Amyloid Goiter in Familial Mediterranean Fever: Description of 42 Cases from a French Cohort and from Literature Review
Abstract
:1. Introduction
2. Materials and Methods
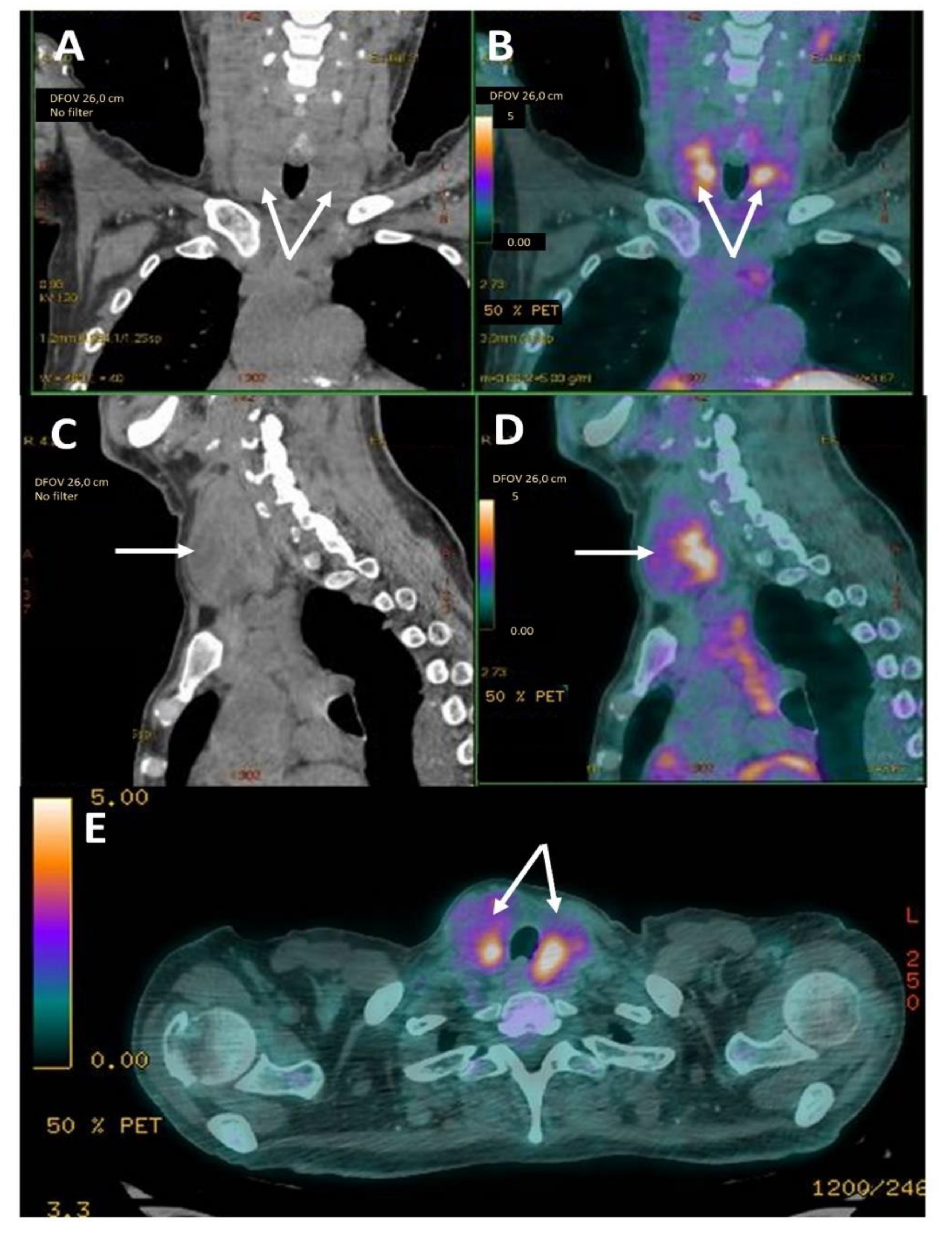
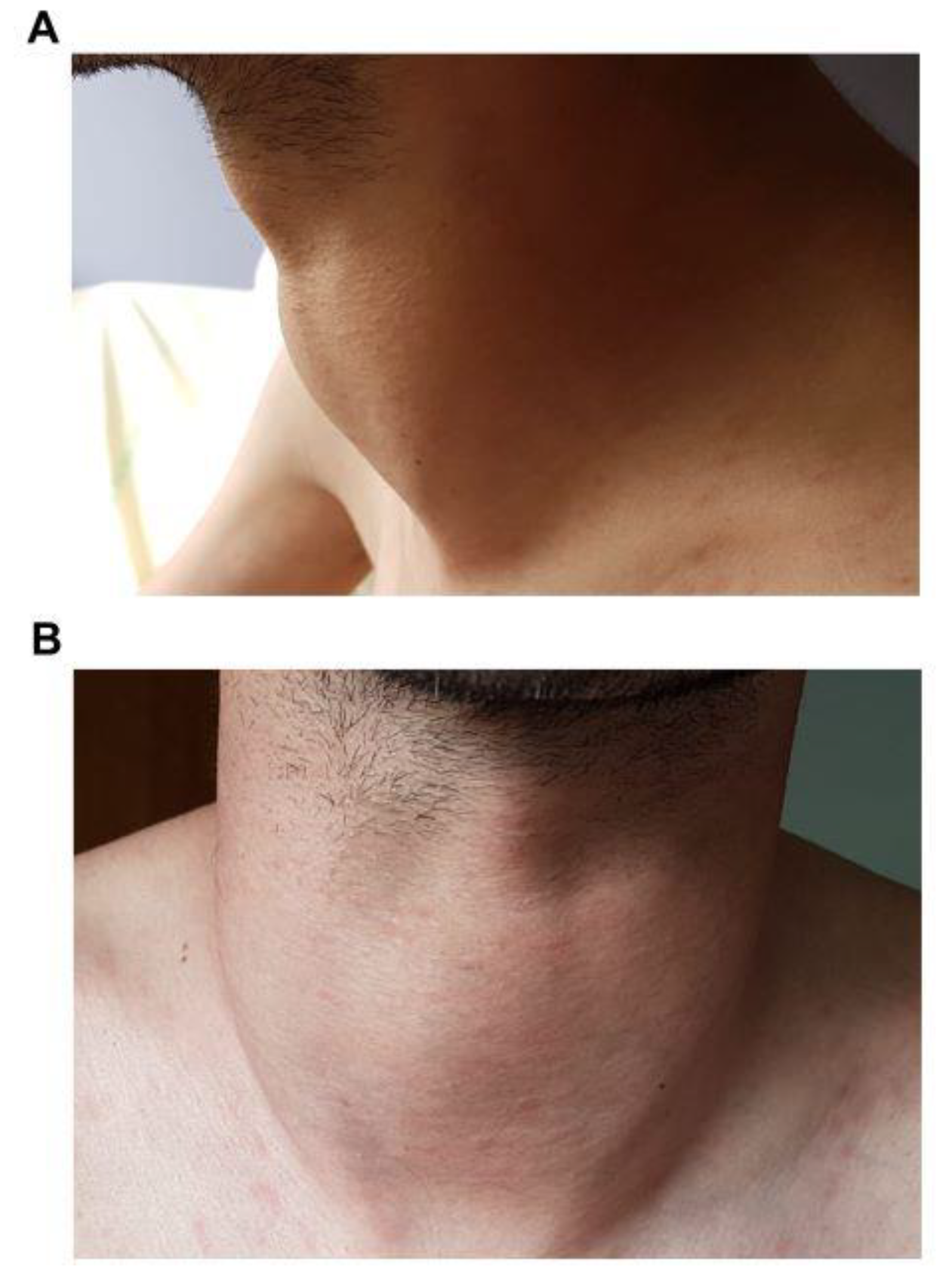
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sohar, E.; Gafni, J.; Pras, M.; Heller, H. Familial Mediterranean fever. A survey of 470 cases and review of the literature. Am. J. Med. 1967, 43, 227–253. [Google Scholar] [CrossRef]
- Özer, F.L.; Kaplaman, E.; Zileli, S. Familial mediterranean fever in Turkey. Am. J. Med. 1971, 50, 336–339. [Google Scholar] [CrossRef]
- von Rokitansky, C. Lehrbuch der Pathologischen Anatomie: Allgemeine Pathologische Anatomie und Anomalien des Blutes; Braumüller: Vienna, Austria, 1855. [Google Scholar]
- Dahlin, D.C. Secondary Amyloidosis. Ann. Intern. Med. 1949, 31, 105. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, B.H.; Akman, B.; Özdemir, F.N. Amyloid goiter in familial mediterranean fever (fmf): A clinicopathologic study of 10 cases. Ren. Fail. 2001, 23, 659–667. [Google Scholar] [CrossRef]
- Altıparmak, M.R.; Pamuk, Ö. N.; Pamuk, G.E.; Apaydın, S.; Ataman, R.; Serdengeçti, K. Amyloid Goitre in Familial Mediterranean Fever: Report on Three Patients and Review of the Literature. Clin. Rheumatol. 2002, 21, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Sbaï, A.; Wechsler, B.; Leenhardt, L.; Beaufils, H.; Hoang, C.; Ménégaux, F.; Piette, J. Amyloid Goiter as the Initial Manifestation of Systemic Amyloidosis due to Familial Mediterranean Fever with Homozygous MEFV Mutation. Thyroid 2001, 11, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Duman, N.; Ateş, K.; Ekinci, C.; Erbay, B.; Karatan, O.; Sak, S.D.; Ertürk, Ş.; Erdoĝan, G.; Ertuĝ, E. Amyloid deposition in the thyroid gland in patients with amyloidosis: An incidence study with fine-needle aspiration biopsy of the thyroid. Nephrol. Dial. Transplant. 1995, 10, 1473. [Google Scholar] [CrossRef]
- Hamed, G.; Heffess, C.S.; Shmookler, B.M.; Wenig, B.M. Amyloid goiter. A clinicopathologic study of 14 cases and review of the literature. Am. J. Clin. Pathol. 1995, 104, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Gharib, H.; Goellner, J.R. Diagnosis of Amyloidosis by Fine-Needle Aspiration Biopsy of the Thyroid. N. Engl. J. Med. 1981, 305, 586. [Google Scholar] [CrossRef]
- Geramizadeh, B.; Akbarzadeh, E.; Nikeghbalian, S. Photoclinic. Amyloid goiter. Arch. Iran Med. 2012, 15, 389–390. [Google Scholar]
- Eren, R.; Sayar, H.; Çiralik, H.; Ezberci, F. Diffuse lipid infiltration and squamous metaplasia accompanying amyloid goiter: Case report. Turk Patoloji Derg. 2013, 29, 146–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emeksiz, H.; Bakkaloglu, S.; Çamurdan, O.; Boyraz, M.; Söylemezoglu, O.; Hasanoğlu, E.; Buyan, N. Acute adrenal crisis mimicking familial Mediterranean fever attack in a renal transplant FMF patient with amyloid goiter. Rheumatol. Int. 2010, 30, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Cigerli, O.; Unal, A.D.; Parildar, H.; Demiralay, E.; Tarcin, O. Amyloid Goiter Due to Familial Mediterranean Fever in a Patient with Byler Syndrome: A Case Report. Balk. Med. J. 2014, 31, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Bakan, S.; Kandemirli, S.G.; Akbas, S.; Cingoz, M.; Guzelbey, B.O.; Kantarci, F.; Akman, C. Amyloid Goiter: A Diagnosis to Consider in Diffuse Fatty Infiltration of the Thyroid. J. Ultrasound Med. 2017, 36, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Danovitch, G.M.; Le Roith, D.; Sobel, R.; Sikulerd, E.; Straus, R. AMYLOID GOITRE IN FAMILIAL MEDITERRANEAN FEVER. Clin. Endocrinol. 1979, 11, 595–601. [Google Scholar] [CrossRef]
- Turhan İyidir, Ö.; Altay, M.; Konca Degertekin, C.; Altınova, A.; Karakoç, A.; Ayvaz, G.; Arslan, M.; Öneç, K.; Arınsoy, T.; Cesur, N.; et al. Diffuse amyloid deposition in thyroid gland: A cause for concern in familial Mediterranean fever. Amyloid 2012, 19, 161–162. [Google Scholar] [CrossRef]
- Ori, Y.; Halpern, M.; Sadov, R.; Feinmesser, R.; Ramadan, R.; Korzets, A. Familial Mediterranean fever, an amyloid thyroid goiter and an enlarged parathyroid gland. Isr. Med. Assoc. J. IMAJ 2012, 14, 232–233. [Google Scholar]
- Mache, C.; Schwingshandl, J.; Ring, E.; Pfleger, A.; Borkenstein, M. Amyloid Goiter in a Child with Familial Mediterranean Fever. J. Pediatr. Endocrinol. Metab. 1994, 7, 371–372. [Google Scholar] [CrossRef]
- Yildiz, L.; Kefeli, M.; Kose, B.; Baris, S. Amyloid goiter: Two cases and a review of the literature. Ann. Saudi Med. 2009, 29, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Kavukcu, S.; Türkmen, M.; Eroğlu, Y.; Canda, T.; Yörükoğlu, K.; Iğci, E.; Büyükgebiz, A. Renal, gastric and thyroidal amyloidosis due to familial Mediterranean fever. Pediatr. Nephrol. 1997, 11, 210–212. [Google Scholar] [CrossRef]
- Law, J.H.; Dean, D.S.; Scheithauer, B.; Earnest, F.; Sebo, T.J.; Fatourechi, V. Symptomatic Amyloid Goiters: Report of Five Cases. Thyroid 2013, 23, 1490–1495. [Google Scholar] [CrossRef]
- Cotoi, L.; Borcan, F.; Sporea, I.; Amzar, D.; Schiller, O.; Schiller, A.; Dehelean, C.A.; Pop, G.N.; Borlea, A.; Stoian, D. Thyroid Pathology in End-Stage Renal Disease Patients on Hemodialysis. Diagnostics 2020, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Jusufovic, S.; Hodzic, E. Functional Thyroid Disorders are More Common in Patients on Chronic HemodIalysis Compared with the General Population. Mater. Socio Med. 2011, 23, 206–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaptein, E.M. Thyroid Hormone Metabolism and Thyroid Diseases in Chronic Renal Failure. Endocr. Rev. 1996, 17, 19. [Google Scholar] [CrossRef]
- Nelson, S.R.; Tennent, G.A.; Sethi, D.; Gower, P.E.; Ballardie, F.W.; Amatayakul-Chantler, S.; Pepys, M.B. Serum amyloid P component in chronic renal failure and dialysis. Clin. Chim. Acta 1991, 200, 191–199. [Google Scholar] [CrossRef]
- Cobo, G.; Lindholm, B.; Stenvinkel, P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol. Dial. Transplant. 2018, 33, iii35–iii40. [Google Scholar] [CrossRef] [Green Version]
- Kimura, H.; Yamashita, S.; Ashizawa, K.; Yokoyama, N.; Nagataki, S. Thyroid dysfunction in patients with amyloid goitre. Clin. Endocrinol. 1997, 46, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, P.; Díez, J.J. Thyroid dysfunction and kidney disease. Eur. J. Endocrinol. 2009, 160, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Aksu, A.O.; Ozmen, M.N.; Oguz, K.K.; Akinci, D.; Yasavun, U.; Firat, P. Diffuse fatty infiltration of the thyroid gland in amyloidosis: Sonographic, computed tomographic, and magnetic resonance imaging findings. J. Ultrasound Med. 2010, 29, 1251–1255. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, B.H.; Uyar, P.; Özdemir, F.N. Diagnosing amyloid goitre with thyroid aspiration biopsy. Cytopathology 2006, 17, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Coca-Pelaz, A.; Vivanco-Allende, B.; Álvarez-Marcos, C.; Suarez-Nieto, C. Multifocal papillary thyroid carcinoma associated with primary amyloid goiter. Auris Nasus Larynx 2012, 39, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, M.L.; Russell, W.O.; Albores-Saavedra, J.; Lampertico, P.; White, E.C.; Clark, R.L. Thyroid carcinoma—Biologic behavior and mortality:Postmortem findings in 42 cases, including 27 in which the disease was fatal. Cancer 1966, 19, 1039–1052. [Google Scholar] [CrossRef]




| Patients with FMF and Amyloid Goiter | |
|---|---|
| Males (n = 42), n (%) | 24 (57) |
| Ethnicity (n = 42) | |
| Turkey (n) | 23 |
| Israel (n) | 5 |
| Iran (n) | 1 |
| Georgia (n) | 1 |
| Armenia (n) | 1 |
| Unknow (n) | 11 |
| Disease characteristics | |
| Age at diagnosis of FMF (n = 28), median (IQR) | 10 (8.75–24.75) |
| Age at amyloidosis diagnosis (n = 26), median (IQR) | 23.5 (15.25–36.75) |
| Renal failure (n = 35), n (%) | 34 (97.10) |
| Hemodialysis (n = 31), n (%) | 28 (90) |
| Kidney transplant (n = 32), n (%) | 9 (28) |
| Goiter characteristics | |
| Age at diagnosis of goiter (n = 34), median (IQR) | 30 (23–45) |
| Symptoms (n = 29) | |
| Asymptomatic, n (%) | 8 (27) |
| Dyspnea, n (%) | 17 (58) |
| Dysphagia, n (%) | 13 (44.8) |
| Dyspnea and dysphagia, n (%) | 9 (31) |
| Pain, n (%) | 2 (6.8) |
| Unknow, n | 13 |
| Thyroid function (n = 37) | |
| Euthyroidism, n (%) | 28 (75.6) |
| Hypothyroidism, n (%) | 2 (5.4) |
| Subclinical hypothyroidism, n (%) | 4 (10.8) |
| Hyperthyroidism, n (%) | 2 (5.4) |
| Dysthyroidism with no precision, n (%) | 1 (2.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergneault, H.; Terré, A.; Buob, D.; Buffet, C.; Dumont, A.; Ardois, S.; Savey, L.; Pardon, A.; Michel, P.-A.; Boffa, J.-J.; et al. Amyloid Goiter in Familial Mediterranean Fever: Description of 42 Cases from a French Cohort and from Literature Review. J. Clin. Med. 2021, 10, 1983. https://doi.org/10.3390/jcm10091983
Vergneault H, Terré A, Buob D, Buffet C, Dumont A, Ardois S, Savey L, Pardon A, Michel P-A, Boffa J-J, et al. Amyloid Goiter in Familial Mediterranean Fever: Description of 42 Cases from a French Cohort and from Literature Review. Journal of Clinical Medicine. 2021; 10(9):1983. https://doi.org/10.3390/jcm10091983
Chicago/Turabian StyleVergneault, Hélène, Alexandre Terré, David Buob, Camille Buffet, Anael Dumont, Samuel Ardois, Léa Savey, Agathe Pardon, Pierre-Antoine Michel, Jean-Jacques Boffa, and et al. 2021. "Amyloid Goiter in Familial Mediterranean Fever: Description of 42 Cases from a French Cohort and from Literature Review" Journal of Clinical Medicine 10, no. 9: 1983. https://doi.org/10.3390/jcm10091983
APA StyleVergneault, H., Terré, A., Buob, D., Buffet, C., Dumont, A., Ardois, S., Savey, L., Pardon, A., Michel, P.-A., Boffa, J.-J., Grateau, G., & Georgin-Lavialle, S. (2021). Amyloid Goiter in Familial Mediterranean Fever: Description of 42 Cases from a French Cohort and from Literature Review. Journal of Clinical Medicine, 10(9), 1983. https://doi.org/10.3390/jcm10091983







