Development and Validation of an Early Mortality Risk Score for Older Patients Treated with Chemotherapy for Cancer
Abstract
:1. Introduction
2. Materials and Methods
Study Schema
3. Statistical Analysis
4. Results
5. Predictive Variables Associated with the Risk of Death in the First 6 Months
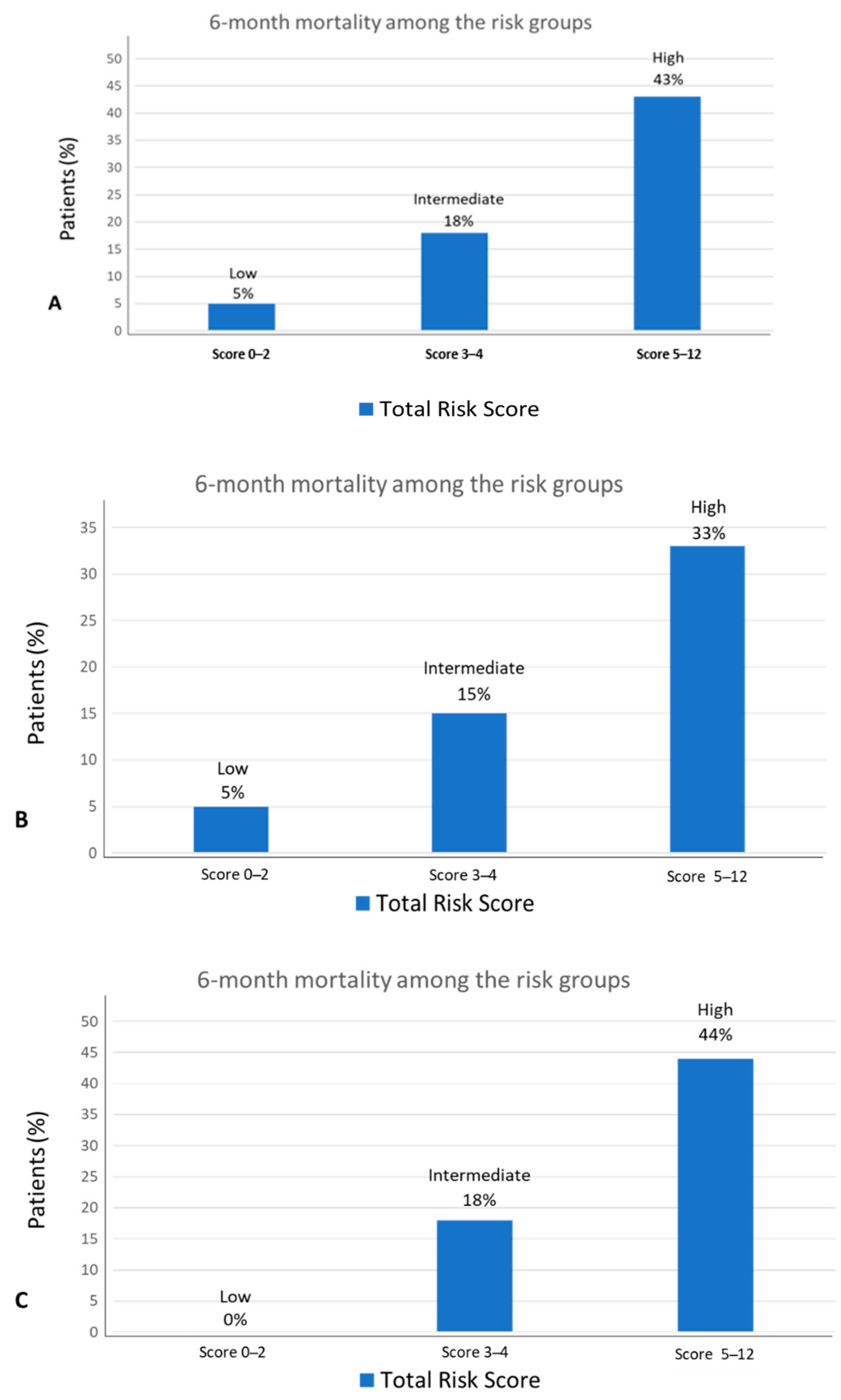
6. Model Prediction of Early Mortality
7. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soubeyran, P.; Fonck, M.; Blanc-Bisson, C.; Blanc, J.F.; Ceccaldi, J.; Mertens, C.; Rainfray, M. Predictors of early death risk in older patients treated with first-line chemotherapy for cancer. J. Clin. Oncol. 2012, 30, 1829–1834. [Google Scholar] [CrossRef] [PubMed]
- Walter, L.C.; Brand, R.J.; Counsell, S.R.; Palmer, R.M.; Landefeld, C.S.; Fortinsky, R.H.; Covinsky, K.E. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA 2001, 285, 2987–2994. [Google Scholar] [CrossRef] [PubMed]
- Kanesvaran, R.; Li, H.; Koo, K.; Poon, D. Analysis of prognostic factors of comprehensive geriatric assessment and development of a clinical scoring system in elderly asian patients with cancer. J. Clin. Oncol. 2011, 29, 3620–3627. [Google Scholar] [CrossRef] [PubMed]
- Puts, M.T.E.; Santos, B.; Hardt, J.; Monette, J.; Girre, V.; Atenafu, E.G.; Alibhai, S.M.H. An update on a systematic review of the use of geriatric assessment for older adults in oncology. Ann. Oncol. 2014, 25, 307–315. [Google Scholar] [CrossRef]
- Mohile, S.G.; Dale, W.; Somerfield, M.R.; Schonberg, M.A.; Boyd, C.M.; Burhenn, P.S.; Hurria, A. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J. Clin. Oncol. 2018, 36, 2326–2347. [Google Scholar] [CrossRef]
- Hamaker, M.E.; Vos, A.G.; Smorenburg, C.H.; De Rooij, S.E.; Van Munster, B.C. The value of geriatric assessments in predicting treatment tolerance and all-cause mortality in older patients with cancer. Oncologist 2012, 17, 1439–1449. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Lindquist, K.; Segal, M.R.; Covinsky, K.E. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA 2006, 295, 801–808. [Google Scholar] [CrossRef] [Green Version]
- Carey, E.C.; Covinsky, K.E.; Lui, L.Y.; Eng, C.; Sands, L.P.; Walter, L.C. Prediction of mortality in community-living frail elderly people with long-term care needs. J. Am. Geriatr. Soc. 2008, 56, 68–75. [Google Scholar] [CrossRef]
- Brunello, A.; Fontana, A.; Zafferri, V.; Panza, F.; Fiduccia, P.; Basso, U.; Zagonel, V. Development of an oncological-multidimensional prognostic index (Onco-MPI) for mortality prediction in older cancer patients. J. Cancer Res. Clin. Oncol. 2016, 142, 1069–1077. [Google Scholar] [CrossRef] [Green Version]
- Bourdel-Marchasson, I.; Diallo, A.; Bellera, C.; Blanc-Bisson, C.; Durrieu, J.; Germain, C.; Doussau, A. One-year mortality older patients with cancer: Development and external validation of an MNA-based prognostic score. PLoS ONE 2016, 11, e0148523. [Google Scholar] [CrossRef]
- Boulahssass, R.; Gonfrier, S.; Ferrero, J.M.; Sanchez, M.; Mari, V.; Moranne, O.; Guerin, O. Predicting early death in older adults with cancer. Eur. J. Cancer 2018, 100, 65–74. [Google Scholar] [CrossRef]
- Angeli, E.; Chouahnia, K.; Canoui-Poitrine, F.; Duchemann, B.; Aparicio, T.; Paillaud, E.; Pamoukdjian, F. Development, Validation and Clinical Impact of a Prediction Model for 6-month Mortality in Older Cancer Patients: The GRADE. Aging 2020, 12, 4230–4246. [Google Scholar] [CrossRef]
- Moth, E.B.; Blinman, P.; Stefanic, N.; Naganathan, V.; Grimison, P.; Stockler, M.R.; Kiely, B.E. Estimating survival time in older adults receiving chemotherapy for advanced cancer. J. Geriatric Oncol. 2020, 11, 617–625. [Google Scholar] [CrossRef]
- Roila, F.; Lupattelli, M.; Sassi, M.; Basurto, C.; Bracarda, S.; Picciafuoco, M.; Del Favero, A. Intra and interobserver variability in cancer patients’ performance status assessed according to Karnofsky and ECOG scales. Ann. Oncol. 1991, 2, 437–439. [Google Scholar] [CrossRef]
- Linn, B.S.; Linn, M.W.; Gurel, L. Cumulative illness rating scale. J. Am. Geriatr. Soc. 1968, 16, 622–626. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, 85–94. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Ferrucci, L.; Simonsick, E.M.; Salive, M.E.; Wallace, R.B. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N. Engl. J. Med. 1995, 332, 556–561. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer, E.A. A short portable mental status questionnaire for the assessment of organic brain deficits in elderly patients. J. Am. Geriatr. Soc. 1975, 22, 433. [Google Scholar] [CrossRef]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of illness in the aged: The index of ADL—A standardized measure of biological and psychosocial function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Zigmod, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scan. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.S.; Skinner, K.; Lee, A.; Kazis, L. Social support, social selection and self-assessed health status: Results from the veterans health study in the United States. Soc. Sci. Med. 1999, 48, 1721–1734. [Google Scholar] [CrossRef]
- Costa-Requena, G.; Salamero, M.; Gil, F. Validity of the questionnaire MOS-SSS of social support in neoplastic patients. Med. Clin. 2007, 128, 687–691. [Google Scholar]
- Luciani, A.; Ascione, G.; Bertuzzi, C.; Marussi, D.; Codecà, C.; Di Maria, G.; Foa, P. Detecting Disabilities in Older Patients with Cancer: Comparison between Comprehensive Geriatric Assessment and Vulnerable Elders Survey-13. J. Clin. Oncol. 2010, 28, 2046–2050. [Google Scholar] [CrossRef]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Chiarantini, D.; Volpato, S.; Sioulis, F.; Bartalucci, F.; Del Bianco, L.; Mangani, I.; Di Bari, M. Lower extremity performance measures predict long-term prognosis in older patients hospitalized for heart failure. J. Card. Fail. 2010, 16, 390–395. [Google Scholar] [CrossRef]
- Fox, K.R.; Ku, P.W.; Hillsdon, M.; Davis, M.G.; Simmonds, B.A.; Thompson, J.L.; Coulson, J.C. Objectively assessed physical activity and lower limb function and prospective associations with mortality and newly diagnosed disease in UK older adults: An OPAL four-year follow-up study. Age Ageing 2015, 44, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Fuentes-Abolafio, I.J.; Stubbs, B.; Pérez-Belmonte, L.M.; Bernal-López, M.R.; Gómez-Huelgas, R.; Cuesta-Vargas, A.I. Physical functional performance and prognosis in patients with heart failure: A systematic review and meta-analysis. BMC Cardiovasc Disord. 2020, 20, 512. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. (Eds.) Applied Logistic Regression; John Wiley and Sons: New York, NY, USA, 1989. [Google Scholar]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Concato, J.; Feinstein, A.R.; Holford, T.R. The risk of determining risk with multivariable models. Ann. Intern. Med. 1993, 118, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Hastie, T.; Tibshirani, R.; Friedman, J. (Eds.) The Elements of Statistical Learning: Data Mining, Inference, and Prediction, 2nd ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Extermann, M.; Albrand, G.; Chen, H.; Zanetta, S.; Schonwetter, R.; Zulian, G.B.; Droz, J.P. Are older French patients as willing as older American patients to undertake chemotherapy? J. Clin. Oncol. 2003, 2, 3214–3219. [Google Scholar] [CrossRef] [PubMed]
- Yellen, S.B.; Cella, D.F.; Leslie, W.T. Age and clinical decision making in oncology patients. J. Natl. Inst. 1994, 86, 1766–1770. [Google Scholar] [CrossRef] [PubMed]
- Söderström, L.; Rosenblad, A.; Adolfsson, E.T.; Saletti, A.; Bergkvist, L. Nutritional status predicts preterm death in older people: A prospective cohort study. Clin. Nutr. 2014, 33, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Edwards, B.J. Malnutrition in Older Adults with Cancer. Curr. Oncol. Rep. 2019, 21, 1–12. [Google Scholar] [CrossRef]
- Ferrat, E.; Paillaud, E.; Laurent, M.; Le Thuaut, A.; Caillet, P.; Tournigand, C.; Lagrange, J.-L.; Canouï-Poitrine, F.; Bastuji-Garin, S.; The ELPACA Study Group. Predictors of 1-Year Mortality in a Prospective Cohort of Elderly Patients With Cancer. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1148–1155. [Google Scholar] [CrossRef]
- Feliu, J.; Jiménez-Gordo, A.M.; Madero, R.; Rodríguez-Aizcorbe, J.R.; Espinosa, E.; Castro, J.; González-Barón, M. Development and validation of a prognostic nomogram for terminally ill cancer patients. J. Natl. Cancer Inst. 2011, 103, 1613–1620. [Google Scholar] [CrossRef] [Green Version]
- Arkenau, H.T.; Olmos, D.; Ang, J.E.; Barriuso, J.; Karavasilis, V.; Ashley, S.; Kaye, S. 90-Days mortality in patients treated within the context of a phase-I trial: How should we identify patients who should not go on trial? Eur. J. Cancer 2008, 44, 1536–1540. [Google Scholar] [CrossRef]
- Repetto, L.; Fratino, L.; Audisio, R.A.; Venturino, A.; Gianni, W.; Vercelli, M.; Zagonel, V. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: An Italian Group for Geriatric Oncology Study. J. Clin. Oncol. 2002, 20, 494–502. [Google Scholar] [CrossRef]
- Falandry, C.; Weber, B.; Savoye, A.M.; Tinquaut, F.; Tredan, O.; Sevin, E.; Freyer, G. Development of a geriatric vulnerability score in elderly patients with advanced ovarian cancer treated with first-line carboplatin: A GINECO prospective trial. Ann. Oncol. 2013, 24, 2808–2813. [Google Scholar] [CrossRef]
- Telles Sales, L.; Telles O Lima, J.; Mello, M.J.G.; Torres, L.; Pires, M.L.L.; Rebello, M.; Orange, F. Baseline laboratory tests as predictors of early death risk in 746 eldely oncologic patients: A prospective cohort study. J. Clin. Oncol. 2019, 37, e23039. [Google Scholar] [CrossRef]
- Steinberg, D. Anemia and cancer. CA Cancer J. Clin. 1989, 39, 296–304. [Google Scholar] [CrossRef]
- Buck, I.; Morceau, F.; Grigorakaki, C.; Dicato, M.; Diederich, M. Linking anemia to inflammation and cancer: The crucial role of TNFalpha. Biochem. Pharmacol. 2009, 77, 1572–1579. [Google Scholar] [CrossRef] [Green Version]

| Training Set No. (%) | Validation Set No. (%) | p Value | |
|---|---|---|---|
| No. of patients | 342 | 401 | |
| No. of early deaths (≤6 months) | 76 (22) | 75 (19) | 0.10 |
| Sex | 0.81 | ||
| Female | 140 (41) | 157 (39) | |
| Male | 202 (59) | 244 (61) | |
| Age (Median and range) | 78 (70–92) | 77 (70–89) | 0.74 |
| Primary cancer sites | 0.001 | ||
| Gastrointestinal | 205 (60) | 193 (48) | |
| Urinary organs | 41 (12) | 27 (7) | |
| Lung | 31 (9) | 85 (22) | |
| Pancreatic cancer | 24 (7) | 12 (3) | |
| Breast | 15 (4) | 22 (5) | |
| Female reproductive | 12 (4) | 19 (5) | |
| Others | 14 (4) | 43 (11) | |
| Stage IV | 185 (54) | 221 (55) | 0.78 |
| ECOG performance status 2 | 44 (13) | 61 (15) | 0.19 |
| Weight change in the last 6 months ≥ 10% | 42 (12) | 67 (9) | 0.09 |
| Activity of daily living ≤ 5 | 74 (22) | 88 (20) | 0.61 |
| Instrumental activity of daily living ≤ 7 | 203 (59) | 197 (49) | 0.005 |
| Body mass index (Mean and SD) | 26.1 (4.1) | 25.6 (6.1) | 0.2 |
| Charlson score ≥ 2 | 125 (37) | 129 (32) | 0.20 |
| Number of falls in the past 6 months ≥ 1 | 69 (20) | 57 (14) | 0.16 |
| SPPB < 6 | 63 (18) | 64 (16) | 0.19 |
| Pfeiffer test ≥ 3 errors | 45 (13) | 40 (10) | 0.17 |
| Hospital Anxiety Scale ≥ 8 | 72 (21) | 76 (19) | 0.47 |
| Hospital Depression Scale ≥ 8 | 79 (23) | 80 (20) | 0.29 |
| MOS Social Support Survey ≤ 15 | 40 (12) | 24 (6) | 0.005 |
| VES-13 ≥ 3 | 184 (54) | 195 (49) | 0.15 |
| Baseline laboratory values (Mean and SD) | |||
| Hemoglobin (g/dL) | 12.5 (1.6) | 12.6 (1.6) | 0.22 |
| Albumin (g/dL) | 3.9 (0.4) | 3.8 (0.5) | 0.29 |
| Creatinine clearance (mL/min) | 47 (22) | 69 (20) | 0.01 |
| Gamma glutamyl transferase (IU/L) | 108 (191) | 82 (170) | 0.002 |
| Variable | Patients | Died (n = 76) | Alive (n = 266) | OR (95% CI) | p-Value |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | |||
| ECOG PS | 2.28 (1.18–4.40) | 0.013 | |||
| 0–1 | 298 (87) | 58 (19) | 240 (81) | ||
| 2 | 44 (13) | 18 (41) | 26 (59) | ||
| ADL | 1.76 (1.01–3.09) | 0.046 | |||
| 6 | 268 (78) | 51 (19) | 217 (81) | ||
| ≤5 | 74 (22) | 25 (34) | 49 (65) | ||
| IADL | 1.67 (1.01–2.76) | 0.042 | |||
| 8 | 139 (41) | 26 (19) | 113 (81) | ||
| ≤7 | 203 (59) | 50 (65) | 153 (75) | ||
| SPPB | 1.99 (1.11–3.58) | 0.021 | |||
| >7 | 279 (82) | 53 (19) | 226 (66) | ||
| ≤6 | 63 (18) | 23 (37) | 40 (63) | ||
| Tumour site | 1.53 (1.06–2.19) | 0.021 | |||
| Lung | 31 (9) | 13 (42) | 18 (58) | ||
| Other | 311 (91) | 63 (20) | 248 (80) | ||
| VES 13 | 1.59 (1.01–2.61) | 0.05 | |||
| 0–2 | 168 (49) | 28 (17) | 140 (83) | ||
| >3 | 174 (51) | 48 (28) | 126 (72) | ||
| Stage | 4.71 (2.62–8.46) | <0.001 | |||
| I-III | 157 (46) | 15 (10) | 142 (90) | ||
| IV | 185 (54) | 61 (33) | 124 (67) | ||
| Neutrophils (×103/µL) | |||||
| ≤8 | 195 (57) | 27 (14) | 168 (86) | 1.48 (1.15–1.90) | 0.002 |
| >8 | 147 (43) | 49 (33) | 98 (67) | ||
| GGT (IU/L) | 1.53 (1.17–2.04) | 0.002 | |||
| ≤130 | 261 (76) | 45 (17) | 216 (83) | ||
| >130 | 81 (24) | 31 (38) | 50 (62) | ||
| Alkaline Phosphatase (IU/L) | 2.81 (1.59–4.97) | <0.001 | |||
| ≤150 | 276 (81) | 49 (18) | 227 (82) | ||
| >150 | 66 (19) | 27 (41) | 39 (59) | ||
| Albumin (g/dL) | 4.74 (2.64–8.51) | <0.001 | |||
| ≥3.5 | 281 (82) | 44 (16) | 237 (84) | ||
| <3.5 | 61 (18) | 32 (52) | 29 (48) | ||
| Hemoglobin (g/dL) | 2.51 (1.43–4.40) | 0.001 | |||
| ≥11 | 272 (80) | 48 (18) | 224 (82) | ||
| <11 | 70 (20) | 28 (40) | 42 (60) | ||
| Weight loss % | 2.06 (1.04–4.06) | 0.037 | |||
| <10% | 301 (88) | 60 (20) | 241 (80) | ||
| ≥10% | 41 (12) | 16 (39) | 25 (61) | ||
| BMI (kg/m2) | 2.14 (1.20–3.81) | 0.01 | |||
| ≥23 | 271 (79) | 50 (18) | 221 (82) | ||
| <23 | 71 (21) | 26 (34) | 45 (17) |
| Variable | β | SE | p† | OR (95% CI) | Score |
|---|---|---|---|---|---|
| BMI (<23 kg/m2) | 0.912 | 0.343 | 0.008 | 2.489 (1.271–4.875) | 2 |
| Stage IV | 1.573 | 0.353 | 0.000 | 4.820 (2.414–9.626) | 3 |
| Albumin (≤3.5 g/dL) | 1.210 | 0.347 | 0.000 | 3.353 (1.698–6.622) | 2 |
| Hemoglobin (<11 g/dL) | 0.868 | 0.344 | 0.012 | 2.383 (1.213–4.681) | 2 |
| ADL (≤5) | 0.565 | 0.351 | 0.041 | 1.760 (1.085–3.501) | 1 |
| ECOG PS 2 | 0.868 | 0.402 | 0.031 | 2.382 (1.084–5.232) | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feliu, J.; Pinto, A.; Basterretxea, L.; López-San Vicente, B.; Paredero, I.; Llabrés, E.; Jiménez-Munárriz, B.; Antonio-Rebollo, M.; Losada, B.; Espinosa, E.; et al. Development and Validation of an Early Mortality Risk Score for Older Patients Treated with Chemotherapy for Cancer. J. Clin. Med. 2021, 10, 1615. https://doi.org/10.3390/jcm10081615
Feliu J, Pinto A, Basterretxea L, López-San Vicente B, Paredero I, Llabrés E, Jiménez-Munárriz B, Antonio-Rebollo M, Losada B, Espinosa E, et al. Development and Validation of an Early Mortality Risk Score for Older Patients Treated with Chemotherapy for Cancer. Journal of Clinical Medicine. 2021; 10(8):1615. https://doi.org/10.3390/jcm10081615
Chicago/Turabian StyleFeliu, Jaime, Alvaro Pinto, Laura Basterretxea, Borja López-San Vicente, Irene Paredero, Elisenda Llabrés, Beatriz Jiménez-Munárriz, Maite Antonio-Rebollo, Beatriz Losada, Enrique Espinosa, and et al. 2021. "Development and Validation of an Early Mortality Risk Score for Older Patients Treated with Chemotherapy for Cancer" Journal of Clinical Medicine 10, no. 8: 1615. https://doi.org/10.3390/jcm10081615






