Experimental Methods to Simulate and Evaluate Postsurgical Peripheral Nerve Scarring
Abstract
:1. Introduction
2. Materials and Methods
3. Scar Simulation: Animal and Nerve Choice
4. Scar simulation: Experimental Methods to Induce Scar Formation
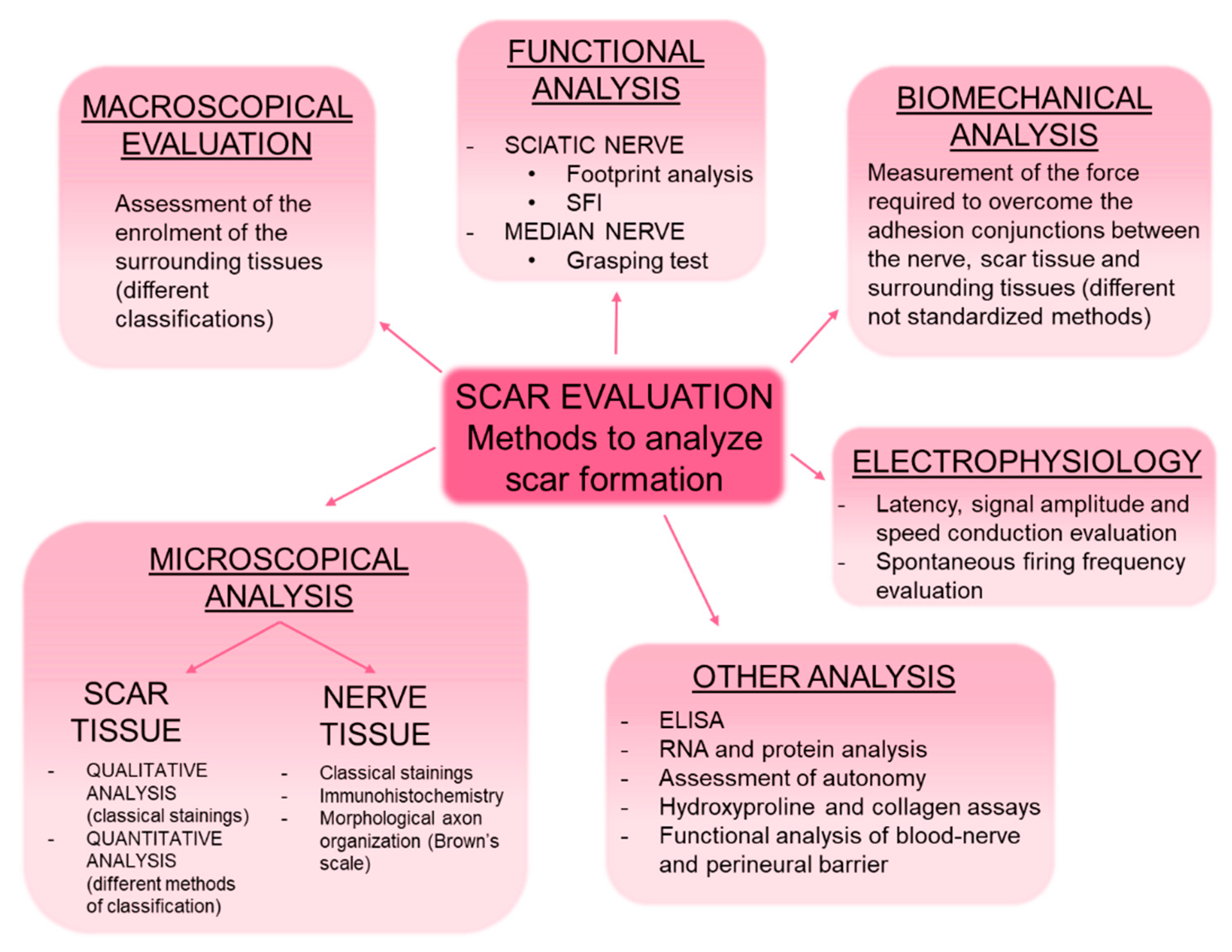
5. Scar Evaluation: Methods to Evaluate Scar Formation in Experimental Models
5.1. Macroscopical Analysis
5.2. Microscopical Analysis: Histological Staining and Immunohistochemistry
5.2.1. Analysis of the Scar Tissue
5.2.2. Analysis of the Nerve Tissue
5.3. Functional Analysis
5.4. Electrophysiological Study
5.5. Biomechanical Analysis
5.6. Other Analysis
6. How to Prevent Scar Formation? An Overview on Different Antiadhesion Devices
6.1. Polysaccharide-Based Devices
6.1.1. Hyaluronic acid
6.1.2. Carboxymethylcellulose
6.1.3. Chitosan
6.1.4. Other polysaccharides
6.2. Collagen-Based Devices
6.3. Autologous Devices
6.3.1. Amniotic Membrane
6.3.2. Fat Grafting
6.3.3. Vein Wrapping and Buccal Mucosa Graft
6.4. Drugs
6.5. Others
7. Discussion
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Tos, P.; Crosio, A.; Pugliese, P.; Adani, R.; Toia, F.; Artiaco, S. Topic: Peripheral Nerve Repair and Regeneration Painful scar neuropathy: Principles of diagnosis and treatment. Plast. Aesthet. Res. 2015, 2. Available online: www.parjournal.net (accessed on 18 May 2020). [CrossRef] [Green Version]
- Jones, N.F.; Ahn, H.C.; Eo, S. Revision surgery for persistent and recurrent carpal tunnel syndrome and for failed carpal tunnel release. Plast. Reconstr. Surg. 2012, 129, 683–692. [Google Scholar] [CrossRef]
- Siemionow, M.; Brzezicki, G. Chapter 8 Current Techniques and Concepts in Peripheral Nerve Repair. Int. Rev. Neurobiol. 2009, 87, 141–172. [Google Scholar] [CrossRef] [PubMed]
- Wiberg, M.; Terenghi, G. Will it be possible to produce peripheral nerves? Surg. Technol. Int. 2003, 11, 303–310. [Google Scholar]
- Geuna, S. Appreciating the difference between design-based and model-based sampling strategies in quantitative morphology of the nervous system. J. Comp. Neurol. 2000, 427, 333–339. [Google Scholar] [CrossRef]
- Siemionow, M.; Mendiola, A. Methods of assessment of cortical plasticity in patients following amputation, replantation, and composite tissue allograft transplantation. Ann. Plast. Surg. 2010, 65, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Amadio, P.C. Interventions for recurrent/persistent carpal tunnel syndrome after carpal tunnel release. J. Hand Surg. 2009, 34, 1320–1322. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, S. Nerves and Nerve Injuries, 2nd ed.; Churchill Livingstone: Edinburgh, UK, 1978. [Google Scholar]
- Wang, M.L.; Rivlin, M.; Graham, J.G.; Beredjiklian, P.K. Peripheral nerve injury, scarring, and recovery. Connect. Tissue Res. 2019, 60, 3–9. [Google Scholar] [CrossRef]
- Smit, X.; van Neck, J.W.; Afoke, A.; Hovius, S.E.R. Reduction of neural adhesions by biodegradable autocrosslinked hyaluronic acid gel after injury of peripheral nerves: An experimental study. J. Neurosurg. 2004, 101, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, G.; Morano, M.; Fregnan, F.; Pugliese, P.; Crosio, A.; Tos, P.; Geuna, S.; Haastert-Talini, K.; Gambarotta, G. The median nerve injury model in pre-clinical research—A critical review on benefits and limitations. Front. Cell. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [Green Version]
- Diogo, C.C.; Camassa, J.A.; Pereira, J.E.; da Costa, L.M.; Filipe, V.; Couto, P.A.; Geuna, S.; Maurício, A.C.; Varejão, A.S. The use of sheep as a model for studying peripheral nerve regeneration following nerve injury: Review of the literature. Neurol. Res. 2017, 39, 926–939. [Google Scholar] [CrossRef]
- Isla, A.; Martinez, J.; Perez-Lopez, C.; Conde, C.P.; Morales, C.; Budke, M. A reservable antiadhesion barrier gel reduces the perineural adhesions in rats after anastomosis/Comment. J. Neurosurg. Sci. 2003, 47, 195–199. Available online: http://search.proquest.com/openview/298ef0c6b31f2fb391e0685f5ab5c636/1?pq-origsite=gscholar&cbl=49236 (accessed on 19 May 2020). [PubMed]
- Kim, S.S.; Sohn, S.K.; Lee, K.Y.; Lee, M.J.; Roh, M.S.; Kim, C.H. Use of human amniotic membrane wrap in reducing perineural adhesions in a rabbit model of ulnar nerve neurorrhaphy. J. Hand Surg. Eur. Vol. 2010, 35, 214–219. [Google Scholar] [CrossRef]
- Ip, W.Y.; Shibata, T.; Tang, F.H.; Mak, A.F.T.; Chow, S.P. Adhesion formation after nerve repair: An experimental study of early protected mobilization in the rabbit. J. Hand Surg. 2000, 25, 582–584. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.R.; Fazio, A.; Costa, A.L.; Galletti, F.; lo Giudice, R.; Galletti, B.; Galletti, C.; lo Giudice, G.; Orabona, G.D.; Papalia, I.; et al. The Use of a Hypoallergenic Dermal Matrix for Wrapping in Peripheral Nerve Lesions Regeneration: Functional and Quantitative Morphological Analysis in an Experimental Animal Model. BioMed Res. Int. 2019, 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, Y.; Doi, K.; Kawai, S. An experimental model of peripheral nerve adhesion in rabbits. Br. J. Plast. Surg. 2005, 58, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Zanjani, L.O.; Firouzi, M.; Nabian, M.-H.; Nategh, M.; Rahimi-Movaghar, V.; Kamrani, R.S. Comparison and Evaluation of Current Animal Models for Perineural Scar Formation in Rat. Iran. J. Basic Med. Sci. 2013, 16, 890. [Google Scholar]
- Okuhara, Y.; Shinomiya, R.; Peng, F.; Kamei, N.; Kurashige, T.; Yokota, K.; Ochi, M. Direct effect of radiation on the peripheral nerve in a rat model. J. Plast. Surg. Hand Surg. 2014, 48, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Crosio, A.; Valdatta, L.; Cherubino, M.; Izzo, M.; Pellegatta, I.; Pascal, D.; Geuna, S.; Tos, P. A simple and reliable method to perform biomechanical evaluation of postoperative nerve adhesions. J. Neurosci. Methods 2014, 233, 73–77. [Google Scholar] [CrossRef]
- Lemke, A.; Penzenstadler, C.; Ferguson, J.; Lidinsky, D.; Hopf, R.; Bradl, M.; Redl, H.; Wolbank, S.; Hausner, T. A novel experimental rat model of peripheral nerve scarring that reliably mimics post-surgical complications and recurring adhesions. Dis. Models Mech. 2017, 10, 1015–1025. [Google Scholar] [CrossRef] [Green Version]
- Zuijdendorp, H.; Smit, X.; Blok, J.; Caruelle, J.; Barritault, D.; Hovius, S.; van Neck, J. Significant reduction in neural adhesions after administration of the regenerating agent OTR4120, a synthetic glycosaminoglycan mimetic, after peripheral nerve injury. J. Neurosurg. 2008, 109, 967–973. Available online: https://thejns.org/view/journals/j-neurosurg/109/5/article-p967.xml (accessed on 19 May 2020). [CrossRef] [PubMed] [Green Version]
- Palatinsky, E.A.; Maier, K.H.; Touhalisky, D.K.; Mock, J.L.; Hingson, M.T.; Coker, G.T. ADCON®-T/N reduces in vivo perineural adhesions in a rat sciatic nerve reoperation model. J. Hand Surg. Eur. Vol. 1997, 22, 331–335. [Google Scholar] [CrossRef]
- Ikeda, K.; Yamauchi, D.; Osamura, N.; Hagiwara, N.; Tomita, K. Hyaluronic acid prevents peripheral nerve adhesion. Br. J. Plast. Surg. 2003, 56, 342–347. [Google Scholar] [CrossRef]
- Xu, J.; Varitimidis, S.E.; Fisher, K.J.; Tomaino, M.M.; Sotereanos, D.G. The effect of wrapping scarred nerves with autogenous vein graft to treat recurrent chronic nerve compression. J. Hand Surg. 2000, 25, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Animals NRC (US). C on R and A of P in L. Recognition and Alleviation of Pain in Laboratory Animals; National Academies Press: Washington, DC, USA, 2010. [Google Scholar] [CrossRef]
- Petersen, J.; Russell, L.; Andrus, K.; MacKinnon, M.; Silver, J.; Kliot, M. Reduction of extraneural scarring by ADCON-T/N after surgical intervention. Neurosurgery 1996, 38, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Dam-Hieu, P.; Lacroix, C.; Said, G.; Devanz, P.; Liu, S.; Tadie, M. Reduction of postoperative perineural adhesions by hyaloglide gel: An experimental study in the rat sciatic nerve. Neurosurgery 2005, 56 (Suppl. S4). [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.C.U.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef]
- Albayrak, B.S.; Ismailoglu, O.; Ilbay, K.; Yaka, U.; Tanriover, G.; Gorgulu, A.; Demir, N. Doxorubicin for prevention of epineurial fibrosis in a rat sciatic nerve model: Outcome based on gross postsurgical, histopathological, and ultrastructural findings—Laboratory investigation. J. Neurosurg. Spine 2010, 12, 327–333. [Google Scholar] [CrossRef]
- Özay, R.; Bekar, A.; Kocaeli, H.; Karli, N.; Filiz, G.; Ulus, I.H. Citicoline improves functional recovery, promotes nerve regeneration, and reduces postoperative scarring after peripheral nerve surgery in rats. Surg. Neurol. 2007, 68, 615–622. [Google Scholar] [CrossRef]
- Ilbay, K.; Etus, V.; Yildiz, K.; Ilbay, G.; Ceylan, S. Topical application of mitomycin C prevents epineural scar formation in rats. Neurosurg. Rev. 2005, 28, 148–153. [Google Scholar] [CrossRef]
- Görgülü, A.; Imer, M.; Şimşek, O.; Sencer, A.; Kutlu, K.; Çobanoǧlu, S. The effect of aprotinin on extraneural scarring in peripheral nerve surgery: An experimental study. Acta Neurochir. 1998, 140, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Görgülü, A.; Uzal, C.; Doǧanay, L.; Imer, M.; Eliuz, K.; Çobanoǧlu, S.; Loeffler, J.S.; Gerszten, P.C.; Kondziolka, D.; Kline, D.G. The effect of low-dose external beam radiation on extraneural scarring after peripheral nerve surgery in rats. Neurosurgery 2003, 53, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Özgenel, G.Y. Effects of hyaluronic acid on peripheral nerve scarring and regeneration in rats. Microsurgery 2003, 23, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Mekaj, A.Y.; Manxhuka-Kerliu, S.; Morina, A.A.; Duci, S.B.; Shahini, L.; Mekaj, Y.H. Effects of hyaluronic acid and tacrolimus on the prevention of perineural scar formation and on nerve regeneration after sciatic nerve repair in a rabbit model. Eur. J. Trauma Emerg. Surg. 2017, 43, 497–504. [Google Scholar] [CrossRef]
- Marcol, W.; Larysz-Brysz, M.; Kucharska, M.; Niekraszewicz, A.; Slusarczyk, W.; Kotulska, K.; Wlaszczuk, P.; Wlaszczuk, A.; Jedrzejowska-Szypulka, H.; Lewin-Kowalik, J. Reduction of post-traumatic neuroma and epineural scar formation in rat sciatic nerve by application of microcrystallic chitosan. Microsurgery 2011, 31, 642–649. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, J.H.; Han, C.S.; Chung, D.W.; Kim, G.Y. Effect of hyaluronic acid-carboxymethylcellulose solution on perineural scar formation after sciatic nerve repair in rats. Clin. Orthop. Surg. 2011, 3, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Meng, H.; Li, M.; You, F.; Du, J.; Luo, Z. Assessment of processed human amniotic membrane as a protective barrier in rat model of sciatic nerve injury. Neurosci. Lett. 2011, 496, 48–53. [Google Scholar] [CrossRef]
- Özgenel, G.Y.; Fílíz, G. Combined application of human amniotic membrane wrapping and hyaluronic acid injection in epineurectomized rat sciatic nerve. J. Reconstr. Microsurg. 2004, 20, 153–157. [Google Scholar] [CrossRef]
- Vural, E.; Yilmaz, M.; Ilbay, K.; Ilbay, G. Prevention of epineural fibrosis in rats by local administration of mitomycin C or daunorubicin. Turk. Neurosurg. 2016, 26, 291–296. [Google Scholar] [CrossRef]
- Sakurai, M.; Miyasaka, Y. Neural fibrosis and the effect of neurolysis. J. Bone Joint Surg. Ser. B 1986, 68, 483–488. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Revel, M.; Loty, B. A quantitative model of post-laminectomy scar formation: Effects of a nonsteroidal anti-inflammatory drug. Spine 1995, 20, 557–563. [Google Scholar] [CrossRef]
- Hinton, J.L.; Warejcka, D.J.; Mei, Y.; McLendon, R.E.; Laurencin, C.; Lucas, P.A.; Robinson, J.S. Inhibition of epidural scar formation after lumbar laminectomy in the rat. Spine 1995, 20, 564–570. [Google Scholar] [CrossRef]
- Ornelas, L.; Padilla, L.; di Silvio, M.; Schalch, P.; Esperante, S.; Infante, R.L.; Bustamante, J.C.; Avalos, P.; Varela, D.; López, M. Fibrin glue: An alternative technique for nerve coaptation—Part II. Nerve regeneration and histomorphometric assessment. J. Reconstr. Microsurg. 2006, 22, 123–128. [Google Scholar] [CrossRef]
- Baltu, Y.; Uzun, H.; Özgenel, G.Y. The reduction of extraneural scarring with buccal mucosa graft wrapping around the sciatic nerve: An experimental study in a rat model. J. Plast. Surg. Hand Surg. 2017, 51, 259–263. [Google Scholar] [CrossRef]
- Mathieu, L.; Adam, C.; Legagneux, J.; Bruneval, P.; Masmejean, E. Reduction of neural scarring after peripheral nerve suture: An experimental study about collagen membrane and autologous vein wrapping. Chir. Main 2012, 31, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, H.; Huang, H.; Bi, W.; Yan, R.; Tan, X.; Wen, W.; Wang, C.; Song, W.; Zhang, Y.; et al. Chitosan conduit combined with hyaluronic acid prevent sciatic nerve scar in a rat model of peripheral nerve crush injury. Mol. Med. Rep. 2018, 17, 4360–4368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adanali, G.; Verdi, M.; Tuncel, A.; Erdogan, B.; Kargi, E. Effects of hyaluronic acid-carboxymethylcellulose membrane on extraneural adhesion formation and peripheral nerve regeneration. J. Reconstr. Microsurg. 2003, 19, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Ngeow, W.C.; Atkins, S.; Morgan, C.R.; Metcalfe, A.D.; Boissonade, F.M.; Loescher, A.R.; Robinson, P.P. A comparison between the effects of three potential scar-reducing agents applied at a site of sciatic nerve repair. Neuroscience 2011, 181, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Ngeow, W.C.; Atkins, S.; Morgan, C.R.; Metcalfe, A.D.; Boissonade, F.M.; Loescher, A.R.; Robinson, P.P. The effect of Mannose-6-Phosphate on recovery after sciatic nerve repair. Brain Res. 2011, 1394, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Atkins, S.; Smith, K.G.; Loescher, A.R.; Boissonade, F.M.; Ferguson, M.W.J.; Robinson, P.P. The effect of antibodies to TGF-β1 and TGF-β2 at a site of sciatic nerve repair. J. Peripher. Nerv. Syst. 2006, 11, 286–293. [Google Scholar] [CrossRef]
- Atkins, S.; Loescher, A.R.; Boissonade, F.M.; Smith, K.G.; Occleston, N.; O’Kane, S.; Ferguson, M.W.J.; Robinson, P.P. Interleukin-10 reduces scarring and enhances regeneration at a site of sciatic nerve repair. J. Peripher. Nerv. Syst. 2007, 12, 269–276. [Google Scholar] [CrossRef]
- Geuna, S. The revolution of counting “tops”: Two decades of the disector principle in morphological research. Microsc. Res. Tech. 2005, 66, 270–274. [Google Scholar] [CrossRef]
- Brown, R.E.; Erdmann, D.; Lyons, S.F.; Suchy, H. The use of cultured Schwann cells in nerve repair in a rabbit hind-limb model. J. Reconstr. Microsurg. 1996, 12, 149–152. [Google Scholar] [CrossRef]
- Aslan, E.; Kocaeli, H.; Bekar, A.; Tolunay, Ş.; Ulus, I.H. CDP-choline and its endogenous metabolites, cytidine and choline, promote the nerve regeneration and improve the functional recovery of injured rat sciatic nerves. Neurol. Res. 2011, 33, 766–773. [Google Scholar] [CrossRef]
- Shintani, K.; Uemura, T.; Takamatsu, K.; Yokoi, T.; Onode, E.; Okada, M.; Nakamura, H. Protective effect of biodegradable nerve conduit against peripheral nerve adhesion after neurolysis. J. Neurosurg. 2018, 129, 815–824. [Google Scholar] [CrossRef] [Green Version]
- Que, J.; Cao, Q.; Sui, T.; Du, S.; Kong, D.; Cao, X. Effect of FK506 in reducing scar formation by inducing fibroblast apoptosis after sciatic nerve injury in rats. Cell Death Dis. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Servet, E.; Bekler, H.; Kilinçoğlu, V.; Özler, T.; Özkut, A. Effect of bleeding on nerve regeneration and epineural scar formation in rat sciatic nerves: An experimental study. Acta Orthop. Traumatol. Turc. 2016, 50, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.W.; Jiao, J.B.; Liu, X.F.; Jiang, Y.T.; Yang, G.; Li, C.Y.; Yin, W.T.; Ling, L. Inhibition of peripheral nerve scarring by calcium antagonists, also known as calcium channel blockers. Artif. Organs 2016, 40, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Tapadia, M.D.; Palispis, W.; Luu, M.; Wang, W.; Gupta, R. Attenuation of robust glial scar formation facilitates functional recovery in animal models of chronic nerve compression injury. J. Bone Joint Surg. Am. Vol. 2017, 99, e132. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Kuniyoshi, K.; Iwakura, N.; Matsuura, Y.; Suzuki, T.; Takahashi, K.; Ohtori, S. Vein wrapping for chronic nerve constriction injury in a rat model: Study showing increases in VEGF and HGF production and prevention of pain-associated behaviors and nerve damage. J. Bone Joint Surg. Am. Vol. 2014, 96, 859–867. [Google Scholar] [CrossRef]
- Bain, J.R.; Mackinnon, S.E.; Hunter, D.A. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast. Reconstr. Surg. 1989, 83, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Atsuta, Y. The effects of experimental neurolysis on ectopic firing in a rat chronic constriction nerve injury model. J. Hand Surg. 2006, 31, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Stößel, M.; Rehra, L.; Haastert-Talini, K. Reflex-based grasping, skilled forelimb reaching, and electrodiagnostic evaluation for comprehensive analysis of functional recovery—The 7-mm rat median nerve gap repair model revisited. Brain Behav. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Dumanian, G.A.; McClinton, M.; Brushart, T.M. The effects of free fat grafts on the stiffness of the rat sciatic nerve and perineural scar. J. Hand Surg. 1999, 24, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Endo, N.; Ito, M.; Okui, N.; Koh, S.; Kaneko, H.; Hirata, H. Novel polysaccharide-derived hydrogel prevents perineural adhesions in a rat model of sciatic nerve adhesion. J. Orthop. Res. 2010, 28, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Urano, H.; Iwatsuki, K.; Yamamoto, M.; Ohnisi, T.; Kurimoto, S.; Endo, N.; Hirata, H. Novel anti-adhesive CMC-PE hydrogel significantly enhanced morphological and physiological recovery after surgical decompression in an animal model of entrapment neuropathy. PLoS ONE 2016, 11, e0164572. [Google Scholar] [CrossRef] [PubMed]
- Ohsumi, H.; Hirata, H.; Nagakura, T.; Tsujii, M.; Sugimoio, T.; Miyamoto, K.; Horiuchi, T.; Nagao, M.; Nakashima, T.; Uchida, A. Enhancement of perineurial repair and inhibition of nerve adhesion by viscous injectable pure alginate sol. Plast. Reconstr. Surg. 2005, 116, 823–830. [Google Scholar] [CrossRef]
- Cherubino, M.; Pellegatta, I.; Crosio, A.; Valdatta, L.; Geuna, S.; Gornati, R.; Tos, P. Use of human fat grafting in the prevention of perineural adherence: Experimental study in athymic mouse. PLoS ONE 2017, 12, e0176393. [Google Scholar] [CrossRef] [Green Version]
- Tos, P.; Crosio, A.; Pellegatta, I.; Valdatta, L.; Pascal, D.; Geuna, S.; Cherubino, M. Efficacy of anti-adhesion gel of carboxymethylcellulose with polyethylene oxide on peripheral nerve: Experimental results on a mouse model. Muscle Nerve 2016, 53, 304–309. [Google Scholar] [CrossRef]
- Magill, C.K.; Tuffaha, S.H.; Yee, A.; Luciano, J.P.; Hunter, D.A.; Mackinnon, S.E.; Borschel, G.H. The short- and long-term effects of Seprafilm® on peripheral nerves: A histological and functional study. J. Reconstr. Microsurg. 2009, 25, 345–354. [Google Scholar] [CrossRef]
- Hachinota, A.; Tada, K.; Yamamoto, D.; Nakajima, T.; Nakada, M.; Tsuchiya, H. Preventive effect of alginate gel formulation on perineural adhesion. J. Hand Surg. Asian Pac. Vol. 2020, 25, 164–171. Available online: https://pubmed-ncbi-nlm-nih-gov.bibliopass.unito.it/32312202/ (accessed on 14 December 2020). [CrossRef] [PubMed]
- Hernández-Cortés, P.; Peregrina, M.; Aneiros-Fernández, J.; Tassi, M.; Pajares-López, M.; Toledo, M.; O’Valle, F. Oxidized regenerated cellulose does not prevent the formation of experimental postoperative perineural fibrosis assessed by digital analysis. Histol. Histopathol. 2010, 25, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.D.; Hayes, A.; Amin, F.; Akelina, Y.; Hays, A.P.; Rosenwasser, M.P. Collagen nerve protector in rat sciatic nerve repair: A morphometric and histological analysis. Microsurgery 2010, 30, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Parisi, T.J.; Friedrich, P.F.; Bishop, A.T.; Shin, A.Y. Does the addition of a nerve wrap to a motor nerve repair affect motor outcomes? Microsurgery 2014, 34, 562–567. [Google Scholar] [CrossRef]
- Jay, R.M.; Huish, J.P.; Wray, J.H. Amniotic membrane in clinical medicine: History, current status, and future use. In Extracellular Matrix-Derived Implants in Clinical Medicine; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 151–176. [Google Scholar] [CrossRef]
- Kaplan, T.; Kafa, I.M.; Cansev, M.; Bekar, A.; Karli, N.; Taskapilioglu, M.O.; Kanar, F. Investigation of the dose-dependency of citicoline effects on nerve regeneration and functional recovery in a rat model of sciatic nerve injury. Turk. Neurosurg. 2014, 24, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Shahraki, M.; Mohammadi, R.; Najafpour, A. Influence of tacrolimus (FK506) on nerve regeneration using allografts: A rat sciatic nerve model. J. Oral Maxillofac. Surg. 2015, 73, 1438.e1–1438.e9. [Google Scholar] [CrossRef]
- Davis, B.; Hilgart, D.; Erickson, S.; Labroo, P.; Burton, J.; Sant, H.; Shea, J.; Gale, B.; Agarwal, J. Local FK506 delivery at the direct nerve repair site improves nerve regeneration. Muscle Nerve 2019, 60, 613–620. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Macchi, V.; Tiengo, C.; Petrelli, L.; Rambaldo, A.; Borean, A.; Capelli, S.; Filippi, A.; Romanato, F.; et al. New bioresorbable wraps based on oxidized polyvinyl alcohol and leukocyte-fibrin-platelet membrane to support peripheral nerve neurorrhaphy: Preclinical comparison versus NeuraWrap. Sci. Rep. 2019, 9. Available online: https://pubmed-ncbi-nlm-nih-gov.bibliopass.unito.it/31748615/ (accessed on 14 December 2020). [CrossRef]
- Kikuchi, K.; Setoyama, K.; Takada, S.; Otsuka, S.; Nakanishi, K.; Norimatsu, K.; Tani, A.; Sakakima, H.; Kawahara, K.I.; Hosokawa, K.; et al. E8002 inhibits peripheral nerve adhesion by enhancing fibrinolysis of l-ascorbic acid in a rat sciatic nerve model. Int. J. Mol. Sci. 2020, 21, 3972. Available online: https://pubmed-ncbi-nlm-nih-gov.bibliopass.unito.it/32492845/ (accessed on 14 December 2020). [CrossRef]
- Okui, N.; Yamamoto, M.; Fukuhira, Y.; Kaneko, H.; Hirata, H. Artificial perineurium to enhance nerve recovery from damage after neurolysis. Muscle Nerve 2010, 42, 570–575. [Google Scholar] [CrossRef]
- Ikeda, K.; Yamauchi, D.; Tomita, K. Preliminary study for prevention of neural adhesion using an absorbable oxidised regenerated cellulose sheet. Hand Surg. 2002, 7, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Masear, V.R.; Tullos, J.; St Mary, E.; Mayer, R. Venous wrapping of nerves to prevent to prevent scarring. J. Hand Surg. 1990, 15, 817–818. Available online: https://ci.nii.ac.jp/naid/10026286269/ (accessed on 23 March 2021).
- Millesi, H.; Zoch, G.; Reihsner, R. Mechanical properties of peripheral nerves. Clin. Orthop. Relat. Res. 1995, 314, 76–83. Available online: https://europepmc.org/article/med/7634654 (accessed on 23 March 2021). [CrossRef]
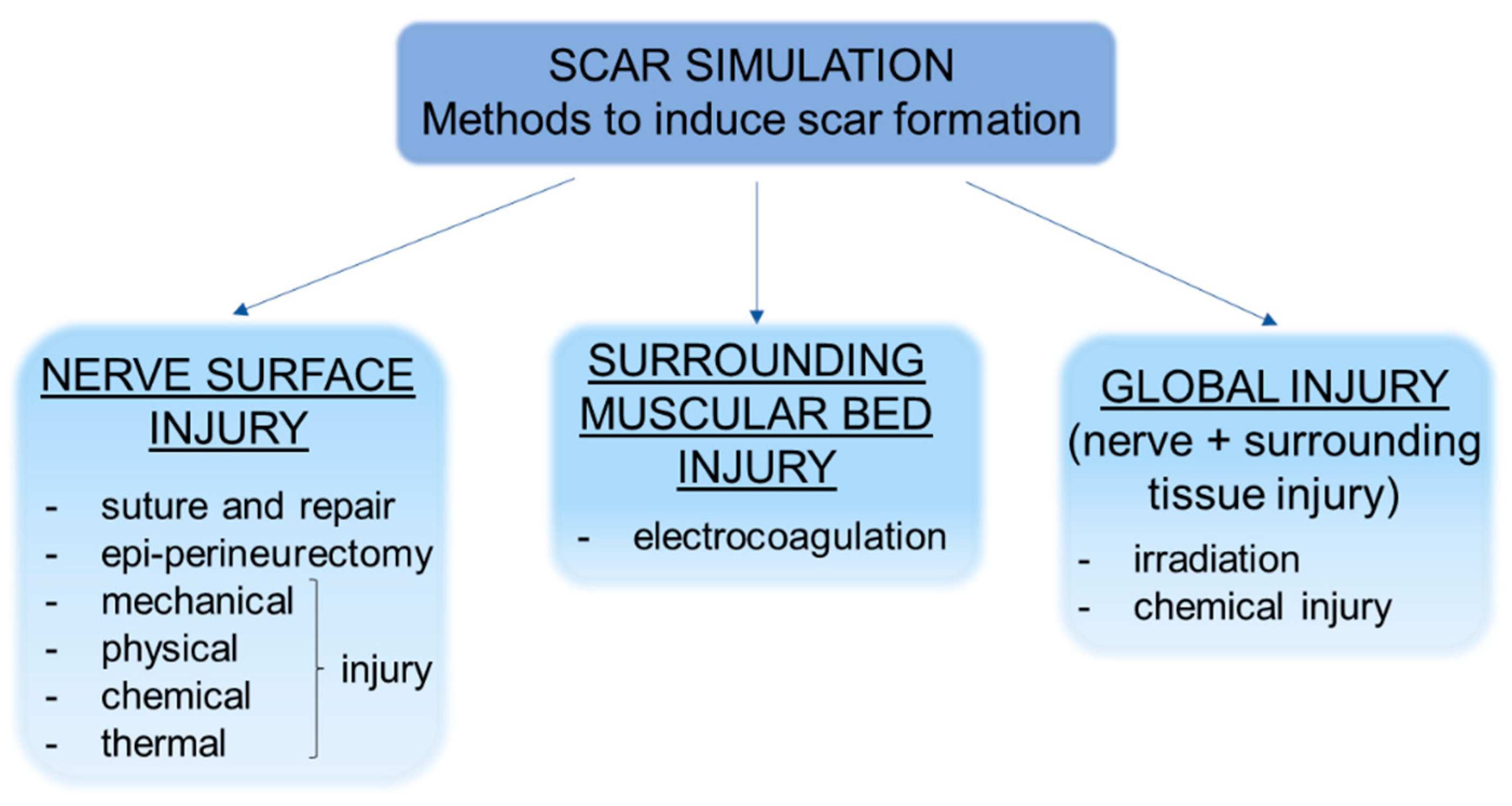
| Reference | Method to Induce Scar Formation | Animal and Nerve Model | Analyses | Results |
|---|---|---|---|---|
| Lemke et al., 2017 [21] | Application of “glutaraldehyde glue” on the nerve and surrounding muscle or scratching | Female Sprague–Dawley Rat Sciatic nerve |
| Severe intra- and perineural scarring, vigorous nerve inflammation and nerve degeneration and functional deficit. |
| Crosio et al., 2014 [20] | Burning or scratching | Male Mouse Sciatic nerve |
| Both methods produced fibrotic reactions with no differences in biomechanical results between the two methods; histology showed a different distribution pattern of the scar tissue. |
| Okuhara Y et al., 2014 [19] | Irradiation of the nerve with X-radiation | Female LEW/CrlCrlj Rat Sciatic nerve |
| Scar formation around the radiated nerve. No differences in SFI between groups, but axonal degeneration in the irradiated nerve. |
| Zanjani et al., 2013 [18] | Laceration, crush, mince, and burn of the surrounding muscles | Female Wistar Rat Sciatic nerve |
| Scar tissue formation surrounding the sciatic nerve in gross examination and histological analysis; no differences in functional assessment compared to control. |
| Abe et al., 2005 [17] | Nerve bed cauterization and suturing the nerve in place | Male Japanese White Rabbit Sciatic nerve |
| Adhesion of peripheral nerve to surrounding tissues results in fibrosis in the nerve. Compound muscle action potentials were reduced in amplitude, and blood flow was significantly decreased at adhesion sites in Group IIb. |
| Reference | Method to Induce Scar Formation | Agent | Animal and Nerve Model | Analyses | Results |
|---|---|---|---|---|---|
| Hachinota et al., 2020 [73] | Section of transvers carpal ligament, excision of median nerve bed, suture of carpal ligament | Alginic acid-based gel formulation | Japanese White Rabbit Median nerve |
| Longer latency, not significant, in the control group. Lower adhesion score values in the treatment group at 2–3–6 weeks, more scar tissue in the control group. More severe perineural fibrosis in the control group. |
| Li et al., 2018 [48] | Crush injury | Chitosan conduit (CC); hyaluronic acid (HA); CC + HA | Sprague–Dawley RatSciatic nerve |
| Both chitosan and HA inhibited extraneural scarring, promoted nerve regeneration, increased nerve conduction velocity, and improved the recovery of nerve function. |
| Mekaj et al., 2017 [36] | Section + suture | HA | Male European Rabbit Sciatic nerve |
| Reduction scar around the nerve, both macroscopically and microscopically. Increased nerve diameter. Higher gastrocnemius mass. Improved microstructural organization. Higher expression of S100. |
| Tos et al., 2016 [71] | Burning | Carboxymethylcellulose (CMC)-polyethylene oxide (PEO) gel | CD1 Mouse Sciatic nerve |
| Reduction in scar tissue after CMC–PEO gel application. The qualitative histological analysis supported the biomechanical findings depicting the pattern of scar tissue. |
| Urano et al., 2016 [68] | Enwrapping with silicon tube (nerve compression) | CMC-phosphatidyl-ethanolamine (PE) | Male Lewis RatSciatic nerve |
| Electrophysiology showed significantly quicker recovery; mean wet muscle weight was constantly higher; the axon area at one month was twice as large as control. |
| Marcol et al., 2011 [37] | Section + suture | Chitosan | Male Wistar Rat Sciatic nerve |
| High incidence of amputations (about 100%, no sig. diff.). Reduction in microscopical analysis of neuroma in chitosan; significant reduction of scar around nerve; increased mast cells and macrophages in chitosan.Application of the microcrystalic chitosan gel is easy and requires no special equipment but does not influence the features of neuropathic pain. |
| Park et al., 2011 [38] | Section + suture | HA-CMC | Sprague–Dawley Rat Sciatic nerve |
| Macroscopical scar reduction. Reduction of inflammation cells and fibroblasts. Reduction of scar formation index. Better axonal organization. |
| Hernández-Cortés et al., 2010 [74] | Tissue aggression (cauterization of muscle bed) | Oxidized regenerated cellulose | Male Sprague-Dawley RatSciatic nerve |
| No statistical differences in intra- and perineural scars, which demonstrate no antifibrogenic effect of oxidized regenerated cellulose. Inflammatory phenomena and foreign body granulomatous reactions were more frequently detected in oxidized regenerated cellulose-treated samples. |
| Yamamoto et al., 2010 [67] | Burning muscle + epi- and perineurium removal | CMC–PE | Lewis Rat Sciatic nerve |
| CMC–PE hydrogel offered superior efficacy to 1% HA and caused no delay in wound healing. Reduction of macroscopical scar, reduction of scar in biomechanical testing. Electrophysiological and muscle weight analyses demonstrated the effectiveness of CMC–PE treatment after extensive internal neurolysis. |
| HA | |||||
| Magill et al., 2009 [72] | Section + suture | HA-CMC (Seprafilm) | Male Lewis Rat Sciatic nerve |
| Qualitatively less perineural scar tissue was observed using Seprafilm. No functional or histological deleterious effects were detected with Seprafilm placed on intact nerves or cut and repaired nerves. |
| Zuijdendorp et al., 2008 [22] | Crush injury | Regenerating agents (sulfated glycosaminoglycan) | Female Wistar RatSciatic nerve |
| Reduction of biomechanical resistance. No differences in magnetoneurography and functional analysis were detected. |
| Dam-Hieu et al., 2005 [28] | Abrasive injury/section + suture | Auto cross-linked polysaccharide (ACP) with different viscosity | Male Sprague–Dawley Rat Sciatic nerve |
| Significant reduction of scar tissue formation was observed through macro and micro analyses. |
| Ohsumi et al., 2005 [69] | Burning | Alginate sol | Lewis Rat Sciatic nerve |
| Strong inhibition of perineurial granulation, recovering of the perineurial barrier function, antiadhesive effect. |
| Smit et al., 2004 [10] | Section + suture | HA | Female Wistar Rat Sciatic nerve |
| Significant biomechanical reduction of adhesion after HA application. |
| Ikeda et al., 2003 [24] | Burning muscular bed + suture | HA (after 6 weeks) | White Japanese RabbitSciatic nerve |
| Significant latency reduction. Qualitative reduction of scar in microscopical analysis. No significant reduction in biomechanical analysis. |
| Ozgenel et al., 2003 [35] | Section + suture | HA | Male Sprague–Dawley Rat Sciatic nerve |
| Significant reduction in scarring, better conduction velocities, increased axon and fiber diameter, and faster functional recovery. |
| Adanali et al., 2003 [49] | Section + suture | HA–CMC | White New Zealand Rabbit Sciatic nerve |
| Macroscopically reduction of scar tissue around the nerve. Increased quality of myelin sheets and the number of axons. |
| Reference | Method to Induce Scar Formation | Agent | Animal and Nerve Model | Analyses | Results |
|---|---|---|---|---|---|
| Colonna et al., 2019 [16] | Section + suture | Collagen sheath derived from an acellular hypoallergenic dermal matrix (OrACELL) | Female Wistar Rat Median nerve |
| Axon diameter was higher in the treated group. No significant differences in the functional test were observed. |
| Lee et al., 2014 [76] | Section + suture | Collagen-based film (NeuraGen) | Male Wistar Rat Sciatic nerve |
| Reduction of scar in microscopical analysis, although the scar-decreasing effect of bioabsorbable nerve wrap did not translate into a better motor nerve recovery. |
| Mathieu et al., 2012 [47] | Section + suture | Collagen membrane and vein wrapping | Female Wistar Rat Sciatic nerve |
| The collagen membrane was effective in reducing neural scar formation. Autologous vein wrapping also showed a favorable effect in this indication despite less successful histological outcomes. |
| Kim et al., 2010 [75] | Section + suture | Collagen wrap | Sprague–Dawley Rat Sciatic nerve |
| Significant reduction of inner epineurium thickness in the treated group. |
| Isla et al., 2003 [13] | Section + suture or repair with silastic tube | ADCON/TN | Male Wistar Rat Ulnar nerve |
| Significant reduction of fibrosis. No differences in terms of fiber density. |
| Palatinsky et al., 1997 [23] | Scratch; a second neurolysis performed 4 weeks later | ADCON/TN (applied after the second neurolysis) | Sprague Dawley Rat Sciatic nerve |
| Significant reduction of composite score (macroscopical evaluation). No statistical difference in axons diameter. |
| Petersen et al., 1996 [27] | External neurolysis, abrasive injury on muscle and nerve, section + suture | ADCON/TN | Lewis’s Albino Rat Sciatic nerve |
| Significant reduction of scar tissue; no differences in morphometrical analysis. |
| Reference | Method to Induce Scar Formation | Agent | Animal and Nerve Model | Analyses | Results |
|---|---|---|---|---|---|
| Cherubino et al., 2017 [70] | Burning | Fat Graft | CD1 nude Mouse Sciatic nerve |
| No significant difference in biomechanical analysis. Reduction of scar observed through microscopical analysis |
| Baltu et al., 2017 [46] | Epineurectomy | Buccal mucosa graft | Female Sprague-Dawley Rat Sciatic nerve |
| Buccal mucosa graft decreases postoperative adhesion and scar tissue formation. Higher inflammation at 4 weeks. |
| Murakami et al., 2014 [62] | Ligature on sciatic nerve | Vein Wrapping | Male Wistar Rat Sciatic nerve |
| Significant allodynia reduction. Significant increase in VEGF and HGF. Reduction of immunoreactive cells in dorsal root ganglia. |
| Meng et al., 2011 [39] | Section + suture | Amniotic membrane | Male Sprague–Dawley Rat Sciatic nerve |
| Significant reduction of scar index. No functional and morphological differences were observed. |
| Kim et al., 2010 [14] | Section + suture | Amniotic membrane | White New Zealand Rabbit Ulnar nerve |
| Four-point evaluation system was significant in the treatment group. Significant reduction of scar thickness. |
| Ozgenel et al., 2004 [40] | Epineurectomy | Amniotic membrane + HA | Male Sprague–Dawley Rat Sciatic nerve |
| Significant reduction in scarring was observed through microscopical analysis. |
| Xu et al., 2000 [25] | Silastic tube around the nerve | Vein wrapping (after 8 months from nerve compression) | Sprague–Dawley Rat Sciatic nerve |
| Significant improvement in functional analysis. Electromyography and microscopical analysis showed no significant scar reduction. |
| Dumanian et al., 1999 [66] | Epineurectomy | Fat graft | Sprague–Dawley Rat Sciatic nerve |
| Significant reduction of nerve stiffness in biomechanical analysis. Insignificant reduction of scar thickness in microscopical analysis. Higher but not significant incidence of neuropathy in fat-graft group. |
| Reference | Method to Induce Scar Formation | Agent | Animal and Nerve model | Analyses | Results |
|---|---|---|---|---|---|
| Mekaj et al., 2017 [36] | Section + suture | Tacrolimus (FK506) | Male European Rabbit Sciatic nerve |
| Scar reduction around the nerve, both macroscopically and microscopically. Increased nerve diameter. Higher gastrocnemius mass. Improved microstructural organization. Higher expression of S100. |
| Zhu et al., 2017 [61] | Silicone tube around the nerve | Decompression and chondroitinase ABC (6 weeks after compression injury) | Male Sprague–Dawley Rat and Male C57BL/6 Mouse Sciatic nerve |
| Surgical decompression alone does not reverse the functional changes to the nerve, whereas the administration of chondroitinase-ABC, in addition to decompression, resulted in functional improvement. |
| Vural et al., 2016 [41] | Abrasion | Mitomycin C/ Daunorubicin | Male Wistar Rat Sciatic nerve |
| Macroscopically, mitomycin C, and daunorubicin decreased adhesion. Scar tissue thickness and fibroblast/fibrocyte cell number were reduced. |
| Xue et al., 2016 [60] | Section + suture | Verapamil (calcium channel blockers) | Sprague–Dawley Rat Sciatic nerve |
| The collagen content of nerve scar was apparently less than that of the control group; more cytoplasmic vesicles in the fibroblasts of the treated group were observed. |
| Kaplan et al., 2014 [78] | Section + suture | Citicoline | Female Wistar Albino Rat Sciatic nerve |
| Improvement of SFI. Significant reduction in scarring. Significant increase in myelinated axons in C900 and reduction of scar in the treated group. |
| Que et al., 2013 [58] | Section + suture | Tacrolimus (FK506) | Male Sprague–Dawley Rat Sciatic nerve |
| FK506 has a valid effect on scar formation reduction in sciatic nerve-injured rat by inducing fibroblast apoptosis. |
| Ngeow et al., 2011 [50] | Section + suture | Triamcinolone acetonide, Interleukin-10 (IL 10), mannose-6-phosphate (M6P) | C57 Black-6 Mouse Sciatic nerve |
| The percentage of scarring was not significantly different between methods in microscopical analysis. Reduction of compound action potential in triamcinolone and M6P 200 was observed through EMG. |
| Ngeow et al., 2011 [51] | Section + suture | Mannose-6-phosphate | C57 Black-6 mice Sciatic nerve |
| Larger compound action potential and better functional recovery in early evaluation. Reduction in collagen staining. |
| Aslan et al., 2011 [56] | Section + suture (immediate or 3 days later) | CDP-choline, cytidine, choline, or cytidine–choline (during nerve repair) | Female Sprague–Dawley Rat Sciatic nerve |
| Treatment with CDP-choline or cytidine–choline reduced scar formation and decreased nerve adherence. |
| Albayrak et al., 2010 [30] | Abrasion | Doxorubicin | Male Wistar Albino Rat Sciatic nerve |
| Topical application of doxorubicin effectively reduced epineural scar formation. |
| Atkins et al., 2007 [53] | Section + suture | IL-10 | C57 Black-6 MouseSciatic nerve |
| Compound action potential and area of staining for collagen not significantly different compared to controls. Higher number of myelinated fibers compared to control but no difference with the other groups. |
| Ozay et al., 2007 [31] | Section + suture | Citicoline | Female Sprague-Dawley Rat Sciatic nerve |
| Rats treated with citicoline showed significantly better SFI and improvement at 12 weeks of electromyography. Nerves were surrounded by only a very thin, lucent membrane and showed thin dark bands of connective tissue surrounding the nerve. |
| Ilbay et al., 2005 [32] | Scratch | Mitomycin C | Male Wistar Rat Sciatic nerve |
| Macroscopical and microscopical reduction of perineural adhesions in the treated groups; lower number of fibroblast/fibrocytes. |
| Gorgulu et al., 1998 [33] | External neurolysis, abrasive injury, anastomosis | Aprotinin | Male Sprague-Dawley RatSciatic nerve |
| Scar reduction after aprotinin application. No differences in neurological tests were observed. |
| Reference | Method to induce scar formation | Agent | Animal and Nerve model | Analyses | Results |
|---|---|---|---|---|---|
| Kikuchi et al., 2020 [82] | Burning | Polylactic acid (PLA)-based biodegradable three-layered membrane (E8002) with or without L-ascorbic acid (AA) | Male Sprague–Dawley Rat Sciatic nerve |
| AA in E8002 has an antiadhesional effect by enhancing fibrinolysis. Adhesion formation was lower in the group containing AA. Motor function and mechanical sensitivity were not impaired after surgery, and no differences were detected among groups. |
| Stocco et al., 2019 [81] | Section + suture | Wraps made of a synthetic 1% oxidized polyvinyl alcohol (OxPVA) and a leukocyte-fbrin-platelet membrane (LFPm) compared to NeuroWrap | Sprague–Dawley rats Sciatic nerve |
| LFPm wraps were completely resorbed, while residues of OxPVA and NeuraWrap were observed. Functional recovery was achieved in all groups. Additionally, at the morphological level, scar tissue formation and inflammatory infiltrate were not observed. Both myelinic and unmyelinic axons were observed. |
| Shintani et al., 2018 [57] | Burning | Polylactide (PLA)-poly(e-caprolactone) PCL conduit and HA | Lewis Rat Sciatic nerve |
| Morphological properties of axons were preserved with PLA-PCL conduit. HA was less effective for nerve protection from adhesion. |
| Servet et al., 2016 [59] | Section + suture | Ankaferd blood stopper (ABS) hemostatic agent | Male Sprague–Dawley Rat Sciatic nerve |
| Significant improvement of latency and speed in the ABS group. Other results were not statistically different. |
| Okui et al., 2010 [83] | Neurolysis and burning | PLA | Male Lewis Rat Sciatic nerve |
| PLA film has the potential to prevent adhesion even after internal neurolysis, and it is a useful substitute for perineurium. |
| Atkins et al., 2006 [52] | Section + suture | TGF-β1and TGF-β2 | Male Sprague–Dawley Rat Sciatic nerve |
| No differences in the percentage of collagen staining area were observed. Compound action potential ratios significantly smaller; increased number of myelinated fibers distally (no differences between TGF-β1 and TGF-β2) |
| Gorgulu et al.,2003 [34] | Neurolysis vs. scratching vs. suture vs. radiation treatment | Low-dose radiation therapy (24 h after surgery) | Male Sprague–Dawley Rat Sciatic nerve |
| Significant reduction of scar tissue in radiation + surgery groups. No increase in scar tissue formation after radiation in normal nerves was observed. |
| Ikeda et al., 2002 [84] | Burning | Absorbable oxidized regenerated cellulose sheet | White Japanese Rabbit Sciatic nerve |
| No significant differences between groups in electrophysiological evaluation. High adhesion between nerve and surrounding tissue in the damage group. |
| Ip et al., 2000 [15] | Section + suture | Early mobilization | Albino Rabbit Peroneal nerve |
| No difference in the biomechanical features of the adhesions |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crosio, A.; Ronchi, G.; Fornasari, B.E.; Odella, S.; Raimondo, S.; Tos, P. Experimental Methods to Simulate and Evaluate Postsurgical Peripheral Nerve Scarring. J. Clin. Med. 2021, 10, 1613. https://doi.org/10.3390/jcm10081613
Crosio A, Ronchi G, Fornasari BE, Odella S, Raimondo S, Tos P. Experimental Methods to Simulate and Evaluate Postsurgical Peripheral Nerve Scarring. Journal of Clinical Medicine. 2021; 10(8):1613. https://doi.org/10.3390/jcm10081613
Chicago/Turabian StyleCrosio, Alessandro, Giulia Ronchi, Benedetta Elena Fornasari, Simonetta Odella, Stefania Raimondo, and Pierluigi Tos. 2021. "Experimental Methods to Simulate and Evaluate Postsurgical Peripheral Nerve Scarring" Journal of Clinical Medicine 10, no. 8: 1613. https://doi.org/10.3390/jcm10081613
APA StyleCrosio, A., Ronchi, G., Fornasari, B. E., Odella, S., Raimondo, S., & Tos, P. (2021). Experimental Methods to Simulate and Evaluate Postsurgical Peripheral Nerve Scarring. Journal of Clinical Medicine, 10(8), 1613. https://doi.org/10.3390/jcm10081613








