL | Mean | SD | SE | Lower | Upper | p-Value | Cohen’s d | Effect |
|---|---|---|---|---|---|---|---|---|
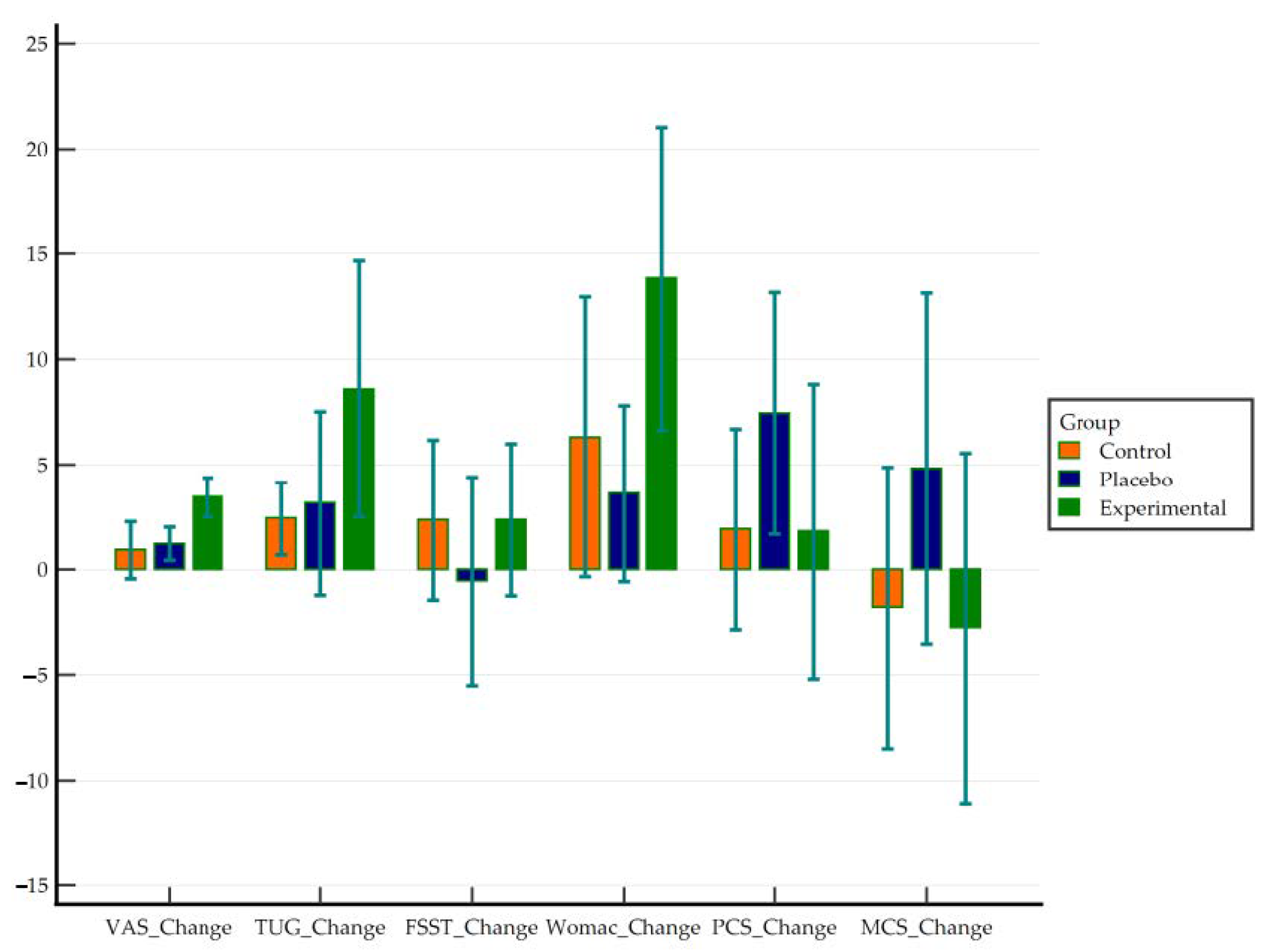
| VAS | 0.92 | 2.35 | 0.63 | −0.43 | 2.28 | 0.166 | 0.39 | Small |
| TUG | 2.42 | 2.87 | 0.79 | 0.69 | 4.15 | 0.01 * | 0.84 | Large |
| FSST | 2.36 | 6.61 | 1.77 | −1.46 | 6.17 | 0.205 | 0.36 | Small |
| WOMAC | 6.31 | 11 | 3.05 | −0.34 | 12.95 | 0.061 | 0.57 | Medium |
| PCS | 1.91 | 7.91 | 2.19 | −2.87 | 6.69 | 0.401 | 0.24 | Small |
| MCS | −1.8 | 11.07 | 3.07 | −8.49 | 4.88 | 0.567 | −0.16 | Negligible |
| PLACEBO | Mean | SD | SE | Lower | Upper | p-Value | Cohen’s d | Effect |
| VAS | 1.21 | 1.37 | 0.37 | 0.42 | 2 | 0.006 ** | 0.89 | Large |
| TUG | 3.15 | 7.58 | 2.03 | −1.23 | 7.53 | 0.144 | 0.42 | Small |
| FSST | −0.54 | 8.58 | 2.29 | −5.5 | 4.41 | 0.816 | −0.06 | Negligible |
| WOMAC | 3.62 | 6.93 | 1.92 | −0.58 | 7.81 | 0.085 | 0.52 | Medium |
| PCS | 7.43 | 9.52 | 2.64 | 1.68 | 13.18 | 0.016 * | 0.78 | Medium |
| MCS | 4.81 | 13.8 | 3.83 | −3.52 | 13.15 | 0.232 | 0.35 | Small |
| EXPERIMENTAL | Mean | SD | SE | Lower | Upper | p-Value | Cohen’s d | Effect |
| VAS | 3.44 | 1.6 | 0.43 | 2.52 | 4.37 | 0.000 *** | 2.15 | Large |
| TUG | 8.59 | 10.54 | 2.82 | 2.5 | 14.67 | 0.009 ** | 0.81 | Large |
| FSST | 2.36 | 6.27 | 1.67 | −1.26 | 5.98 | 0.183 | 0.38 | Small |
| WOMAC | 13.83 | 11.34 | 3.27 | 6.63 | 21.04 | 0.001 ** | 1.22 | Large |
| PCS | 1.81 | 8.38 | 2.96 | −5.2 | 8.82 | 0.561 | 0.22 | Small |
| MCS | −2.77 | 9.93 | 3.51 | −11.07 | 5.54 | 0.456 | −0.28 | Small |
| Variable | Control | Placebo | Experimental | p-Value | ETA2 | |||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |||
| VAS | 4.25 | 2.64 | 4.15 | 1.68 | 3.04 | 1.95 | 0.009 ** | 0.277 |
| TUG | 11.88 | 2.19 | 13.09 | 5.34 | 13.50 | 6.20 | 0.092 | 0.152 |
| FSST | 14.56 | 1.97 | 15.59 | 4.82 | 14.89 | 5.24 | 0.456 | 0.053 |
| WOMAC | 21.42 | 9.31 | 22.54 | 13.30 | 21.38 | 20.74 | 0.021 * | 0.235 |
| PCS | 39.18 | 4.38 | 37.29 | 10.88 | 37.56 | 5.97 | 0.276 | 0.085 |
| MCS | 41.81 | 7.55 | 32.57 | 9.52 | 37.49 | 7.46 | 0.219 | 0.099 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Marín, M.; Rodríguez-Almagro, D.; Castellote-Caballero, Y.; Achalandabaso-Ochoa, A.; Lomas-Vega, R.; Ibáñez-Vera, A.J. Efficacy of Non-Invasive Radiofrequency-Based Diathermy in the Postoperative Phase of Knee Arthroplasty: A Double-Blind Randomized Clinical Trial. J. Clin. Med. 2021, 10, 1611. https://doi.org/10.3390/jcm10081611
García-Marín M, Rodríguez-Almagro D, Castellote-Caballero Y, Achalandabaso-Ochoa A, Lomas-Vega R, Ibáñez-Vera AJ. Efficacy of Non-Invasive Radiofrequency-Based Diathermy in the Postoperative Phase of Knee Arthroplasty: A Double-Blind Randomized Clinical Trial. Journal of Clinical Medicine. 2021; 10(8):1611. https://doi.org/10.3390/jcm10081611
Chicago/Turabian StyleGarcía-Marín, Manuel, Daniel Rodríguez-Almagro, Yolanda Castellote-Caballero, Alexander Achalandabaso-Ochoa, Rafael Lomas-Vega, and Alfonso Javier Ibáñez-Vera. 2021. "Efficacy of Non-Invasive Radiofrequency-Based Diathermy in the Postoperative Phase of Knee Arthroplasty: A Double-Blind Randomized Clinical Trial" Journal of Clinical Medicine 10, no. 8: 1611. https://doi.org/10.3390/jcm10081611
APA StyleGarcía-Marín, M., Rodríguez-Almagro, D., Castellote-Caballero, Y., Achalandabaso-Ochoa, A., Lomas-Vega, R., & Ibáñez-Vera, A. J. (2021). Efficacy of Non-Invasive Radiofrequency-Based Diathermy in the Postoperative Phase of Knee Arthroplasty: A Double-Blind Randomized Clinical Trial. Journal of Clinical Medicine, 10(8), 1611. https://doi.org/10.3390/jcm10081611









