Chronic Breast Pain Prior to Breast Cancer Surgery Is Associated with Worse Acute Postoperative Pain Outcomes
Abstract
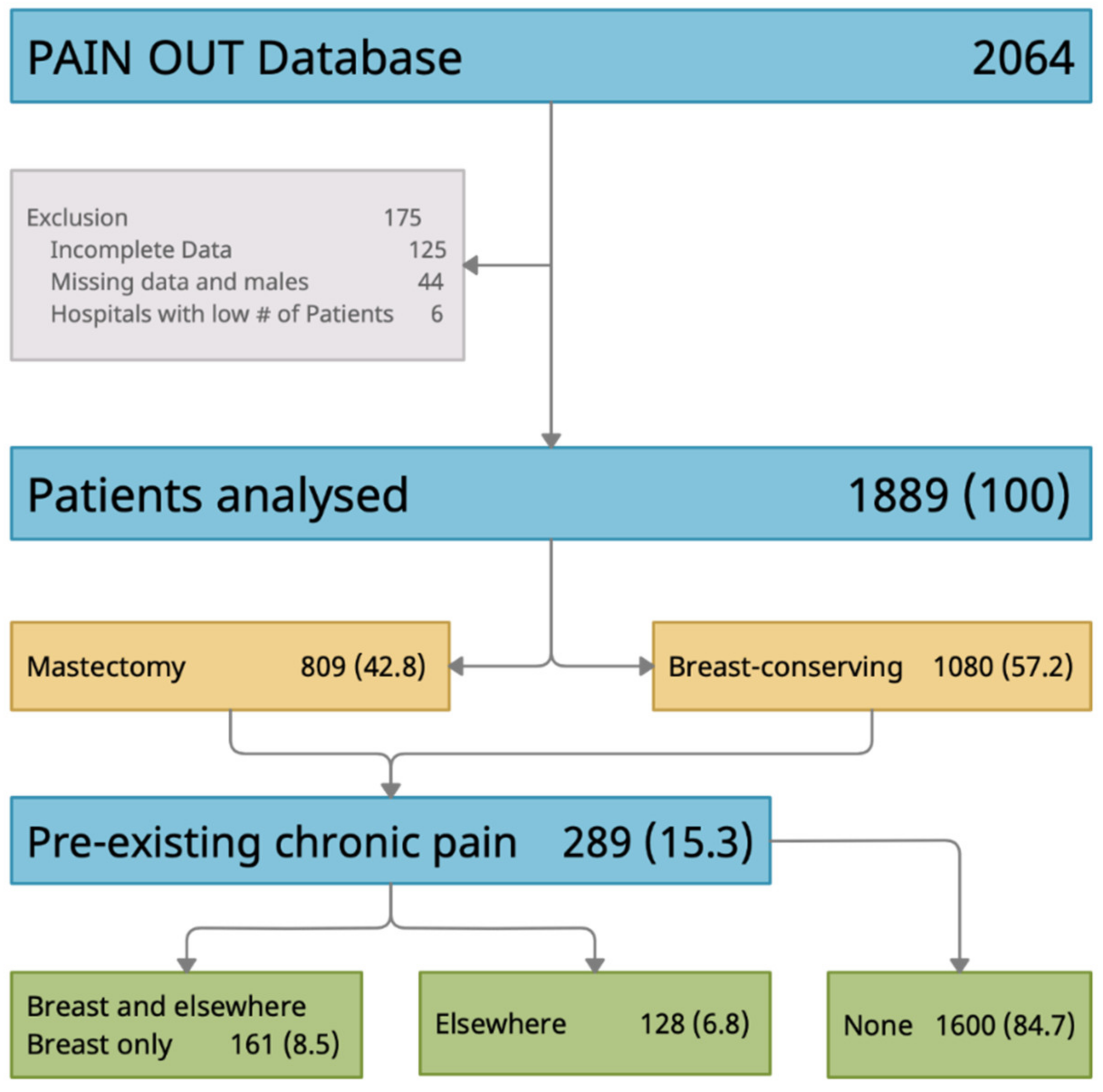
1. Introduction
2. Materials and Methods
2.1. Participants & Setting
2.2. Measures
- ◦
- Pain intensity: Maximum (i.e., worst) and minimum (i.e., least) were rated on 0 “no pain” to 10 “worst pain imaginable” numeric rating scales (NRS) and the percent of time in severe pain since surgery (0% to 100%).
- ◦
- Pain interference: Pain interference with activity in and out of bed, breathing deeply or coughing and sleeping, rated on a 0 “did not interfere” to 10 “completely interferes” NRS.
- ◦
- Pain-related anxiety and helplessness: Anxiety and helplessness caused by pain rated on a 0 “not at all” to 10 “extremely” NRS.
- ◦
- Perception of pain treatment: Pain relief was rated from 0 “no relief” to 100% “complete relief”. Patients were also asked to indicate they would have liked more pain treatment than they received (yes/no).
- ◦
- Preexisting chronic pain: Patients were asked if they had preexisting pain for at least three months prior to surgery (yes/no), intensity of preexisting pain (0 to 10 NRS), and location of preexisting pain (surgical site [i.e., breast], elsewhere in the body, surgical site and elsewhere in the body).
2.3. Statistical Methods
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Acute Postoperative Pain-Related Outcomes
3.3. Opioid Pain Management before Admission and Perioperatively
3.4. Multivariable Linear Regression Findings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Fecho, K.; Miller, N.; Merritt, S.A.; Klauber-Demore, N.; Hultman, C.S.; Blau, W.S. Acute and persistent postoperative pain after breast surgery. Pain Med. 2009, 10, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.J. Poorly controlled postoperative pain: Prevalence, consequences, and prevention. J. Pain Res. 2017, 10, 2287–2298. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.S.; Ullricht, K.; Regan, S.; Broussard, C.; Schwenkglenks, M.; Taylor, R.J.; Gordon, D.B.; Zaslansky, R.; Meissner, W.; Rothaug, J.; et al. The impact of early postoperative pain on health-related quality of life. Pain Pract. 2013, 13, 515–523. [Google Scholar] [CrossRef]
- Meretoja, T.J.; Andersen, K.G.; Bruce, J.; Haasio, L.; Sipila, R.; Scott, N.W.; Ripatti, S.; Kehlet, H.; Kalso, E. Clinical Prediction Model and Tool for Assessing Risk of Persistent Pain After Breast Cancer Surgery. J. Clin. Oncol. 2017, 35, 1660–1667. [Google Scholar] [CrossRef]
- Poleshuck, E.L.; Katz, J.; Andrus, C.H.; Hogan, L.A.; Jung, B.F.; Kulick, D.I.; Dwornik, R.H. Risk factors for chronic pain following breast cancer surgery: A prospective study. J. Pain 2006, 7, 626–634. [Google Scholar] [CrossRef]
- Andersen, K.G.; Duriaud, H.M.; Jensen, H.E.; Kroman, N.; Kehlet, H. Predictive factors for the development of persistent pain after breast cancer surgery. Pain 2015, 156, 2413–2422. [Google Scholar] [CrossRef]
- Tasmuth, T.; Estlander, A.-M.; Kalso, E. Effect of present pain and mood on the memory of past postoperative pain in women treated surgically for breast cancer. Pain 1996, 68, 343–347. [Google Scholar] [CrossRef]
- Berger, J.M.; Longhitano, Y.; Zanza, C.; Sener, S.F. Factors affecting the incidence of chronic pain following breast cancer surgery: Preoperative history, anesthetic management, and surgical technique. J. Surg. Oncol. 2020, 122, 1307–1314. [Google Scholar] [CrossRef]
- Fassoulaki, A.; Melemeni, A.; Staikou, C.; Triga, A.; Sarantopoulos, C. Acute postoperative pain predicts chronic pain and long-term analgesic requirements after breast surgery for cancer. Acta Anaesthesiol. Belg. 2008, 59, 241–248. [Google Scholar]
- Sun, E.C.; Darnall, B.D.; Baker, L.C.; Mackey, S. Incidence of and Risk Factors for Chronic Opioid Use Among Opioid-Naive Patients in the Postoperative Period. JAMA Intern. Med. 2016, 176, 1286–1293. [Google Scholar] [CrossRef]
- Yang, M.M.H.; Hartley, R.L.; Leung, A.A.; Ronksley, P.E.; Jetté, N.; Casha, S.; Riva-Cambrin, J. Preoperative predictors of poor acute postoperative pain control: A systematic review and meta-analysis. BMJ Open 2019, 9, e025091. [Google Scholar] [CrossRef]
- Edgley, C.; Hogg, M.; De Silva, A.; Braat, S.; Bucknill, A.; Leslie, K. Severe acute pain and persistent post-surgical pain in orthopaedic trauma patients: A cohort study. Br. J. Anaesth. 2019, 123, 350–359. [Google Scholar] [CrossRef]
- Kinjo, S.; Sands, L.P.; Lim, E.; Paul, S.; Leung, J.M. Prediction of postoperative pain using path analysis in older patients. J. Anesth. 2012, 26, 1–8. [Google Scholar] [CrossRef]
- Roth, R.S.; Qi, J.; Hamill, J.B.; Kim, H.M.; Ballard, T.N.; Pusic, A.L.; Wilkins, E.G. Is chronic postsurgical pain surgery-induced? A study of persistent postoperative pain following breast reconstruction. Breast 2018, 37, 119–125. [Google Scholar] [CrossRef]
- Rehberg, B.; Mathivon, S.; Combescure, C.; Mercier, Y.; Savoldelli, G.W. Prediction of Acute Postoperative Pain Following Breast Cancer Surgery Using the Pain Sensitivity Questionnaire: A Cohort Study. Clin. J. Pain 2017, 33, 57–66. [Google Scholar] [CrossRef]
- McCann, B.; Miaskowsky, C.; Koetters, T.; Baggott, C.; West, C.; Levine, J.D.; Elboim, C.; Abrams, G.; Hamolsky, D.; Dunn, L.; et al. Associations between pro- and anti-inflammatory cytokine genes and breast pain in women prior to breast cancer surgery. J. Pain 2012, 13, 425–437. [Google Scholar] [CrossRef]
- Langford, D.J.; West, C.; Elboim, C.; Cooper, B.A.; Abrams, G.; Paul, S.M.; Schmidt, B.L.; Levine, J.D.; Merriman, J.D.; Dhruva, A.; et al. Variations in potassium channel genes are associated with breast pain in women prior to breast cancer surgery. J. Neurogenet. 2014, 28, 122–135. [Google Scholar] [CrossRef]
- Bruce, J.; Thornton, A.J.; Scott, N.W.; Marfizo, S.; Powell, R.; Johnston, M.; Wells, M.; Heys, S.D.; Thompson, A.M. Chronic preoperative pain and psychological robustness predict acute postoperative pain outcomes after surgery for breast cancer. Br. J. Cancer 2012, 107, 937–946. [Google Scholar] [CrossRef]
- Sipila, R.M.; Haasio, L.; Meretoja, T.J.; Ripatti, S.; Estlander, A.-M.; Kalso, E.A. Does expecting more pain make it more intense? Factors associated with the first week pain trajectories after breast cancer surgery. Pain 2017, 158, 922–930. [Google Scholar] [CrossRef]
- Langford, D.J.; Schmidt, B.; Levine, J.D.; Abrams, G.; Elboim, C.; Esserman, L.; Hamolsky, D.; Mastick, J.; Paul, S.M.; Coopper, B.; et al. Preoperative Breast Pain Predicts Persistent Breast Pain and Disability After Breast Cancer Surgery. J. Pain Symptom Manag. 2015, 49, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Mustonen, L.; Aho, T.; Harno, H.; Sipila, R.; Meretoja, T.; Kalso, E. What makes surgical nerve injury painful? A 4-year to 9-year follow-up of patients with intercostobrachial nerve resection in women treated for breast cancer. Pain 2019, 160, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Benett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Tunks, E.R.; Crook, J.; Weir, R. Epidemiology of chronic pain with psychological comorbidity: Prevalence, risk, course, and prognosis. Can. J. Psychiatry 2008, 53, 224–234. [Google Scholar] [CrossRef]
- Murphy, B.L.; Thiels, C.A.; Hanson, K.T.; McLaughlin, S.; Jakub, J.W.; Gray, R.J.; Ubl, D.S.; Habermann, E.B. Pain and opioid prescriptions vary by procedure after breast surgery. J. Surg. Oncol. 2019, 120, 593–602. [Google Scholar] [CrossRef]
- Sipila, R.; Estlander, A.-M.; Tasmuth, T.; Kataja, M.; Kalso, E. Development of a screening instrument for risk factors of persistent pain after breast cancer surgery. Br. J. Cancer 2012, 107, 1459–1466. [Google Scholar] [CrossRef]
- Dereu, D.; Savoldelli, G.L.; Combescure, C.; Mathivon, S.; Rehberg, B. Development of a Simple Preoperative Risk Score for Persistent Pain After Breast Cancer Surgery: A Prospective Observational Cohort Study. Clin. J. Pain 2018, 34, 559–565. [Google Scholar] [CrossRef]
- Wang, L.; Guyatt, G.H.; Kennedy, S.A.; Romerosa, B.; Kwon, H.Y.; Kaushal, A.; Chang, Y.; Craigie, S.; de Almeida, C.P.B.; Couban, R.J.; et al. Predictors of persistent pain after breast cancer surgery: A systematic review and meta-analysis of observational studies. CMAJ 2016, 188, E352–E361. [Google Scholar] [CrossRef]
- Kulkarni, A.R.; Pusic, A.L.; Hamill, J.B.; Kim, H.M.; Qi, J.; Wilkins, E.G.; Roth, R.S. Factors Associated with Acute Postoperative Pain Following Breast Reconstruction. JPRAS Open 2017, 11, 1–13. [Google Scholar] [CrossRef]
- Marfizo, S.; Thornton, A.J.; Scott, N.W.; Thompson, A.M.; Hays, S.D.; Bruce, J. Intensity and features of acute postoperative pain after mastectomy and breast-conserving surgery. Breast Cancer Res. 2010, 12, P56. [Google Scholar] [CrossRef]
- Chou, R.; Gordon, D.B.; de Leon-Casasola, O.A.; Rosenberg, J.M.; Bickler, S.; Brennan, T.; Carter, T.; Cassidy, C.L.; Chittenden, E.H.; Degenhardt, E.; et al. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J. Pain 2016, 17, 131–157. [Google Scholar]
- Chaichana, K.L.; Mukherjee, D.; Adogwa, O.; Cheng, J.S.; McGirt, M.J. Correlation of preoperative depression and somatic perception scales with postoperative disability and quality of life after lumbar discectomy. J. Neurosurg. Spine 2011, 14, 261–267. [Google Scholar] [CrossRef]
- Coronado, R.A.; George, S.Z.; Devin, C.J.; Wegener, S.T.; Archer, K.R. Pain Sensitivity and Pain Catastrophizing Are Associated With Persistent Pain and Disability After Lumbar Spine Surgery. Arch. Phys. Med. Rehabil. 2015, 96, 1763–1770. [Google Scholar] [CrossRef]
- Konstantatos, A.H.; Zhong, T.; Paul, E.; Tsang, S.; Tian, S.; Liu, M.; Liang, Y.; Tian, Y.; Qiao, S.; Wu, W.K.K.; et al. Effect of cultural background and healthcare environment on postoperative opioid requirement. Can. J. Anaesth. 2019, 66, 309–317. [Google Scholar] [CrossRef]
| Total Sample N = 1889 | No Chronic Pain Group 0 n = 1600 (84.7%) | Pain Elsewhere Group 1 n = 128 (6.8%) | Pain in Breast * Group 2 n = 161 (8.5%) | Statistics Pairwise Post Hoc Contrasts | |
|---|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||
| Age | 56.6 (56.0–57.3) | 56.2 (55.6–56.9) | 62.1 (59.9–64.3) | 56.5 (54.1–58.90 | F = 10.9, p < 0.001 ** 1 > 0 and 2 |
| Body Mass Index | 26.4 (26.2–26.7) | 26.3 (26.0–26.6) | 27.2 (26.2–28.3) | 27.5 (26.4–28.6) | F = 4.0, p = 0.019 No sig pw contrasts |
| # of Comorbidities | 1.6 (1.6–1.6) | 1.5 (1.5–1.6) | 1.9 (1.7, 2.1) | 1.9 (1.7, 2.1) | F = 13.2, p < 0.001 0 < 1 and 2 |
| n (%) | n (%) | n (%) | n (%) | ||
| Any comorbidity | 1304 (80.9) | 1091 (80.3) | 108 (91.5) | 105 (77.8) | X2 = 9.8, p= 0.008 1 > 0 and 2 |
| Hypertension | 476 (36.1) | 389 (35.3) | 52 (47.7) | 35 (33.0) | X2 = 7.1, p = 0.028 1 > 0 |
| Smoker | 156 (11.8) | 130 (11.8) | 10 (9.2) | 16 (15.1) | X2 = 1.8, p = 0.402 |
| Affective disorder | 94 (7.1) | 70 (6.3) | 8 (7.3) | 16 (15.1) | X2 = 11.2, p = 0.004 2 > 0 |
| Asthma | 61 (4.6) | 44 (4.0) | 8 (7.3) | 9 (8.5) | X2 = 6.4, p = 0.040 No sig pw contrasts |
| Coronary disease | 56 (4.2) | 42 (3.8) | 8 (7.3) | 6 (5.7) | X2 = 3.6, p = 0.165 |
| Surgery Mastectomy Breast Conserving | 809 (42.8) 1080 (57.2) | 679 (42.4) 921 (57.6) | 54 (42.2) 74 (57.8) | 76 (47.2) 85 (52.8) | X2 = 1.4, p = 0.501 |
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||
| Surgery duration (min) | 97.6 (94.9–100.3) | 97.6 (94.7–100.5) | 94.6 (86.6–102.55) | 103.3 (93.7–113.0) | X2 = 0.9, p = 0.395 |
| Pain Outcome | Total Sample N = 1889 | No Chronic Pain Group 0 n = 1600 (84.7%) | Pain Elsewhere Group 1 n = 128 (6.8%) | Pain in Breast Group 2 n = 161 (8.5%) | Statistics Pairwise Post Hoc Contrasts |
|---|---|---|---|---|---|
| M (95% CI) | M (95% CI) | M (95% CI) | M (95% CI) | ||
| Pain Intensity + Interference Composite Score | 2.4 (2.3–2.5) | 2.3 (2.2–2.4) | 2.3 (2.0–2.5) | 3.3 (2.9–3.6) | F = 20.8, p < 0.001 2 > 0 and 1 |
| Worst Pain Intensity | 4.0 (3.8–4.1) | 3.9 (3.7–4.0) | 4.2 (3.7–4.6) | 4.9 (4.4–5.4) | F = 11.9, p < 0.001 2 > 0 |
| Least Pain Intensity | 1.4 (1.3–1.5) | 1.3 (1.2–.4) | 1.2 (1.0–1.5) | 2.1 (1.8–2.5) | F = 20.0, p < 0.001 2 > 0 and 1 |
| % Time in Severe Pain since Surgery | 18.1 (17.2–19.0) | 17.4 (16.5–18.4) | 16.8 (13.6–9.9) | 26.2 (23.4–30.1) | F = 14.5, p < 0.001 2 > 0 and 1 |
| Pain Interference with Activities in Bed Pain Interference with Breathing Deeply/Coughing Pain Interference with Sleeping Pain Interference with Activities Out of Bed | 3.3 (2.2–3.4) 1.4 (1.3–1.6) 1.9 (1.8–2.0) 2.1 (2.0–2.2) | 3.2 (3.1–3.4) 1.4 (1.3–1.5) 1.8 (1.7–2.0) 2.0 (1.9–2.1) | 3.4 (2.9–3.9) 1.3 (0.9–1.7) 1.9 (1.5–2.3) 2.2 (1.7–2.7) | 4.6 (4.1–5.0) 2.3 (2.2–3.2) 2.7 (2.2–3.2) 3.3 (2.8–3.7) | F = 16.6, p < 0.001 2 > 0 and 1 F = 10.6, p < 0.001 2 > 0 and 1 F = 7.6, p = 0.001 2 > 0 and 1 F =20.4, p < 0.001 2 > 0 and 1 |
| Pain-Related Anxiety | 1.7 (1.6–1.8) | 1.6 (1.5–1.7) | 1.6 (1.2–2.1) | 2.9 (1.8–2.8) | F = 22.1, p < 0.001 2 > 0 and 1 |
| Pain-Related Helplessness | 1.4 (1.3–1.5) | 1.3 (1.2–1.4) | 1.5 (1.1–2.0) | 2.3 (1.8–2.8) | F = 12.4, p < 0.001 2 > 0 and 1 |
| Pain Relief (%) | 73.9 (72.7–75.2) | 74.0 (72.6–75.4) | 74.7 (70.0–79.5) | 72.6 (68.3–76.9) | F = 0.2, p = 0.784 |
| n (%) | n (%) | n (%) | n (%) | ||
| Would have liked more pain treatment than received | 154 (8.3%) | 127 (8.1%) | 7 (5.5%) | 20 (12.6%) | X2 = 5.2, p = 0.073 |
| Opioids | Total Sample N = 1889 | No Chronic Pain Group 0 n = 1600 (84.7%) | Pain Elsewhere Group 1 n = 128 (6.8%) | Pain in Breast Group 2 n = 161 (8.5%) | Statistics Pairwise Post Hoc Contrasts |
|---|---|---|---|---|---|
| Before Admission MED n (%) Mean (95% CI) | 32 (1.7) 0.6 (0.2–1.0) | 12 (0.8) 0.2 (0.03–0.3) | 13 (10.4) 4.5 (−1.2–10.2) | 7 (4.6) 1.6 (−0.1–3.4) | F = 13.8, p < 0.001 1 > 0 and 2 |
| Intraoperative MED n (%) Mean (95% CI) | 559 (29.6) 2.2 (1.6–2.7) | 478 (29.9) 2.2 (1.6–2.8) | 39 (30.5) 2.3 (1.6–2.9) | 42 (26.1) 1.7 (1.2–2.3) | F = 0.1, p = 0.895 |
| Postoperative MED n (%) Mean (95% CI) | 816 (43.2) 4.9 (4.3–5.4) | 679 (42.5) 4.5 (4.0–5.0) | 59 (46.1) 6.1 (2.0–10.1) | 78 (48.4) 7.2 (5.1–9.3) | F = 4.7, p = 0.009 2 > 0 |
| Unadjusted Model (n = 1884)/F = 20.8, p < 0.001 (R2 = 0.022) | |||||||
|---|---|---|---|---|---|---|---|
| B | Robust SE | Std. B | t | p | 95% Confidence Interval | ||
| Preexisting Chronic Pain | |||||||
| No Chronic Pain | (ref) | - | - | - | - | - | - |
| Pain Elsewhere | 0.02 | 0.15 | 0.002 | 0.11 | 0.914 | −0.28 | 0.32 |
| Pain in Breast | 0.96 | 0.18 | 0.15 | 5.36 | <0.001 | 0.61 | 1.31 |
| Adjusted Model (n = 1285)/F=19.6, p < 0.001 (R2 = 0.121) | |||||||
| B | Robust SE | Std. B | t | p | 95% Confidence Interval | ||
| Preexisting Chronic Pain | |||||||
| No Chronic Pain | (ref) | - | - | - | - | - | - |
| Pain Elsewhere | 0.08 | 0.16 | 0.01 | 0.51 | 0.611 | −0.23 | 0.39 |
| Pain in Breast | 1.00 | 0.22 | 0.16 | 4.62 | <0.001 | 0.58 | 1.44 |
| Age (years) | −0.03 | 0.004 | −0.20 | −6.59 | <0.001 | −0.04 | −0.02 |
| Number of Comorbidities | 0.09 | 0.06 | 0.05 | 1.56 | 0.118 | −0.02 | 0.21 |
| Region (Europe) | 0.20 | 0.15 | 0.07 | 1.32 | 0.198 | −0.10 | 0.50 |
| Surgery Type (mastectomy) | 0.23 | 0.10 | 0.07 | 2.20 | 0.028 | 0.02 | 0.43 |
| Surgery Duration (min) | 0.002 | 0.001 | −0.11 | 2.32 | 0.021 | 0.000 | 0.004 |
| Intraop Regional Anesthesia (yes) | −0.82 | 0.18 | 0.04 | −4.67 | <0.001 | −1.17 | −0.48 |
| Postop Opioid MED (mg) | 0.02 | 0.009 | 0.15 | 2.32 | 0.02 | 0.03 | 0.04 |
| Unadjusted Model (n = 1889)/F = 4.702, p = 0.009 (R2 = 0.005) | |||||||
|---|---|---|---|---|---|---|---|
| B | Robust SE | Std. B | t | p | 95% Confidence Interval | ||
| Preexisting Chronic Pain | |||||||
| No Chronic Pain | (ref) | - | - | - | - | - | - |
| Pain Elsewhere | 1.55 | 2.06 | 0.03 | 0.75 | 0.452 | −2.49 | 5.58 |
| Pain in Breast | 2.67 | 1.10 | 0.07 | 2.44 | 0.015 | 0.52 | 4.82 |
| Adjusted Model (n = 1564)/F = 8.33, p < 0.001 (R2 = 0.026) | |||||||
| B | Robust SE | Std. B | t | p | 95% Confidence Interval | ||
| Preexisting Chronic Pain | |||||||
| No Chronic Pain | (ref) | - | - | - | - | - | - |
| Pain Elsewhere | 2.09 | 2.31 | 0.05 | 0.90 | 0.366 | −2.44 | 6.63 |
| Pain in Breast | 2.56 | 1.22 | 0.06 | 2.11 | 0.035 | 0.18 | 4.95 |
| Age (years) | −0.04 | 0.02 | −0.05 | −2.48 | 0.013 | −0.08 | −0.009 |
| Surgery Duration (min) | 0.03 | 0.006 | 0.12 | 4.21 | <0.001 | 0.01 | 0.04 |
| Intraop Regional Anesthesia (yes) | 0.08 | 0.03 | −0.05 | 2.57 | 0.010 | 0.02 | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raza, M.M.; Zaslansky, R.; Gordon, D.B.; Wildisen, J.M.; Komann, M.; Stamer, U.M.; Langford, D.J. Chronic Breast Pain Prior to Breast Cancer Surgery Is Associated with Worse Acute Postoperative Pain Outcomes. J. Clin. Med. 2021, 10, 1887. https://doi.org/10.3390/jcm10091887
Raza MM, Zaslansky R, Gordon DB, Wildisen JM, Komann M, Stamer UM, Langford DJ. Chronic Breast Pain Prior to Breast Cancer Surgery Is Associated with Worse Acute Postoperative Pain Outcomes. Journal of Clinical Medicine. 2021; 10(9):1887. https://doi.org/10.3390/jcm10091887
Chicago/Turabian StyleRaza, Marium M., Ruth Zaslansky, Debra B. Gordon, Jeanne M. Wildisen, Marcus Komann, Ulrike M. Stamer, and Dale J. Langford. 2021. "Chronic Breast Pain Prior to Breast Cancer Surgery Is Associated with Worse Acute Postoperative Pain Outcomes" Journal of Clinical Medicine 10, no. 9: 1887. https://doi.org/10.3390/jcm10091887
APA StyleRaza, M. M., Zaslansky, R., Gordon, D. B., Wildisen, J. M., Komann, M., Stamer, U. M., & Langford, D. J. (2021). Chronic Breast Pain Prior to Breast Cancer Surgery Is Associated with Worse Acute Postoperative Pain Outcomes. Journal of Clinical Medicine, 10(9), 1887. https://doi.org/10.3390/jcm10091887






