Impact of Age and Comorbidity on Multimodal Management and Survival from Colorectal Cancer: A Population-Based Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Surgical Procedures
2.3. Neoadjuvant and Adjuvant Treatments
2.4. Follow-Up
2.5. Data Collection, Assessment of Age-Adjusted Charlson Comorbidity Index and Outcome Measures
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics and Tumor Stage Distribution According to Age-Adjusted Charlson Comorbidity Index (ACCI) Score
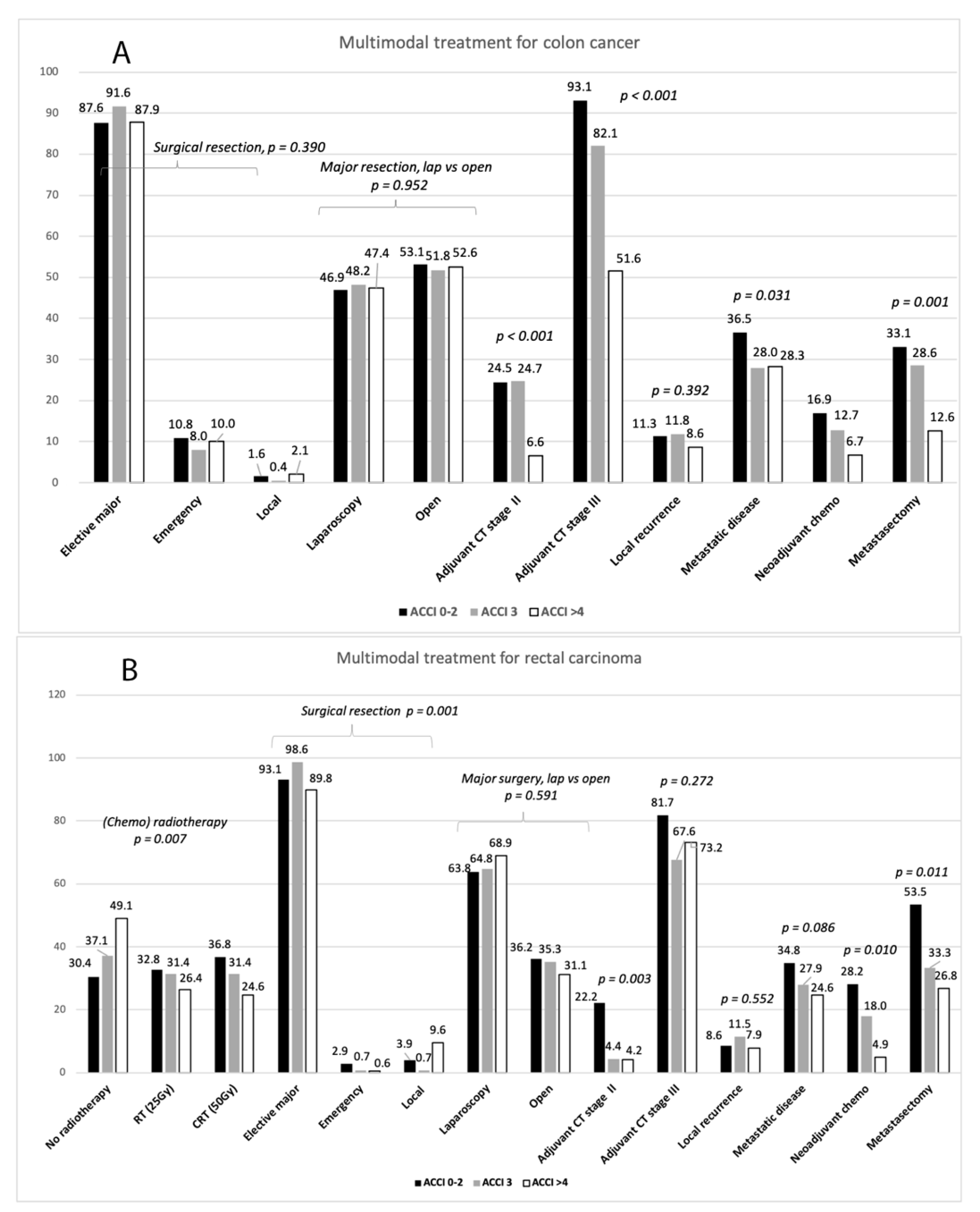
3.2. Multimodal Treatment
3.3. Long-Term Survival
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.; Cologne, K.G. Laparoscopic Approach to Rectal Cancer—The New Standard? Front. Oncol. 2020, 10, 1239. [Google Scholar] [CrossRef]
- Schmoll, H.J.; Van Cutsem, E.; Stein, A.; Valentini, V.; Glimelius, B.; Haustermans, K.; Nordlinger, B.; van de Velde, C.J.; Balmana, J.; Regula, J.; et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann. Oncol. 2012, 23, 2479–2516. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Nordlinger, B.; Arnold, D. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25, iii1–iii9. [Google Scholar] [CrossRef]
- Adam, R.; De Gramont, A.; Figueras, J.; Guthrie, A.; Kokudo, N.; Kunstlinger, F.; Loyer, E.; Poston, G.; Rougier, P.; Rubbia-Brandt, L.; et al. The Oncosurgery Approach to Managing Liver Metastases from Colorectal Cancer: A Multidisciplinary International Consensus. Oncologist 2012, 17, 1225–1239. [Google Scholar] [CrossRef] [Green Version]
- Adam, R.; de Gramont, A.; Figueras, J.; Kokudo, N.; Kunstlinger, F.; Loyer, E.; Poston, G.; Rougier, P.; Rubbia-Brandt, L.; Sobrero, A.; et al. Managing synchronous liver metastases from colorectal cancer: A multidisciplinary international consensus. Cancer Treat. Rev. 2015, 41, 729–741. [Google Scholar] [CrossRef] [Green Version]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv22–iv40. [Google Scholar] [CrossRef]
- Steele, S.R.; Park, G.E.; Johnson, E.K.; Martin, M.J.; Stojadinovic, A.; Maykel, J.A.; Causey, M.W. The Impact of Age on Colorectal Cancer Incidence, Treatment, and Outcomes in an Equal-Access Health Care System. Dis. Colon Rectum 2014, 57, 303–310. [Google Scholar] [CrossRef]
- Lemmens, V.E.P.P.; Janssen-Heijnen, M.L.G.; Verheij, C.D.G.W.; Houterman, S.; van Driel, O.J.R.; Coebergh, J.W.W. Co-morbidity leads to altered treatment and worse survival of elderly patients with colorectal cancer. BJS 2005, 92, 615–623. [Google Scholar] [CrossRef]
- Faivre, J.; Lemmens, V.; Quipourt, V.; Bouvier, A. Management and survival of colorectal cancer in the elderly in population-based studies. Eur. J. Cancer 2007, 43, 2279–2284. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiology 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- De Groot, V.; Beckerman, H.; Lankhorst, G.J.; Bouter, L.M. How to measure comorbiditya critical review of available methods. J. Clin. Epidemiology 2003, 56, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Dias-Santos, D.; Ferrone, C.R.; Zheng, H.; Lillemoe, K.D.; Castillo, C.F.-D. The Charlson age comorbidity index predicts early mortality after surgery for pancreatic cancer. Surgery 2015, 157, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Maezawa, Y.; Aoyama, T.; Kano, K.; Tamagawa, H.; Numata, M.; Hara, K.; Murakawa, M.; Yamada, T.; Sato, T.; Ogata, T.; et al. Impact of the Age-adjusted Charlson comorbidity index on the short- and long-term outcomes of patients undergoing curative gastrectomy for gastric cancer. J. Cancer 2019, 10, 5527–5535. [Google Scholar] [CrossRef] [Green Version]
- Iversen, L.H.; Nørgaard, M.; Jacobsen, J.; Laurberg, S.; Sørensen, H.T. The Impact of Comorbidity on Survival of Danish Colorectal Cancer Patients from 1995 to 2006—A Population-Based Cohort Study. Dis. Colon Rectum 2009, 52, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, T.L.; Hallas, J.; Friis, S.; Herrstedt, J. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. Br. J. Cancer 2012, 106, 1353–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostenfeld, E.B.; Nørgaard, M.; Thomsen, R.W.; Iversen, L.H.; Jacobsen, J.B.; Sogaard, M. Comorbidity and survival of Danish patients with colon and rectal cancer from 2000–2011: A population-based cohort study. Clin. Epidemiology 2013, 5, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-C.; Hsu, T.-W.; Chang, C.-M.; Yu, C.-H.; Lee, C.-C. Age-Adjusted Charlson Comorbidity Index Scores as Predictor of Survival in Colorectal Cancer Patients Who Underwent Surgical Resection and Chemoradiation. Medicine 2015, 94, e431. [Google Scholar] [CrossRef]
- Ouellette, J.R.; Small, D.G.; Termuhlen, P.M. Evaluation of Charlson-Age Comorbidity Index as predictor of morbidity and mortality in patients with colorectal carcinoma. J. Gastrointest. Surg. 2004, 8, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Marventano, S.; Grosso, G.; Mistretta, A.; Bogusz-Czerniewicz, M.; Ferranti, R.; Nolfo, F.; Giorgianni, G.; Rametta, S.; Drago, F.; Basile, F.; et al. Evaluation of four comorbidity indices and Charlson comorbidity index adjustment for colorectal cancer patients. Int. J. Color. Dis. 2014, 29, 1159–1169. [Google Scholar] [CrossRef]
- Hohenberger, W.; Reingruber, B.; Merkel, S. Surgery for Colon Cancer. Scand. J. Surg. 2003, 92, 45–52. [Google Scholar] [CrossRef]
- Hohenberger, W.; Weber, K.; Matzel, K.; Papadopoulos, T.; Merkel, S. Standardized surgery for colonic cancer: Complete mesocolic excision and central ligation—Technical notes and outcome. Color. Dis. 2009, 11, 354–364. [Google Scholar] [CrossRef]
- Heald, R.; Ryall, R. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986, 327, 1479–1482. [Google Scholar] [CrossRef]
- Ehrlich, A.; Kairaluoma, M.; Böhm, J.; Vasala, K.; Kautiainen, H.; Kellokumpu, I. Laparoscopic Wide Mesocolic Excision and Central Vascular Ligation for Carcinoma of the Colon. Scand. J. Surg. 2016, 105, 228–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellokumpu, I.H.; Kairaluoma, M.I.; Nuorva, K.P.; Kautiainen, H.J.; Jantunen, I.T. Short- and Long-term Outcome Following Laparoscopic Versus Open Resection for Carcinoma of the Rectum in the Multimodal Setting. Dis. Colon Rectum 2012, 55, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Sobin, L.H.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 978-1-4443-3241-4. [Google Scholar]
- Van Eeghen, E.E.; Bakker, S.D.; Van Bochove, A.; Loffeld, R.J.L.F. Impact of age and comorbidity on survival in colorectal cancer. J. Gastrointest. Oncol. 2015, 6, 605–612. [Google Scholar]
- Glimelius, B.; Osterman, E. Adjuvant Chemotherapy in Elderly Colorectal Cancer Patients. Cancers 2020, 12, 2289. [Google Scholar] [CrossRef]
- Delgado, M.V.; Serrano, C.S.; De La Fuente, E.C.; García, A.B.; Saez, O.M.; Huertas, R.M.; Domingo, J.S.; Cerrillo, J.M.; Muñoz, F.L.; Olmos, V.P.; et al. Efficacy of adjuvant chemotherapy for elderly patients with colon cancer. Ann. Oncol. 2018, 29, v65. [Google Scholar] [CrossRef] [Green Version]
- Boakye, D.; Walter, V.; Martens, U.M.; Chang-Claude, J.; Hoffmeister, M.; Jansen, L.; Brenner, H. Treatment selection bias for chemotherapy persists in colorectal cancer patient cohort studies even in comprehensive propensity score analyses. Clin. Epidemiology 2019, 2019, 821–832. [Google Scholar] [CrossRef] [Green Version]
- Pisano, M.; Zorcolo, L.; Merli, C.; Cimbanassi, S.; Poiasina, E.; Ceresoli, M.; Agresta, F.; Allievi, N.; Bellanova, G.; Coccolini, F.; et al. 2017 WSES guidelines on colon and rectal cancer emergencies: Obstruction and perforation. World J. Emerg. Surg. 2018, 13, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Rutegård, M.; Haapamäki, M.; Matthiessen, P.; Rutegård, J. Early postoperative mortality after surgery for rectal cancer in Sweden, 2000–2011. Color. Dis. 2014, 16, 426–432. [Google Scholar] [CrossRef]
- Morris, E.J.A.; Taylor, E.F.; Thomas, J.D.; Quirke, P.; Finan, P.J.; Coleman, M.P.; Rachet, B.; Forman, D. Thirty-day postoperative mortality after colorectal cancer surgery in England. Gut 2011, 60, 806–813. [Google Scholar] [CrossRef] [Green Version]
- Alves, A.; Panis, Y.; Mathieu, P.; Mantion, G.; Kwiatkowski, F.; Slim, K. Postoperative Mortality and Morbidity in French Patients Undergoing Colorectal Surgery. Arch. Surg. 2005, 140, 278–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böckelman, C.; Engelmann, B.E.; Kaprio, T.; Hansen, T.F.; Glimelius, B. Risk of recurrence in patients with colon cancer stage II and III: A systematic review and meta-analysis of recent literature. Acta Oncol. 2014, 54, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Van Der Geest, L.G.M.; Lam-Boer, J.; Koopman, M.; Verhoef, C.; Elferink, M.A.G.; De Wilt, J.H.W. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin. Exp. Metastasis 2015, 32, 457–465. [Google Scholar] [CrossRef]
- Van Gestel, Y.R.; de Hingh, I.H.; van Herk-Sukel, M.P.; van Erning, F.N.; Beerepoot, L.V.; Wijsman, J.H.; Slooter, G.D.; Rutten, H.J.; Creemers, G.-J.M.; Lemmens, V.E. Patterns of metachronous metastases after curative treatment of colorectal cancer. Cancer Epidemiology 2014, 38, 448–454. [Google Scholar] [CrossRef]
- Riihimäki, M.; Hemminki, A.; Sundquist, J.; Hemminki, K. Patterns of metastasis in colon and rectal cancer. Sci. Rep. 2016, 6, 29765. [Google Scholar] [CrossRef] [Green Version]
- Seppälä, T.T.; Bohm, J.; Friman, M.; Lahtinen, L.; Väyrynen, V.M.J.; Liipo, T.K.E.; Ristimäki, A.P.; Kairaluoma, M.V.J.; Kellokumpu, I.H.; Kuopio, T.H.I.; et al. Combination of microsatellite instability and BRAF mutation status for subtyping colorectal cancer. Br. J. Cancer 2015, 112, 1966–1975. [Google Scholar] [CrossRef] [Green Version]
- Wirta, E.-V.; Seppälä, T.; Friman, M.; Väyrynen, J.; Ahtiainen, M.; Kautiainen, H.; Kuopio, T.; Kellokumpu, I.; Mecklin, J.-P.; Böhm, J. Immunoscore in mismatch repair-proficient and -deficient colon cancer. J. Pathol. Clin. Res. 2017, 3, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Brulé, S.; Jonker, D.; Karapetis, C.; O’Callaghan, C.; Moore, M.; Wong, R.; Tebbutt, N.; Underhill, C.; Yip, D.; Zalcberg, J.; et al. Location of colon cancer (right-sided versus left-sided) as a prognostic factor and a predictor of benefit from cetuximab in NCIC CO.17. Eur. J. Cancer 2015, 51, 1405–1414. [Google Scholar] [CrossRef] [PubMed]

| Weight | Charlson Comorbid Condition |
|---|---|
| 1 | Myocardial infarction, congestive heart failure, peripheral or cerebral vascular disease, TIA (transient ischemic attack), chronic obstructive pulmonary disease (COPD), connective tissue disease, mild liver disease, peptic ulcer disease, diabetes, dementia |
| 2 | Hemiplegia, moderate/severe renal disease, diabetes with end-organ damage, previous cancer *, leukemia, lymphoma |
| 3 | Moderate or severe liver disease |
| 6 | Metastatic solid cancer *, Acquired Immunodeficiency Syndrome (AIDS) |
| 1 | For each decade over age 50 years, up to 4 points |
| All Patients | ACCI = 0–2 Low | ACCI = 3 Intermediate | ACCI = ≥4 High | p-Value | |
|---|---|---|---|---|---|
| Primary tumour site Right colon Left colon Rectum | 558 (37.7) 410 (27.7) 511 (34.6) | 171 (32.3) 152 (28.7) 207 (39.1) | 124 (34.2) 101 (27.8) 138 (38.0) | 263 (44.9) 157 (26.8) 166 (28.3) | <0.001 |
| Colon Age (years), mean (SD) | 968 (100.0) 70.7 (11.3) | 323 (100.0) 59.1 (8.1) | 225 (100.0) 71.3 (6.2) | 420 (100.0) 79.4 (6.8) | <0.001 |
| Gender, male | 471 (48.7) | 155 (48.0) | 108 (48.0) | 208 (49.5) | 0.894 |
| Charlson comorbidity | 412 (42.6) | 19 (5.9) | 55 (24.4) | 338 (80.5) | <0.001 |
| Disease stage (UICC) I II III IV | 168 (17.4) 365 (37.7) 304 (31.4) 131 (13.5) | 58 (18.0) 106 (32.8) 102 (31.6) 57 (17.6) | 32 (14.2) 93 (41.3) 78 (34.7) 22 (9.8) | 79 (18.6) 166 (39.5) 124 (29.5) 52 (12.4) | 0.044 |
| Rectum Age(years), mean (SD) | 511 (100.0) 67.9 (10.7) | 204 (100.0) 59.0 (7.6) | 140 (100.0) 70.4 (7.0) | 167 (100.0) 76.7 (7.8) | <0.001 |
| Gender male | 331 (64.8) | 134 (65.7) | 94 (67.1) | 103 (61.7) | 0.571 |
| Charlson comorbidity | 185 (36.2) | 9 (4.4) | 49 (35.0) | 127 (76.1) | <0.001 |
| Disease stage (UICC) | |||||
| 0 (pCR) a I II III IV | 10 (2.0) 170 (33.3) 147 (28.8) 138 (27.0) 46 (9.0) | 3 (1.5) 60 (29.4) 54 (26.5) 63 (29.4) 27 (13.2) | 4 (2.9) 46 (32.9) 45 (32.4) 37 (26.4) 8 (5.7) | 3 (1.8) 64 (38.2) 48 (28.7) 41 (24.6) 11 (6.6) | 0.168 |
| Univariate Hazard Ratio (HR) (95% Confidence Interval (CI)) | p-Value * | Multivariable HR | p-Value * | |
|---|---|---|---|---|
| (95% CI) | ||||
| Colon | ||||
| Age-adjusted comorbidity index score (ACCI) | ||||
| Low | 1 | <0.001 | 1 | <0.001 |
| Intermediate | 1.30 (1.02–1.66) | 1.50 (1.17–1.92) | ||
| High | 2.70 (2.21–3.30) | 3.24 (2.65–3.98) | ||
| Gender | ||||
| Male | 1 | 0.032 | 1 | 0.026 |
| Female | 0.84 (0.71–0.98) | 0.82 (0.69–0.97) | ||
| UICC stage | ||||
| I | 1 | <0.001 | 1 | <0.001 |
| II | 1.25 (0.97–1.63) | 1.31 (1.00–1.73) | ||
| III | 1.53 (1.18–1.20) | 1.71 (1.29–2.26) | ||
| IV | 7.34 (5.47–9.83 ) | 8.75 (6.38–11.99) | ||
| Type of surgical resection | ||||
| Elective major surgery | 1 | <0.001 | 1 | <0.001 |
| Emergency surgery | 2.21 (1.72–2.83) | 1.82 (1.41–2.35) | ||
| Local excision | 1.36 (0.75–2.48) | 1.90 (1.0–3.62) | ||
| Adjuvant chemotherapy | ||||
| No | 1 | 0.254 | _ | |
| Yes | 0.90 (0.76–1.07) | |||
| Side of colon | ||||
| Right | 1 | 0.141 | 1 | 0.077 |
| Left | 0.88 (0.75–1.04) | 0.86 (0.72–1.02) | ||
| Time periods | ||||
| 2000–2005 | 1 | 0.707 | ||
| 2006–2010 | 0.96 (0.79–1.17) | _ | ||
| 2011–2015 | 0.96 (0.77–1.19) | |||
| Rectum | ||||
| ACCI | ||||
| Low | 1 | <0.001 | 1 | <0.001 |
| Intermediate | 1.81 (1.33–2.45) | 2.25 (1.65–3.07) | ||
| High | 2.51 (1.89–3.34) | 3.29 (2.42–4.36) | ||
| Gender | ||||
| Male | 1 | 0.320 | _ | |
| Female | 0.88 (0.69–1.13) | |||
| UICC stage | ||||
| I | 1 | <0.001 | 1 | <0.001 |
| II | 1.33 (0.98–1.80) | 1.41 (1.04–1.91) | ||
| III | 1.59 (1.16–2.16) | 1.90 (1.39–2.60) | ||
| IV | 5.51 (3.73–8.15) | 8.42 (5.60–12.67) | ||
| Type of surgical resection | ||||
| Elective major surgery | 1 | 0.543 | _ | |
| Emergency surgery | 3.11 (1.46–6.60) | |||
| Local excision | 1.01 (0.58–1.76) | |||
| Preoperative radiotherapy | ||||
| No radiotherapy | 1 | 0.296 | _ | |
| Short-course (25 Gy) | 0.87 (0.65–1.16) | |||
| Chemoradiotherapy (50 Gy) | 1.17 (0.89–1.55) | |||
| Adjuvant chemotherapy | ||||
| No | 1 | 0.601 | _ | |
| Yes | 1.07 (0.83–1.39) | |||
| Time periods | ||||
| 2000–2005 | 1 | 0.483 | _ | |
| 2006–2010 | 1.01 (0.76–1.34) | |||
| 2011–2015 | 0.89 (0.66–1.22) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kellokumpu, I.; Kairaluoma, M.; Mecklin, J.-P.; Kellokumpu, H.; Väyrynen, V.; Wirta, E.-V.; Sihvo, E.; Kuopio, T.; Seppälä, T.T. Impact of Age and Comorbidity on Multimodal Management and Survival from Colorectal Cancer: A Population-Based Study. J. Clin. Med. 2021, 10, 1751. https://doi.org/10.3390/jcm10081751
Kellokumpu I, Kairaluoma M, Mecklin J-P, Kellokumpu H, Väyrynen V, Wirta E-V, Sihvo E, Kuopio T, Seppälä TT. Impact of Age and Comorbidity on Multimodal Management and Survival from Colorectal Cancer: A Population-Based Study. Journal of Clinical Medicine. 2021; 10(8):1751. https://doi.org/10.3390/jcm10081751
Chicago/Turabian StyleKellokumpu, Ilmo, Matti Kairaluoma, Jukka-Pekka Mecklin, Henrik Kellokumpu, Ville Väyrynen, Erkki-Ville Wirta, Eero Sihvo, Teijo Kuopio, and Toni T. Seppälä. 2021. "Impact of Age and Comorbidity on Multimodal Management and Survival from Colorectal Cancer: A Population-Based Study" Journal of Clinical Medicine 10, no. 8: 1751. https://doi.org/10.3390/jcm10081751
APA StyleKellokumpu, I., Kairaluoma, M., Mecklin, J.-P., Kellokumpu, H., Väyrynen, V., Wirta, E.-V., Sihvo, E., Kuopio, T., & Seppälä, T. T. (2021). Impact of Age and Comorbidity on Multimodal Management and Survival from Colorectal Cancer: A Population-Based Study. Journal of Clinical Medicine, 10(8), 1751. https://doi.org/10.3390/jcm10081751






