Biomechanical Difference between Conventional Transtibial Single-Bundle and Anatomical Transportal Double-Bundle Anterior Cruciate Ligament Reconstruction Using Three-Dimensional Finite Element Model Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Intact Model
2.2. Surgical Procedure
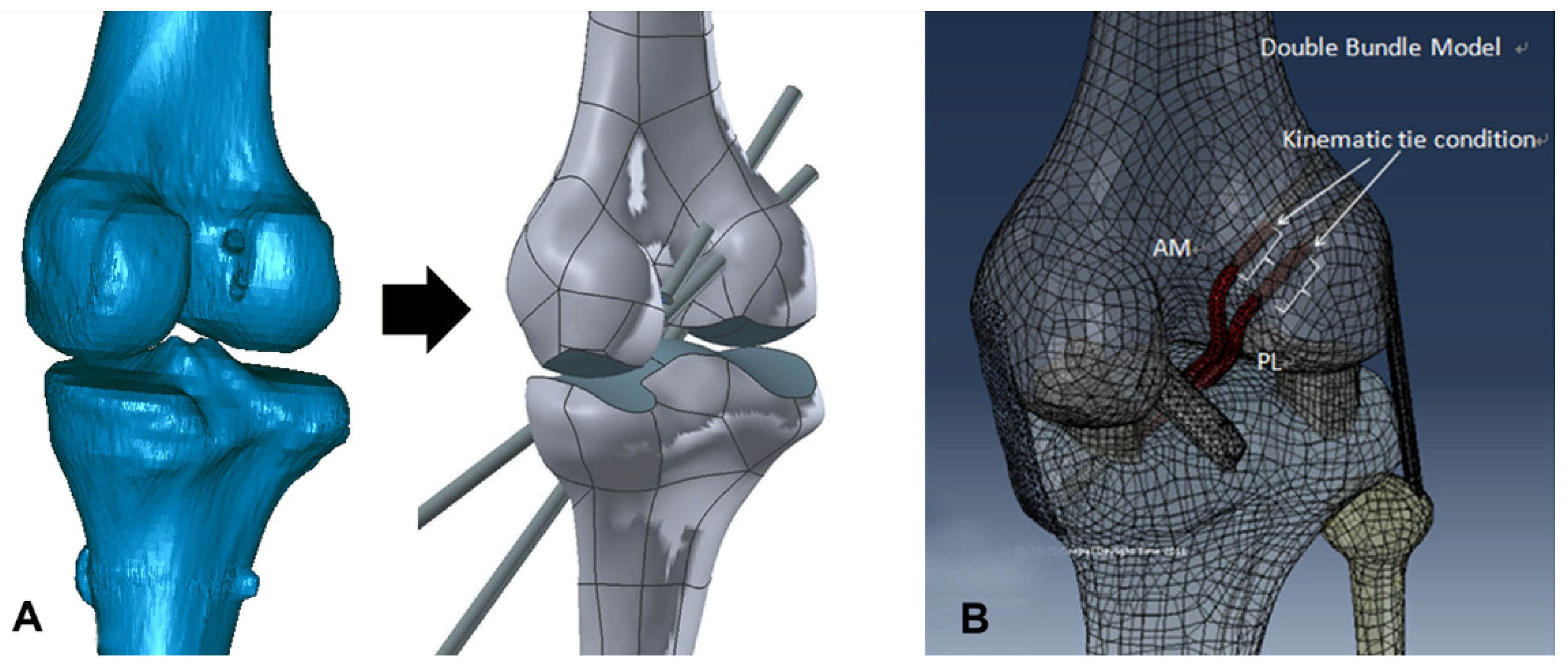
2.2.1. Conventional SB Reconstruction Using the TT Technique
2.2.2. Anatomical DB Reconstruction Using the TP Technique
2.3. Surgical Simulation Model
2.4. Loading and Boundary Conditions and Evaluation
3. Results
3.1. Validation
3.2. Anterior Tibial Translation under a 134 N Anterior Load
3.3. Graft Stress
3.4. Contact Stress Between Graft and Tunnel
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iriuchishima, T.; Horaguchi, T.; Kubomura, T.; Morimoto, Y.; Fu, F.H. Evaluation of the intercondylar roof impingement after anatomical double-bundle anterior cruciate ligament reconstruction using 3D-CT. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 674–679. [Google Scholar] [CrossRef]
- Yasuda, K.; van Eck, C.F.; Hoshino, Y.; Fu, F.H.; Tashman, S. Anatomic single- and double-bundle anterior cruciate ligament reconstruction, part 1: Basic science. Am. J. Sports Med. 2011, 39, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Kanamori, A.; Zeminski, J.; Yagi, M.; Papageorgiou, C.; Fu, F.H. The effectiveness of reconstruction of the anterior cruciate ligament with hamstrings and patellar tendon. A cadaveric study comparing anterior tibial and rotational loads. J. Bone Joint Surg. Am. Vol. 2002, 84, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Brophy, R.H.; Selby, R.M.; Altchek, D.W. Anterior cruciate ligament revision: Double-bundle augmentation of primary vertical graft. Arthrosc. J. Arthrosc. Related Surg. 2006, 22, e681–e685. [Google Scholar] [CrossRef]
- Robinson, J.; Stanford, F.C.; Kendoff, D.; Stuber, V.; Pearle, A.D. Replication of the range of native anterior cruciate ligament fiber length change behavior achieved by different grafts: Measurement using computer-assisted navigation. Am. J. Sports Med. 2009, 37, 1406–1411. [Google Scholar] [CrossRef]
- Fu, F.H.; Shen, W.; Starman, J.S.; Okeke, N.; Irrgang, J.J. Primary anatomic double-bundle anterior cruciate ligament reconstruction: A preliminary 2-year prospective study. Am. J. Sports Med. 2008, 36, 1263–1274. [Google Scholar] [CrossRef]
- Yasuda, K.; Kondo, E.; Ichiyama, H.; Tanabe, Y.; Tohyama, H. Clinical evaluation of anatomic double-bundle anterior cruciate ligament reconstruction procedure using hamstring tendon grafts: Comparisons among 3 different procedures. Arthrosc. J. Arthrosc. Related Surg. 2006, 22, 240–251. [Google Scholar] [CrossRef]
- Oh, J.Y.; Kim, K.T.; Park, Y.J.; Won, H.C.; Yoo, J.I.; Moon, D.K.; Cho, S.H.; Hwang, S.C. Biomechanical comparison of single-bundle versus double-bundle anterior cruciate ligament reconstruction: A meta-analysis. Knee Rurg. Related Res. 2020, 32, 14. [Google Scholar] [CrossRef]
- Lubowitz, J.H.; Konicek, J. Anterior cruciate ligament femoral tunnel length: Cadaveric analysis comparing anteromedial portal versus outside-in technique. Arthrosc. J. Arthrosc. Related Surg. 2010, 26, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Lubowitz, J.H. Anteromedial portal technique for the anterior cruciate ligament femoral socket: Pitfalls and solutions. Arthrosc. J. Arthrosc. Related Surg. 2009, 25, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Kim, J.G.; Lee do, K.; Lim, H.C.; Ahn, J.H. Comparison of femoral graft bending angle and tunnel length between transtibial technique and transportal technique in anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1584–1593. [Google Scholar] [CrossRef]
- Vercillo, F.; Woo, S.L.; Noorani, S.Y.; Dede, O. Determination of a safe range of knee flexion angles for fixation of the grafts in double-bundle anterior cruciate ligament reconstruction: A human cadaveric study. Am. J. Sports Med. 2007, 35, 1513–1520. [Google Scholar] [CrossRef]
- Li, G.; Papannagari, R.; DeFrate, L.E.; Yoo, J.D.; Park, S.E.; Gill, T.J. Comparison of the ACL and ACL graft forces before and after ACL reconstruction: An in-vitro robotic investigation. Acta Orthopaedica 2006, 77, 267–274. [Google Scholar] [CrossRef]
- Tsai, A.G.; Wijdicks, C.A.; Walsh, M.P.; Laprade, R.F. Comparative kinematic evaluation of all-inside single-bundle and double-bundle anterior cruciate ligament reconstruction: A biomechanical study. Am. J. Sports Med. 2010, 38, 263–272. [Google Scholar] [CrossRef]
- Kim, H.Y.; Seo, Y.J.; Kim, H.J.; Nguyenn, T.; Shetty, N.S.; Yoo, Y.S. Tension changes within the bundles of anatomic double-bundle anterior cruciate ligament reconstruction at different knee flexion angles: A study using a 3-dimensional finite element model. Arthrosc. J. Arthrosc. Related Surg. 2011, 27, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.L.; Seon, J.K.; Gadikota, H.R.; Hosseini, A.; Sutton, K.M.; Gill, T.J.; Li, G. In situ forces in the anteromedial and posterolateral bundles of the anterior cruciate ligament under simulated functional loading conditions. Am. J. Sports Med. 2010, 38, 558–563. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Wen, C.; Cheung, J.T.; Zhang, M.; Hu, Y.; Yan, C.; Chiu, K.Y.; Lu, W.W.; Fan, Y. Deterioration of stress distribution due to tunnel creation in single-bundle and double-bundle anterior cruciate ligament reconstructions. Ann. Biomed. Eng. 2012, 40, 1554–1567. [Google Scholar] [CrossRef]
- Yasuda, K.; Ichiyama, H.; Kondo, E.; Miyatake, S.; Inoue, M.; Tanabe, Y. An in vivo biomechanical study on the tension-versus-knee flexion angle curves of 2 grafts in anatomic double-bundle anterior cruciate ligament reconstruction: Effects of initial tension and internal tibial rotation. Arthrosc. J. Arthrosc. Related Surg. 2008, 24, 276–284. [Google Scholar] [CrossRef]
- Kwon, O.R.; Kang, K.T.; Son, J.; Kwon, S.K.; Jo, S.B.; Suh, D.S.; Choi, Y.J.; Kim, H.J.; Koh, Y.G. Biomechanical comparison of fixed- and mobile-bearing for unicomparmental knee arthroplasty using finite element analysis. J. Orthopaedic Res. 2014, 32, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Pena, E.; Calvo, B.; Martinez, M.A.; Doblare, M. A three-dimensional finite element analysis of the combined behavior of ligaments and menisci in the healthy human knee joint. J. Biomech. 2006, 39, 1686–1701. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Xerogeanes, J.W.; Livesay, G.A.; Fu, F.H.; Woo, S.L. Biomechanical function of the human anterior cruciate ligament. Arthrosc. J. Arthrosc. Related Surg. 1994, 10, 140–147. [Google Scholar] [CrossRef]
- Mesfar, W.; Shirazi-Adl, A. Biomechanics of the knee joint in flexion under various quadriceps forces. Knee 2005, 12, 424–434. [Google Scholar] [CrossRef]
- ABAQUS, version 6.11; Simulia: Providence, RI, USA, 2011.
- Forsythe, B.; Kopf, S.; Wong, A.K.; Martins, C.A.; Anderst, W.; Tashman, S.; Fu, F.H. The location of femoral and tibial tunnels in anatomic double-bundle anterior cruciate ligament reconstruction analyzed by three-dimensional computed tomography models. J. Bone Joint Surg. Am. Vol. 2010, 92, 1418–1426. [Google Scholar] [CrossRef] [Green Version]
- Chizari, M.; Wang, B. 3D numerical analysis of an ACL reconstructed knee. SIMULIA Customer Conf. 2009, 2009, 1–13. [Google Scholar]
- Li, G.; Gil, J.; Kanamori, A.; Woo, S.L. A validated three-dimensional computational model of a human knee joint. J. Biomech. Eng. 1999, 121, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Suggs, J.; Gill, T. The effect of anterior cruciate ligament injury on knee joint function under a simulated muscle load: A three-dimensional computational simulation. Ann. Biomed. Eng. 2002, 30, 713–720. [Google Scholar] [CrossRef]
- Kondo, E.; Merican, A.M.; Yasuda, K.; Amis, A.A. Biomechanical comparisons of knee stability after anterior cruciate ligament reconstruction between 2 clinically available transtibial procedures: Anatomic double bundle versus single bundle. Am. J. Sports Med. 2010, 38, 1349–1358. [Google Scholar] [CrossRef]
- Yagi, M.; Wong, E.K.; Kanamori, A.; Debski, R.E.; Fu, F.H.; Woo, S.L. Biomechanical analysis of an anatomic anterior cruciate ligament reconstruction. Am. J. Sports Med. 2002, 30, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Amis, A.A.; Dawkins, G.P. Functional anatomy of the anterior cruciate ligament. Fibre bundle actions related to ligament replacements and injuries. J. Bone Joint Surg. Br. Vol. 1991, 73, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; DeFrate, L.E.; Sun, H.; Gill, T.J. In vivo elongation of the anterior cruciate ligament and posterior cruciate ligament during knee flexion. Am. J. Sports Med. 2004, 32, 1415–1420. [Google Scholar] [CrossRef]
- Sakane, M.; Fox, R.J.; Woo, S.L.; Livesay, G.A.; Li, G.; Fu, F.H. In situ forces in the anterior cruciate ligament and its bundles in response to anterior tibial loads. J. Orthopaedic Res. 1997, 15, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.S.; Jeong, W.S.; Shetty, N.S.; Ingham, S.J.; Smolinski, P.; Fu, F. Changes in ACL length at different knee flexion angles: An in vivo biomechanical study. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 292–297. [Google Scholar] [CrossRef]
- Jordan, S.S.; DeFrate, L.E.; Nha, K.W.; Papannagari, R.; Gill, T.J.; Li, G. The in vivo kinematics of the anteromedial and posterolateral bundles of the anterior cruciate ligament during weightbearing knee flexion. Am. J. Sports Med. 2007, 35, 547–554. [Google Scholar] [CrossRef]
- Song, Y.; Debski, R.E.; Musahl, V.; Thomas, M.; Woo, S.L. A three-dimensional finite element model of the human anterior cruciate ligament: A computational analysis with experimental validation. J. Biomech. 2004, 37, 383–390. [Google Scholar] [CrossRef]
- Hirokawa, S.; Tsuruno, R. Three-dimensional deformation and stress distribution in an analytical/computational model of the anterior cruciate ligament. J. Biomech. 2000, 33, 1069–1077. [Google Scholar] [CrossRef]
- Grontvedt, T.; Pena, F.; Engebretsen, L. Accuracy of femoral tunnel placement and resulting graft force using one- or two-incision drill guides. A cadaver study on ten paired knees. Arthrosc. J. Arthrosc. Related Surg. 1996, 12, 187–192. [Google Scholar] [CrossRef]
- Hoher, J.; Kanamori, A.; Zeminski, J.; Fu, F.H.; Woo, S.L. The position of the tibia during graft fixation affects knee kinematics and graft forces for anterior cruciate ligament reconstruction. Am. J. Sports Med. 2001, 29, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Orsi, A.D.; Canavan, P.K.; Vaziri, A.; Goebel, R.; Kapasi, O.A.; Nayeb-Hashemi, H. The effects of graft size and insertion site location during anterior cruciate ligament reconstruction on intercondylar notch impingement. Knee 2017, 24, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Westermann, R.W.; Wolf, B.R.; Elkins, J.M. Effect of ACL reconstruction graft size on simulated Lachman testing: A finite element analysis. Iowa Orthop. J. 2013, 33, 70–77. [Google Scholar]
- Yoon, K.H.; Kim, Y.H.; Ha, J.H.; Kim, K.; Park, W.M. Biomechanical evaluation of double bundle augmentation of posterior cruciate ligament using finite element analysis. Clin. Biomech. 2010, 25, 1042–1046. [Google Scholar] [CrossRef]






| 0° | 30° | 60° | 90° | |
|---|---|---|---|---|
| Transtibial SB b | 12.0 | 13.1 | 11.4 | 10.0 |
| Transportal DB c | 12.3 | 13.3 | 10.3 | 9.2 |
| AM d | 6.2 | 6.4 | 5.2 | 4.8 |
| PL e | 6.1 | 6.9 | 5.1 | 4.4 |
| 0° | 30° | 60° | 90° | |
|---|---|---|---|---|
| Transtibial SB b | ||||
| Femur | 12.0 | 9.1 | 6.3 | 4.1 |
| Transportal DB c | 12.5 | 9.3 | 6.6 | 4.8 |
| AM d femur | 6.2 | 4.4 | 3.2 | 2.7 |
| PL e femur | 6.3 | 4.9 | 3.4 | 2.1 |
| 0° | 30° | 60° | 90° | |
|---|---|---|---|---|
| Transtibial SB b | ||||
| Tibia | 6.0 | 5.4 | 4.1 | 3.5 |
| Transportal DB c | 6.2 | 5.4 | 4.3 | 3.6 |
| AM d tibia | 2.9 | 2.6 | 2.2 | 1.8 |
| PL e tibia | 3.3 | 2.8 | 2.1 | 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.G.; Kang, K.T.; Wang, J.H. Biomechanical Difference between Conventional Transtibial Single-Bundle and Anatomical Transportal Double-Bundle Anterior Cruciate Ligament Reconstruction Using Three-Dimensional Finite Element Model Analysis. J. Clin. Med. 2021, 10, 1625. https://doi.org/10.3390/jcm10081625
Kim JG, Kang KT, Wang JH. Biomechanical Difference between Conventional Transtibial Single-Bundle and Anatomical Transportal Double-Bundle Anterior Cruciate Ligament Reconstruction Using Three-Dimensional Finite Element Model Analysis. Journal of Clinical Medicine. 2021; 10(8):1625. https://doi.org/10.3390/jcm10081625
Chicago/Turabian StyleKim, Jae Gyoon, Kyoung Tak Kang, and Joon Ho Wang. 2021. "Biomechanical Difference between Conventional Transtibial Single-Bundle and Anatomical Transportal Double-Bundle Anterior Cruciate Ligament Reconstruction Using Three-Dimensional Finite Element Model Analysis" Journal of Clinical Medicine 10, no. 8: 1625. https://doi.org/10.3390/jcm10081625
APA StyleKim, J. G., Kang, K. T., & Wang, J. H. (2021). Biomechanical Difference between Conventional Transtibial Single-Bundle and Anatomical Transportal Double-Bundle Anterior Cruciate Ligament Reconstruction Using Three-Dimensional Finite Element Model Analysis. Journal of Clinical Medicine, 10(8), 1625. https://doi.org/10.3390/jcm10081625






