The Diagnostic Usefulness of Circulating Profile of Extracellular Matrix Components: Sulfated Glycosaminoglycans (sGAG), Hyaluronan (HA) and Extracellular Part of Syndecan-1 (sCD138) in Patients with Crohn’s Disease and Ulcerative Colitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Patients with Ulcerative Colitis (UC)–Inclusion and Exclusion Criteria
2.3. Patients with Crohn’s Disease (CD)—Inclusion and Exclusion Criteria
2.4. Control Subjects
2.5. Biological Material for Research
2.6. Assessing the Serum Hyaluronan (HA) Concentration
2.7. Assessing the Serum Soluble Part of Syndecan-1 (sCD138) Concentration
2.8. Assessing the Serum Sulfated Glycosaminoglycans (sGAG) Concentration
2.9. Statistical Analysis
3. Results
3.1. Research Data
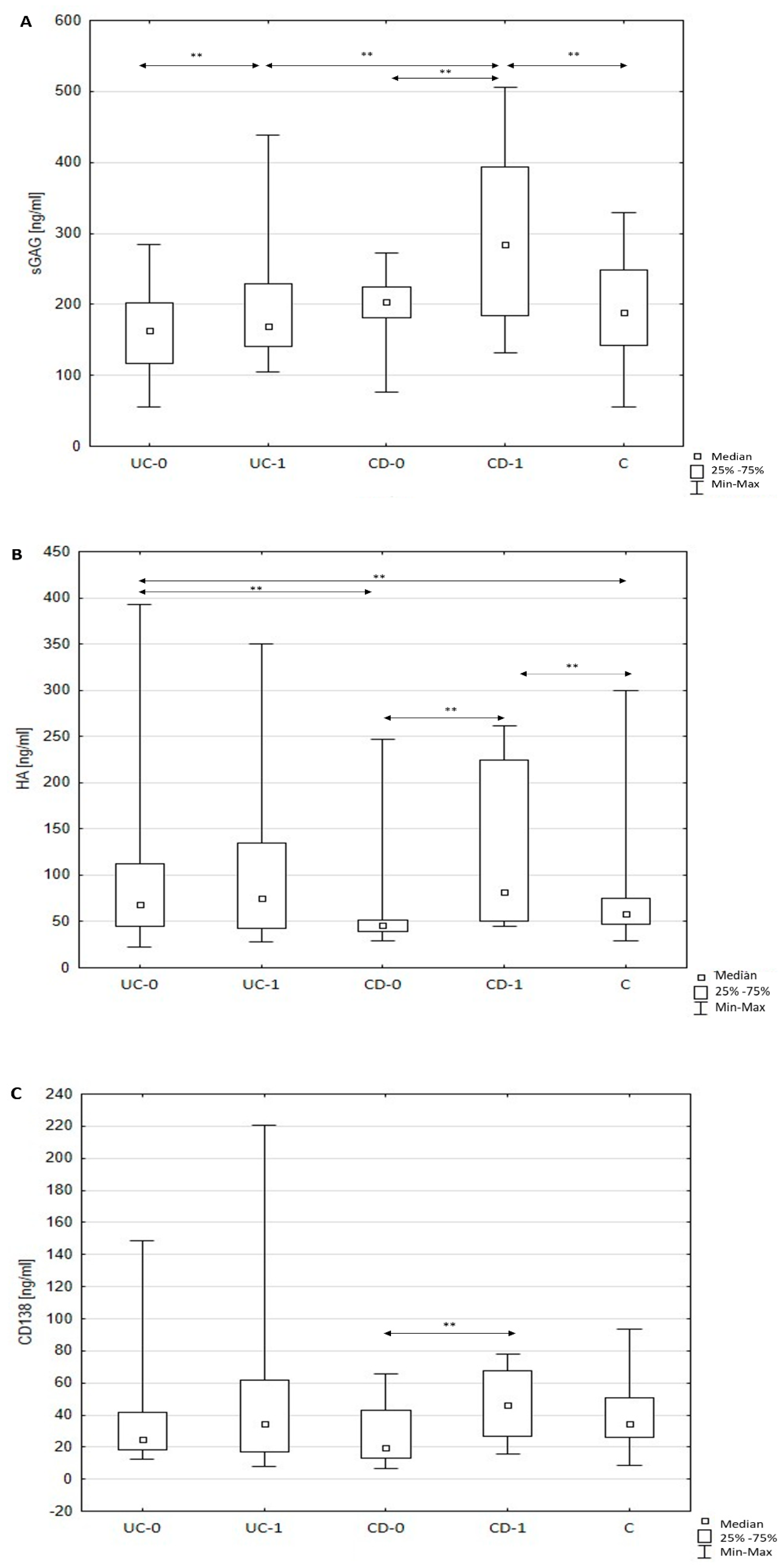
3.2. Quantitative Changes of Serum sGAG, HA, and sCD138 in Patients with Crohn’s Disease
3.3. Quantitative Changes of Serum sGAG, HA, and sCD138 in Patients with Ulcerative Colitis
3.4. The Relationship between Serum ECM Components and Inflammatory Processes and Disease Activity
4. Discussion
4.1. Quantitative Changes of the ECM Components (sGAG, HA, and sCD138) in Serum of Patients Newly Diagnosed with CD or UC
4.2. The Influence of Therapy with Prednisone (Patients with CD) or Biological Treatment with Adalimumab (Patients with UC) on the Circulating Profile of Analyzed ECM Components
4.3. The Influence of Inflammatory Processes and Disease Activity on ECM Components in Patients with IBD
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Actis, G.C.; Pellicano, R.; Fagoonee, S.; Ribaldone, D.G. History of Inflammatory Bowel Diseases. J. Clin. Med. 2019, 8, 1970. [Google Scholar] [CrossRef]
- Ahlawat, S.; Kumar, P.; Mohan, H.; Sandeep Goyal, S.; Kant Sharma, K. Inflammatory bowel disease: Tri-directional relationship between microbiota, immune system and intestinal epithelium. Crit. Rev. Microbiol. 2021, 47, 254–273. [Google Scholar] [CrossRef]
- Gomollón, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: Part 1: Diagnosis and medical management. J. Crohn Colitis 2017, 11, 3–25. [Google Scholar] [CrossRef]
- Tanaka, M.; Saito, H.; Fukuda, S.; Sasaki, Y.; Munakata, A.; Kudo, H. Simple mucosal biopsy criteria differentiating among Crohn disease, ulcerative colitis, and other forms of colitis: Measurement of validity. Scand. J. Gastroenterol. 2000, 35, 281–286. [Google Scholar]
- Kopylov, U.; Rosenfeld, G.; Bressler, B.; Seidman, E. Clinical utility of fecal biomarkers for the diagnosis and management of inflammatory bowel disease. Inflamm. Bowel Dis. 2014, 20, 742–756. [Google Scholar] [CrossRef]
- Konikoff, M.R.; Denson, L.A. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm. Bowel Dis. 2006, 12, 524–534. [Google Scholar] [CrossRef]
- Bennike, T.; Birkelund, S.; Stensballe, A.; Andersen, V. Biomarkers in inflammatory bowel diseases: Current status and proteomics identification strategies. World J. Gastroenterol. 2014, 20, 3231–3244. [Google Scholar] [CrossRef] [PubMed]
- Di Ruscio, M.; Vernia, F.; Ciccone, A.; Frieri, G.; Latella, G. Surrogate fecal biomarkers in inflammatory bowel disease: Rivals or complementary tools of fecal calprotectin? Inflamm. Bowel Dis. 2018, 24, 78–92. [Google Scholar] [CrossRef]
- Petrey, A.C.; De La Motte, C.A. Hyaluronan in inflammatory bowel disease: Cross-linking inflammation and coagulation. Matrix Biol. 2019, 78, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Scott, J.E. Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. FASEB J. 1992, 6, 2639–2645. [Google Scholar] [CrossRef]
- Murch, S.H.; MacDonald, T.T.; Walker-Smith, J.A.; Lionetti, P.; Levin, M.; Klein, N.J. Disruption of sulphated glycosaminoglycans in intestinal inflammation. Lancet 1993, 341, 711–714. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan: From extracellular glue to pericellular cue. Nat. Rev. Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef]
- Derkacz, A.; Olczyk, P.; Olczyk, K.; Komosinska-Vassev, K. The Role of Extracellular Matrix Components in Inflammatory Bowel Diseases. J. Clin. Med. 2021, 10, 1122. [Google Scholar] [CrossRef]
- Grandoch, M.; Bollyky, P.L.; Fischer, J.W. Hyaluronan: A master switch between vascular homeostasis and inflammation. Circ. Res. 2018, 122, 1341–1343. [Google Scholar] [CrossRef] [PubMed]
- Elenius, K.; Vainio, S.; Laato, M.; Salmivirta, M.; Thesleff, I.; Jalkanen, M. Induced expression of syndecan in healing wounds. J. Cell Biol. 1991, 114, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, A.N.; Multhaupt, H.A.; Couchman, J.R. Syndecans in wound healing, inflammation and vascular biology. Int. J. Biochem. Cell Biol. 2007, 39, 505–528. [Google Scholar] [CrossRef]
- Tkachenko, E.; Rhodes, J.M.; Simons, M. Syndecans: New kids on the signaling block. Circ. Res. 2005, 96, 488–500. [Google Scholar] [CrossRef]
- Yablecovitch, D.; Stein, A.; Shabat-Simon, M.; Naftali, T.; Gabay, G.; Laish, I.; Laish, I.; Oren, A.; Konikoff, F.M. Soluble syndecan-1 levels are elevated in patients with inflammatory bowel disease. Dig. Dis. Sci. 2015, 60, 2419–2426. [Google Scholar] [CrossRef]
- Wang, X.; Li, A.; Li, J.; Lin, S.; Zhou, Y.; Wang, X.; Chen, C.; Liu, S.; Chen, Y. Low molecular weight heparin relieves experimental colitis in mice by downregulating IL-1β and inhibiting syndecan-1 shedding in the intestinal mucosa. PLoS ONE 2013, 8, e66397. [Google Scholar] [CrossRef][Green Version]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodeling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef]
- Bosman, F.T.; Stamenkovic, I. Functional structure and composition of the extracellular matrix. J. Pathol. 2003, 200, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Kessler, S.; Rho, H.; West, G.; Fiocchi, C.; Drazba, J.; De La Motte, C. Hyaluronan (HA) deposition precedes and promotes leukocyte recruitment in intestinal inflammation. Clin. Transl. Sci. 2008, 1, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, J.H.; Manon-Jensen, T.; Jensen, M.D.; Hagglund, P.M.; Klinge, L.G.; Kjeldsen, J.; Krag, A.; Karsdal, M.A.; Bay-Jensen, A.C. Ulcerative colitis, Crohn’s disease, and irritable bowel syndrome have different profiles of extracellular matrix turnover, which also reflects disease activity in Crohn’s disease. PLoS ONE 2017, 12, e0185855. [Google Scholar] [CrossRef]
- Chang, S.; Malter, L.; Hudesman, D. Disease monitoring in inflammatory bowel disease. World J. Gastroenterol. 2015, 21, 11246–11259. [Google Scholar] [CrossRef]
- Solem, C.A.; Loftus, E.V., Jr.; Tremaine, W.J.; Harmsen, W.S.; Zinsmeister, A.R.; Sandborn, W.J. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm. Bowel Dis. 2005, 11, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Florin, T.H.J.; Paterson, E.W.J.; Fowler, E.V.; Radford-Smith, G.L. Clinically active Crohn’s disease in the presence of a low C-reactive protein. Scand. J. Gastroenterol. 2006, 41, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Fagan, E.A.; Dyck, R.F.; Maton, P.N.; Hodgson, H.J.; Chadwick, V.S.; Petrie, A.; Pepys, M.B. Serum levels of C-reactive protein in Crohn’s disease and ulcerative colitis. Eur. J. Clin. Investig. 1982, 12, 351–359. [Google Scholar] [CrossRef]
- Greenfield, J.R.; Samaras, K.; Jenkins, A.B.; Kelly, P.J.; Spector, T.D.; Gallimore, J.R.; Pepys, M.B.; Campbell, L.V. Obesity is an important determinant of baseline serum C-reactive protein concentration in monozygotic twins, independent of genetic influences. Circulation 2004, 109, 3022–3028. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Elisei, W.; Faggiani, R.; Allegretta, L.; Della Valle, N.; Forti, G.; Franceschi, M.; Ferronato, A.; Gallina, S.; Larussa, T.; et al. Effectiveness and safety of adalimumab to treat outpatient ulcerative colitis. Medicine 2018, 97, e11897. [Google Scholar] [CrossRef]
| Parameter | Patients with Ulcerative Colitis | p | |
|---|---|---|---|
| before Treatment UC0 | after Treatment UC1 | UC0 Vs. UC1 | |
| Age (years) | 33.38 ± 12.75 | ||
| Mayo score | 3 (2–3) | 2 (1–3) | 0.000 |
| CRP (mg/L) | 3.37 (0.79–26.44) | 2.41 (1.42–7.33) | 0.031 |
| Glucose (mmol/L) | 4.99 ± 0.72 | 4.81 ± 0.81 | 0.331 |
| Cholesterol (mmol/L) | 4.91 ± 0.89 | 4.99 ± 0.86 | 0.264 |
| Triglycerides (mmol/L) | 1.41 ± 0.52 | 1.14 ± 0.38 | 0.030 |
| Indirect bilirubin (μmol/L) | 5.20 (4.61–8.32) | 8.30 (5.50–16.70) | 0.000 |
| Direct bilirubin (μmol/L) | 3.70 (2.90–4.42) | 5.30 (3.51–8.21) | 0.010 |
| ALT (U/l) | 15.02 (10.04–26.00) | 16.01 (10.01–25.03) | 0.814 |
| AST (U/L) | 19.00 (14.02–21.02) | 19.03 (15.02–23.01) | 0.100 |
| Total protein (g/L) | 73.48 ± 5.43 | 74.73 ± 5.63 | 0.235 |
| Albumin (g/L) | 42.00 (40.01–46.03) | 43.02 (40.03–48.00) | 0.264 |
| WBC | 7.90 (4.6–13.6) | 7.90 (3.9–13.7) | 0.61 |
| PLT (× 109/L) | 372.03 (292.00–457.00) | 343.04 (263.02–422.03) | 0.050 |
| BMI | 24.25 ± 3.59 | 24.46 ± 4.23 | 0.455 |
| Parameter | Patients with Crohn’s Disease | p | |
|---|---|---|---|
| before Treatment CD0 | after Treatment CD1 | CD0 Vs. CD1 | |
| Age (years) | 32.10 ± 9.56 | ||
| CDAI | 299.60 ± 47.93 | 274.01 ± 50.71 | 0.323 |
| CRP (mg/L) | 15.70 (4.22–39.05) | 15.20 (5.30–28.90) | 0.665 |
| Glucose (mmol/L) | 4.89 (4.72–5.47) | 4.95 (4.61–5.17) | 0.950 |
| Indirect bilirubin (μmol/L) | 5.75 (4.85–7.43) | 6.25 (5.2–10.65) | 0.073 |
| Direct bilirubin (μmol/L) | 3.75 (2.88–4.05) | 7.9 (4.5–10.8) | 0.020 |
| ALT (U/L) | 24 (16.25–29.05) | 21.5 (14.5–31) | 0.351 |
| AST (U/L) | 21.5 (18.5–24.25) | 21 (16.75–23.50) | 0.373 |
| Total protein (g/L) | 72.13 ± 4.90 | 77.25 ± 5.21 | 0.004 |
| Albumin (g/L) | 43.50 (42–47.25) | 43.5 (42–49) | 0.531 |
| WBC (103/µL) | 7.12 ± 3.20 | 6.68 ± 2.05 | 0.421 |
| PLT (x109/L) | 356.50 (277.50–396.02) | 232.20 (134.21–309.11) | 0.090 |
| BMI | 20.58 ± 3.43 | 19.84 ± 2.84 | 0.190 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derkacz, A.; Olczyk, P.; Jura-Półtorak, A.; Olczyk, K.; Komosinska-Vassev, K. The Diagnostic Usefulness of Circulating Profile of Extracellular Matrix Components: Sulfated Glycosaminoglycans (sGAG), Hyaluronan (HA) and Extracellular Part of Syndecan-1 (sCD138) in Patients with Crohn’s Disease and Ulcerative Colitis. J. Clin. Med. 2021, 10, 1722. https://doi.org/10.3390/jcm10081722
Derkacz A, Olczyk P, Jura-Półtorak A, Olczyk K, Komosinska-Vassev K. The Diagnostic Usefulness of Circulating Profile of Extracellular Matrix Components: Sulfated Glycosaminoglycans (sGAG), Hyaluronan (HA) and Extracellular Part of Syndecan-1 (sCD138) in Patients with Crohn’s Disease and Ulcerative Colitis. Journal of Clinical Medicine. 2021; 10(8):1722. https://doi.org/10.3390/jcm10081722
Chicago/Turabian StyleDerkacz, Alicja, Paweł Olczyk, Agnieszka Jura-Półtorak, Krystyna Olczyk, and Katarzyna Komosinska-Vassev. 2021. "The Diagnostic Usefulness of Circulating Profile of Extracellular Matrix Components: Sulfated Glycosaminoglycans (sGAG), Hyaluronan (HA) and Extracellular Part of Syndecan-1 (sCD138) in Patients with Crohn’s Disease and Ulcerative Colitis" Journal of Clinical Medicine 10, no. 8: 1722. https://doi.org/10.3390/jcm10081722
APA StyleDerkacz, A., Olczyk, P., Jura-Półtorak, A., Olczyk, K., & Komosinska-Vassev, K. (2021). The Diagnostic Usefulness of Circulating Profile of Extracellular Matrix Components: Sulfated Glycosaminoglycans (sGAG), Hyaluronan (HA) and Extracellular Part of Syndecan-1 (sCD138) in Patients with Crohn’s Disease and Ulcerative Colitis. Journal of Clinical Medicine, 10(8), 1722. https://doi.org/10.3390/jcm10081722








