Pelvic Floor Morbidity Following Vaginal Delivery versus Cesarean Delivery: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
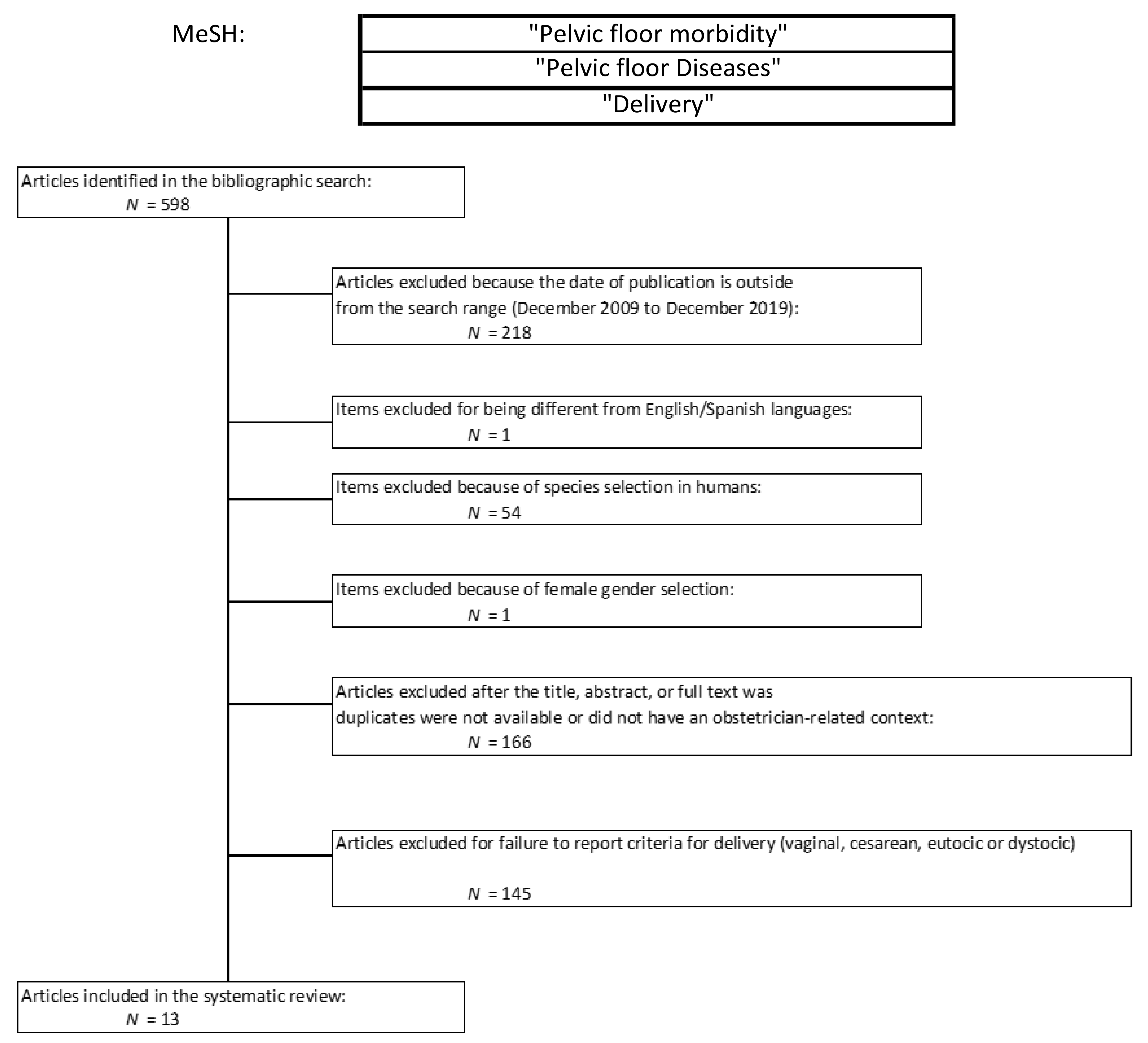
2.1. Protocol, Eligibility Criteria, Information Sources, and Search Strategies
2.2. Study Selection, Data Collection and Data Items
2.3. Risk of Bias and Statistical Analysis
3. Results
3.1. Characteristic of the Included Studies
3.2. Quality Assessment
3.3. Results of the Systematic Review
3.4. Results of the Meta-Analysis
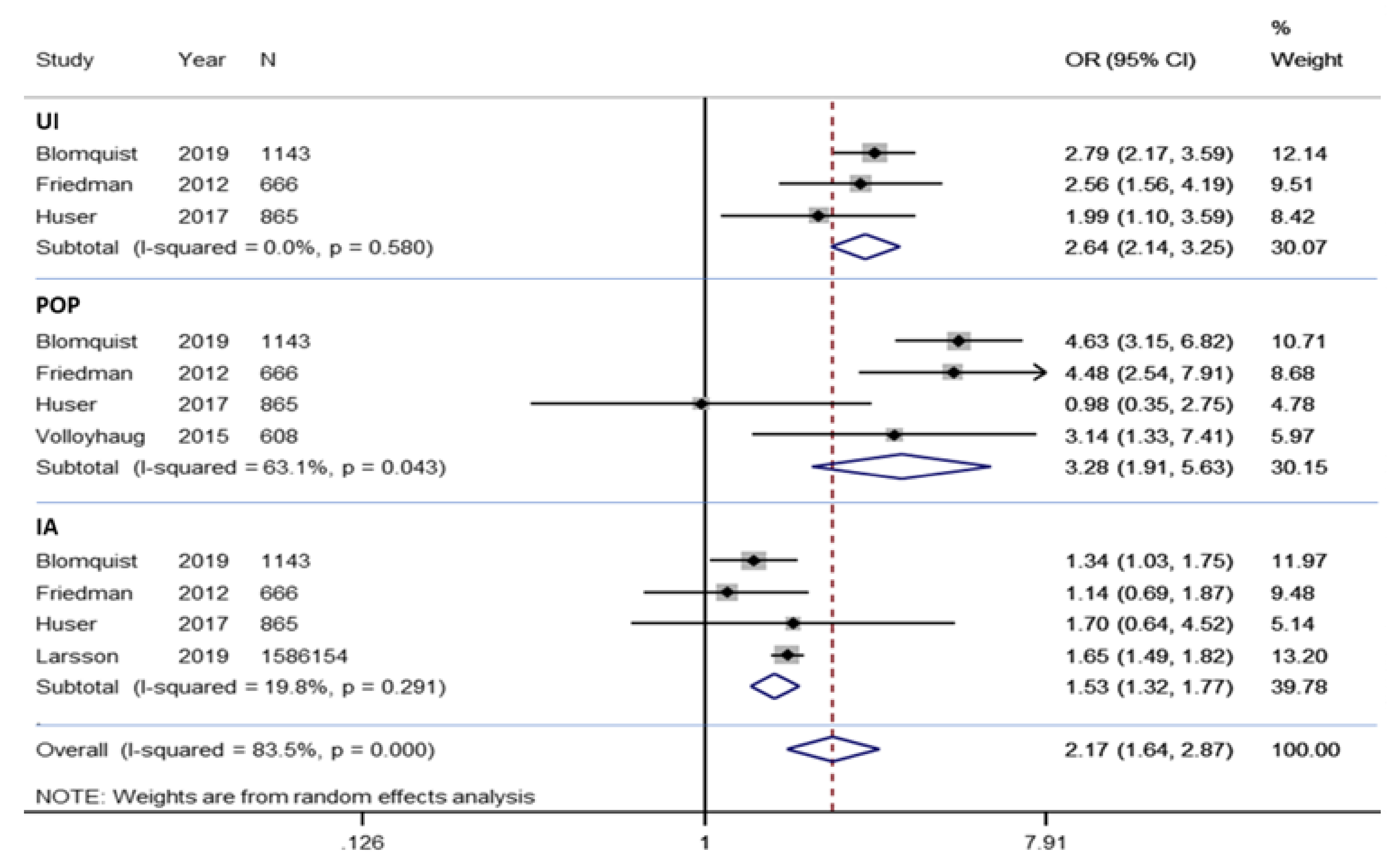
3.4.1. Meta-Analysis of Morbidity
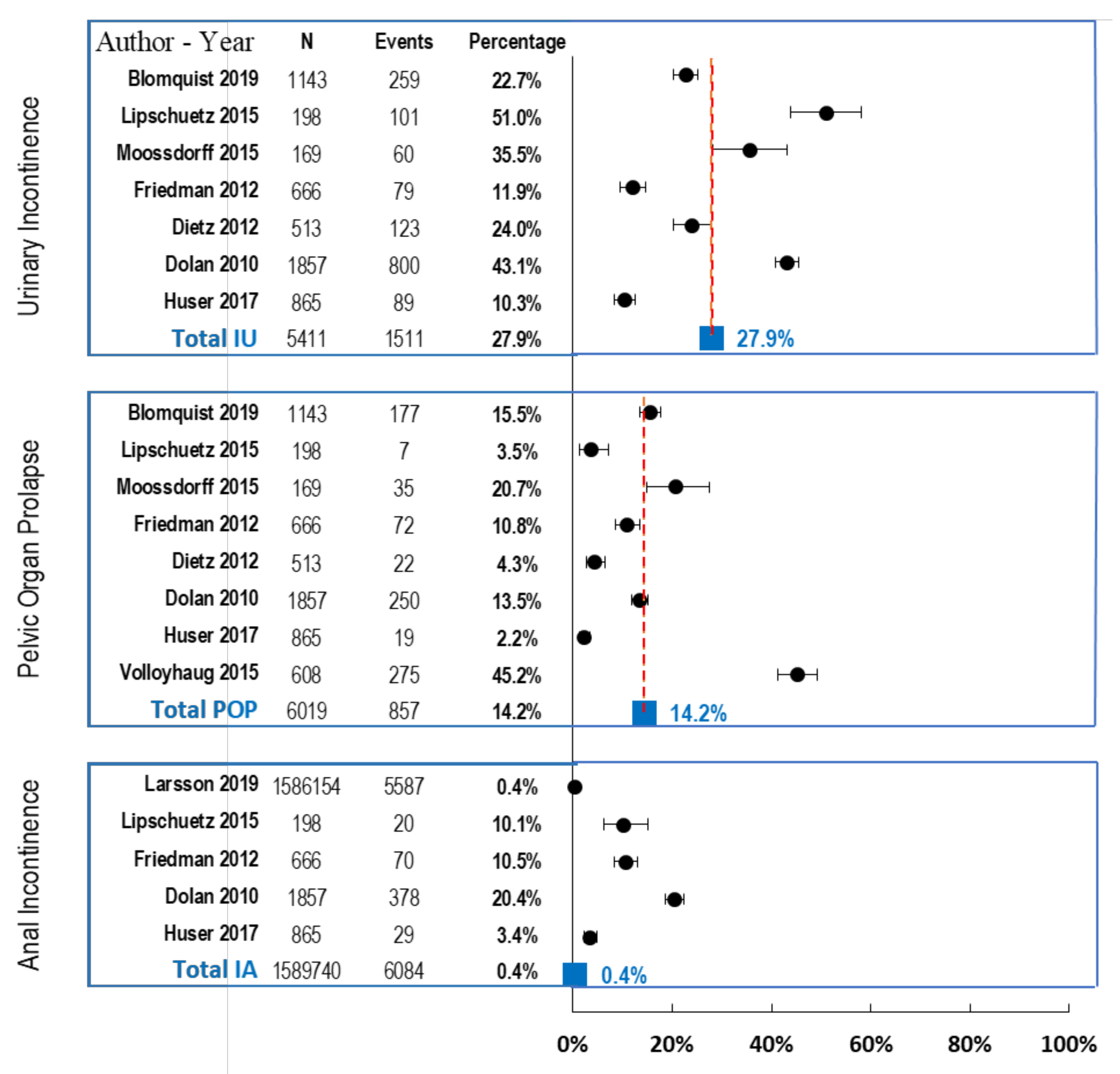
3.4.2. Meta-Analysis of Urinary Incontinence
3.4.3. Meta-Analysis of Pelvic Organ Prolapse
3.4.4. Meta-Analysis of Anal Incontinence
3.5. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VD | Vaginal delivery |
| CD | Cesarean delivery |
| UI | Urinary incontinence |
| POP | Pelvic organ prolapse |
| AI | Anal incontinence |
| OR | Odds Ratio |
| CI | Confidence Interval |
| I2 | I squared |
| PFD | Pelvic floor disorders |
References
- Lan, C.W.; Tavrow, P. Composite measures of women’s empowerment and their association with maternal mortality in low-income countries. BMC Pregnancy Childbirth 2017, 17 (Suppl. 2), 337. [Google Scholar] [CrossRef] [PubMed]
- Kenton, K.; Mueller, E.R. The global burden of female pelvic floor disorders. BJU Int. 2006, 98 (Suppl. S1), 6–7. [Google Scholar] [CrossRef]
- Martin-Martin, S.; Pascual-Fernandez, A.; Alvarez-Colomo, C.; Calvo-Gonzalez, R.; Muñoz-Moreno, M.; Cortiñas-Gonzalez, J.R. Urinary incontinence during pregnancy and postpartum. Associated risk factors and influence of pelvic floor exercises. Arch. Esp. Urol. 2014, 67, 323–330. [Google Scholar]
- Rortveit, G.; Hannestad, Y.S. Association between mode of delivery and pelvic floor dysfunction. Tidsskr. Nor. Legeforening 2014, 134, 1848–1852. [Google Scholar]
- Wu, J.M.; Vaughan, C.P.; Goode, P.S.; Redden, D.T.; Burgio, K.L.; Richter, H.E.; Markland, A.D. Prevalence and trends of symptomatic pelvic floor disorders in U.S. women. Obstet. Gynecol. 2014, 123, 141–1488. [Google Scholar] [CrossRef] [PubMed]
- Maclennan, A.H.; Taylor, A.W.; Wilson, D.H.; Wilson, D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. BJOG 2000, 107, 1460–1470. [Google Scholar] [CrossRef]
- Ashton-Miller, J.A.; Delancey, J.O. On the biomechanics of vaginal birth and common sequelae. Annu. Rev. Biomed. Eng. 2009, 11, 163–176. [Google Scholar] [CrossRef]
- Caudwell-Hall, J.; Atan, I.K.; Rojas, R.G.; Langer, S.; Shek, K.L.; Dietz, H.P. Atraumatic normal vaginal delivery: How many women get what they want? Am. J. Obstet. Gynecol. 2018, 219, 379.e1–379.e8. [Google Scholar] [CrossRef] [PubMed]
- Siahkal, S.F.; Iravani, M.; Mohaghegh, Z.; Sharifipour, F.; Zahedian, M. Maternal, obstetrical and neonatal risk factors’ impact on female urinary incontinence: A systematic review. Int. Urogynecol. J. 2020, 31, 2205–2224. [Google Scholar] [CrossRef] [PubMed]
- Bo, K.; Frawley, H.C.; Haylen, B.T.; Abramov, Y.; Almeida, F.G.; Berghmans, B.; Bortolini, M.; Dumoulin, C.; Gomes, M.; McClurg, D.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Int. Urogynecol. J. 2017, 28, 191–213. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The Prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; Van Kerrebroeck, P.; Victor, A.; Wein, A. The standardisation of terminology in lower urinary tract function: Report from the standardisation sub-committee of the International Continence Society. Neurourol. Urodyn. 2002, 21, 167–178. [Google Scholar] [CrossRef]
- Whitehead, W.E.; Borrud, L.; Goode, P.S.; Meikle, S.; Mueller, E.R.; Tuteja, A.; Weidner, A.; Weinstein, M.; Ye, W.; Pelvic Floor Disorders Network. Fecal incontinence in US adults: Epidemiology and risk factors. Gastroenterology 2009, 137, 512–517.e5172. [Google Scholar] [CrossRef] [PubMed]
- Iglesia, C.B.; Smithling, K.R. Pelvic Organ Prolapse. Am. Fam. Physician 2017, 96, 179–185. [Google Scholar]
- Dietz, H.P.; Lanzarone, V. Levator trauma after vaginal delivery. Obstet. Gynecol. 2005, 106, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Larsson, C.; Hedberg, C.L.; Lundgren, E.; Söderström, L.; Tunón, K.; Nordin, P. Anal incontinence after caesarean and vaginal delivery in Sweden: A national population-based study. Lancet 2019, 393, 1233–1239. [Google Scholar] [CrossRef]
- Blomquist, J.L.; Carroll, M.; Muñoz, A.; Handa, V.L. Pelvic floor muscle strength and the incidence of pelvic floor disorders after vaginal and cesarean delivery. Am. J. Obstet. Gynecol. 2020, 222, 62.e1–62.e8. [Google Scholar] [CrossRef]
- Fairchild, P.S.; Low, L.K.; Kowalk, K.M.; Kolenic, G.E.; DeLancey, J.O.; Fenner, D.E. Defining “normal recovery” of pelvic floor function and appearance in a high-risk vaginal delivery cohort. Int. Urogynecol. J. 2019, 31, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Colla, C.; Paiva, L.L.; Ferla, L.; Trento, M.J.B.; De Vargas, I.M.P.; Dos Santos, B.A.; Ferreira, C.F.; Ramos, J.G.L. Pelvic floor dysfunction in the immediate puerperium, and 1 and 3 months after vaginal or cesarean delivery. Int. J. Gynaecol. Obstet. 2018, 143, 94–100. [Google Scholar] [CrossRef]
- Abdool, Z.; Lindeque, B.G.; Dietz, H.P. The impact of childbirth on pelvic floor morphology in primiparous Black South African women: A prospective longitudinal observational study. Int. Urogynecol. J. 2018, 29, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Huser, M.; Janku, P.; Hudecek, R.; Zbozinkova, Z.; Bursa, M.; Unzeitig, V.; Ventruba, P. Pelvic floor dysfunction after vaginal and cesarean delivery among singleton primiparas. Int. J. Gynaecol. Obstet. 2017, 137, 170–173. [Google Scholar] [CrossRef]
- Yohay, D.; Weintraub, A.Y.; Mauer-Perry, N.; Peri, C.; Kafri, R.; Yohay, Z.; Bashiri, A. Prevalence and trends of pelvic floor disorders in late pregnancy and after delivery in a cohort of Israeli women using the PFDI-20. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 200, 35–39. [Google Scholar] [CrossRef]
- Moossdorff-Steinhauser, H.F.A.; Albers-Heitner, P.; Weemhoff, M.; Spaanderman, M.E.A.; Nieman, F.H.M.; Berghmansa, B. Factors influencing postpartum women’s willingness to participate in a preventive pelvic floor muscle training program: A web-based survey. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 195, 182–187. [Google Scholar] [CrossRef]
- Lipschuetz, M.; Cohen, S.M.; Liebergall-Wischnitzer, M.; Zbedat, K.; Hochner-Celnikier, D.; Lavy, Y.; Yagel, S. Degree of bother from pelvic floor dysfunction in women one year after first delivery. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 191, 90–94. [Google Scholar] [CrossRef]
- Volløyhaug, I.; Mørkved, S.; Salvesen, Ø.; Salvesen, K.Å. Forceps delivery is associated with increased risk of pelvic organ prolapse and muscle trauma: A cross-sectional study 16-24 years after first delivery. Ultrasound Obstet. Gynecol. 2015, 46, 487–495. [Google Scholar] [CrossRef]
- Friedman, S.; Blomquist, J.L.; Nugent, J.M.; McDermott, K.C.; Muñoz, A.; Handa, V.L. Pelvic muscle strength after childbirth. Obstet. Gynecol. 2012, 120, 1021–1028. [Google Scholar] [CrossRef]
- Dietz, H.P.; Shek, K.L.; Chantarasorn, V.; Langer, S.E. Do women notice the effect of childbirth-related pelvic floor trauma? Aust. N. Z. J. Obstet. Gynaecol. 2012, 52, 277–281. [Google Scholar] [CrossRef]
- Dolan, L.M.; Hilton, P. Obstetric risk factors and pelvic floor dysfunction 20 years after first delivery. Int. Urogynecol. J. 2010, 21, 535–544. [Google Scholar] [CrossRef]
- Hage-Fransen, M.A.H.; Wiezer, M.; Otto, A.; Wieffer-Platvoet, M.S.; Slotman, M.H.; der Sanden, M.W.G.N.; Pool-Goudzwaard, A.L. Pregnancy- and obstetric-related risk factors for urinary incontinence, fecal incontinence, or pelvic organ prolapse later in life: A systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2021, 100, 373–382. [Google Scholar] [CrossRef]
- Modroño Freire, M.J.; Sánchez Cougil, M.J.; Gayoso Diz, P.; Valero Paternain, M.; Blanco Ramos, M.; Cuña Ramos, F.O. Estudio de prevalencia de incontinencia urinaria en mujeres de 18 a 65 años y su influencia en la calidad de vida [Study of the prevalence of urinary incontinence in women from 18 to 65 and its influence on their quality of life]. Aten Primaria 2004, 34, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, H.H.; Stafne, S.N.; Falk, R.S.; Stordahl, A.; Wibe, A.; Mørkved, S. Prevalence and predictors of double incontinence 1 year after first delivery. Int. Urogynecol. J. 2018, 29, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, L.; Iran-Pour, E.; Safarinejad, M.R. Sexual function of primiparous women after elective cesarean section and normal vaginal delivery. Urol. J. 2012, 9, 498–504. [Google Scholar]
- Wang, H.; Ghoniem, G. Postpartum stress urinary incontinence, is it related to vaginal delivery? J. Matern. Fetal Neonatal Med. 2017, 30, 1552–1555. [Google Scholar] [CrossRef]
- Blomquist, J.L.; Muñoz, A.; Carroll, M.; Handa, V.L. Association of Delivery Mode with Pelvic Floor Disorders After Childbirth. JAMA 2018, 320, 2438–2447. [Google Scholar] [CrossRef]
- Schreiber Pedersen, L.; Lose, G.; Høybye, M.T.; Elsner, S.; Waldmann, A.; Rudnicki, M. Prevalence of urinary incontinence among women and analysis of potential risk factors in Germany and Denmark. Acta Obstet. Gynecol. Scand. 2017, 96, 939–948. [Google Scholar] [CrossRef]
- Barber, M.D.; Maher, C. Epidemiology and outcome assessment of pelvic organ prolapse. Int. Urogynecol. J. 2013, 24, 1783–1790. [Google Scholar] [CrossRef]
- Mankuta, D.; Shaul, Y.; Leshno, M.; Brezis, M. 233: Spontaneous normal vaginal birth versus elective cesarean section by request—A decision analysis of maternal and perinatal complications. Am. J. Obstet. Gynecol. 2007, 197, S77. [Google Scholar] [CrossRef]
- Gyhagen, M.; Bullarbo, M.; Nielsen, T.F.; Milsom, I. A comparison of the long-term consequences of vaginal delivery versus caesarean section on the prevalence, severity and bothersomeness of urinary incontinence subtypes: A national cohort study in primiparous women. BJOG 2013, 120, 1548–1555. [Google Scholar] [CrossRef]

| Author | Year | Period of Study | Country | Type of Study | Type of Questionnaire | No. of Patients | Mean Age (SD or Range) |
|---|---|---|---|---|---|---|---|
| Blomquist [18] | 2019 | October 2008–December 2013 | USA | Longitudinal study | Prolapse-Incontinence Questionnaire | 1143 | 40 (36.6 43.7) |
| Larsson [17] | 2019 | 1973–2015 | Sweden | Observational study | ICD8-10 diagnosis | 1586.154 | 26.73 (5.57) |
| Fairchild [19] | 2019 | August 2014–July 2016 | USA | Prospective cohort | POP-Q, Ultrasound | 112 | 30.62 (N/A) |
| Colla [20] | 2018 | August 2016–May 2017 | Brazil | Prospective observational | ICIQ_SF, POP-Q, PFM perineometer data | 227 | 27 (26.03–27.68) |
| Abdool [21] | 2017 | November 2015–June 2016 | South Africa | Observational study | ICIQ-VS Questionnaire | 153 | 25.35 (18–38) |
| Huser [22] | 2017 | January 2002–December 2007 | Czech | Prospective cohort | Internet-based survey | 865 | N/A |
| Yohay [23] | 2016 | March–July 2014 | Israel | Prospective longitudinal cohort | PFDI-20 Questionnaire | 117 | 30.84 (5.05) |
| Moossdorff [24] | 2015 | January 2010–December 2010 | Netherlands | Observational study | Web based survey | 169 | 29.74 (N/A) |
| Lipschuetz [25] | 2015 | N/A | Israel | Cross sectional study | PFBQ Questionnaire | 198 | 28 (5.7) |
| Volloyhaug [26] | 2015 | January 1990–December 1997 | Norway | Cross sectional study | POP-Q questionnaire, ultrasound | 608 | 28.5 (N/A) |
| Friedman [27] | 2012 | N/A | USA | Prospective cohort | POP Quantification system | 666 | 32.40 (N/A) |
| Dietz [28] | 2012 | October 2005–March 2010 | Australia | Prospective cohort | Questions interview & ultrasound | 513 | 27.5 (17–45) |
| Dolan [29] | 2010 | January 1983–August 1986 | UK | Prospective cohort | Sheffield pelvic floor questionnaires | 1857 | 26.2 (4.8) |
| Total (N) | 1597.303 |
| Heterogeneity Statistic | Degrees of Freedom | p | I-Squared ** | Tau-Squared | |
|---|---|---|---|---|---|
| UI | 1.09 | 2 | 0.580 | 0.0% | 0.000 |
| POP | 8.13 | 3 | 0.043 | 63.1% | 0.1812 |
| AI | 3.74 | 3 | 0.291 | 19.8% | 0.0058 |
| Overall | 60.46 | 10 | 0.000 | 83.5% | 0.1535 |
| z | p | ||||
| UI | 9.10 | 0.000 | |||
| POP | 4.31 | 0.000 | |||
| AI | 5.64 | 0.000 | |||
| Overall | 5.40 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barca, J.A.; Bravo, C.; Pintado-Recarte, M.P.; Asúnsolo, Á.; Cueto-Hernández, I.; Ruiz-Labarta, J.; Buján, J.; Ortega, M.A.; De León-Luis, J.A. Pelvic Floor Morbidity Following Vaginal Delivery versus Cesarean Delivery: Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 1652. https://doi.org/10.3390/jcm10081652
Barca JA, Bravo C, Pintado-Recarte MP, Asúnsolo Á, Cueto-Hernández I, Ruiz-Labarta J, Buján J, Ortega MA, De León-Luis JA. Pelvic Floor Morbidity Following Vaginal Delivery versus Cesarean Delivery: Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(8):1652. https://doi.org/10.3390/jcm10081652
Chicago/Turabian StyleBarca, Juan A., Coral Bravo, Maria P. Pintado-Recarte, Ángel Asúnsolo, Ignacio Cueto-Hernández, Javier Ruiz-Labarta, Julia Buján, Miguel A. Ortega, and Juan A. De León-Luis. 2021. "Pelvic Floor Morbidity Following Vaginal Delivery versus Cesarean Delivery: Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 8: 1652. https://doi.org/10.3390/jcm10081652
APA StyleBarca, J. A., Bravo, C., Pintado-Recarte, M. P., Asúnsolo, Á., Cueto-Hernández, I., Ruiz-Labarta, J., Buján, J., Ortega, M. A., & De León-Luis, J. A. (2021). Pelvic Floor Morbidity Following Vaginal Delivery versus Cesarean Delivery: Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 10(8), 1652. https://doi.org/10.3390/jcm10081652









