Long-Term Swallowing Outcome and Dysphagia in Advanced Staged Head and Neck Squamous Cell Carcinomas after Radiotherapy
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Cohort
2.2. Clinical Data
2.3. Dysphagia and Swallowing
2.4. Statistical Methods
2.5. Ethics Approval
3. Results
3.1. Study Cohort
3.2. Therapy
3.3. Dysphagia
3.4. Risk Factors for Dysphagia
3.5. Long-Term Dysphagia and Penetration-Aspiration Scale
3.6. Radiation Related Side-Effects
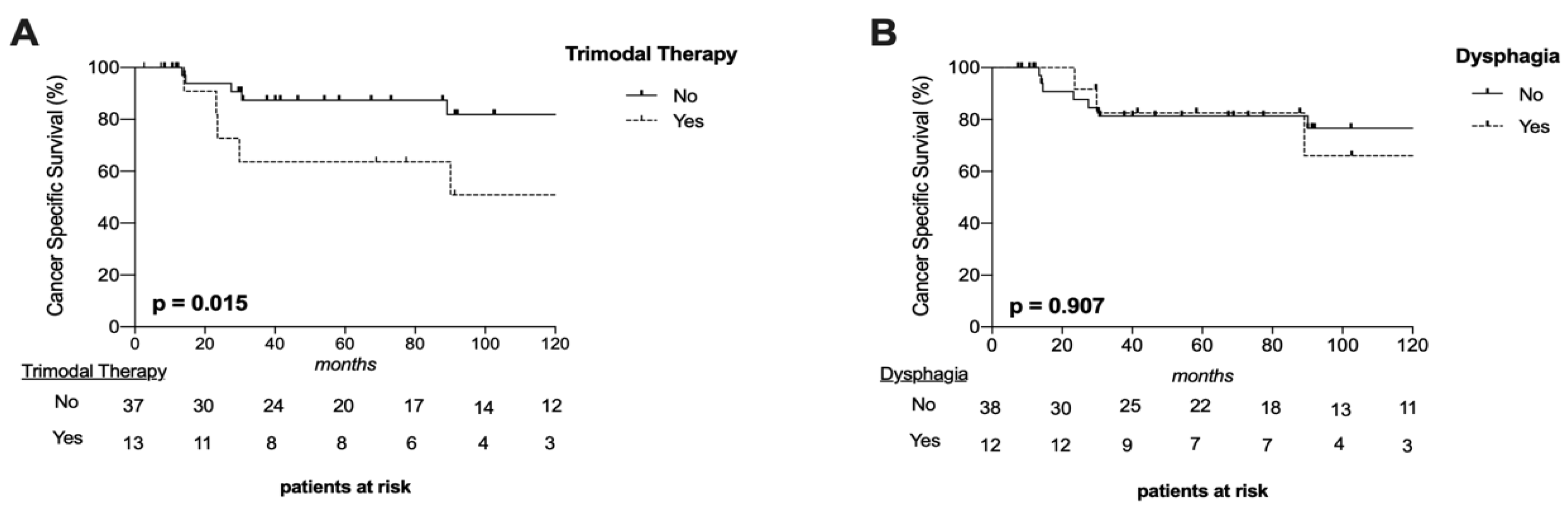
3.7. Oncological Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRT | Chemoradiotherapy |
| CIs | Confidence intervals |
| CSS | Cancer-specific survival |
| ECE | Extracapsular extension |
| FEES | Fiberendoscopic (flexible) examination of swallowing |
| FU | Follow-up |
| HNC | Head and neck cancer |
| HNSCC | Head and neck squamous cell carcinoma |
| OPSCC | Oropharyngeal squamous cell carcinoma |
| HPXSCC | Hypopharynx squamous cell carcinoma |
| PAS | Penetration-Aspiration Scale |
| PORT | Post-operative radiotherapy |
| RT | Radiotherapy |
| SCC | Squamous cell carcinoma |
| VFS | Videofluoroscopy |
References
- Nguyen, N.P.; Frank, C.; Moltz, C.C.; Vos, P.; Smith, H.J.; Karlsson, U.; Dutta, S.; Midyett, A.; Barloon, J.; Sallah, S. Impact of dysphagia on quality of life after treatment of head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Rathod, S.; Gupta, T.; Ghosh-Laskar, S.; Murthy, V.; Budrukkar, A.; Agarwal, J. Quality-of-life (QOL) outcomes in patients with head and neck squamous cell carcinoma (HNSCC) treated with intensity-modulated radiation therapy (IMRT) compared to three-dimensional conformal radiotherapy (3D-CRT): Evidence from a prospective randomized study. Oral. Oncol. 2013, 49, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.S.; Massa, S.T.; Varvares, M.A. Improved overall survival and mortality in head and neck cancer with adjuvant concurrent chemoradiotherapy in national databases. Head Neck 2016, 38, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Rathod, S.; Livergant, J.; Klein, J.; Witterick, I.; Ringash, J. A systematic review of quality of life in head and neck cancer treated with surgery with or without adjuvant treatment. Oral. Oncol. 2015, 51, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Rosenbek, J.C.; Robbins, J.A.; Roecker, E.B.; Coyle, J.L.; Wood, J.L. A penetration-aspiration scale. Dysphagia 1996, 11, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Colodny, N. Interjudge and intrajudge reliabilities in fiberoptic endoscopic evaluation of swallowing (fees) using the penetration-aspiration scale: A replication study. Dysphagia 2002, 17, 308–315. [Google Scholar] [CrossRef]
- Scharitzer, M.; Roesner, I.; Pokieser, P.; Weber, M.; Denk-Linnert, D.M. Simultaneous Radiological and Fiberendoscopic Evaluation of Swallowing (“SIRFES”) in Patients After Surgery of Oropharyngeal/Laryngeal Cancer and Postoperative Dysphagia. Dysphagia 2019, 34, 852–861. [Google Scholar] [CrossRef]
- Givens, D.J.; Karnell, L.H.; Gupta, A.K.; Clamon, G.H.; Pagedar, N.A.; Chang, K.E.; Van Daele, D.J.; Funk, G.F. Adverse Events Associated with Concurrent Chemoradiation Therapy in Patients With Head and Neck Cancer. Arch. Otolaryngol. Head Neck Surg. 2009, 135, 1209–1217. [Google Scholar] [CrossRef] [Green Version]
- Machtay, M.; Moughan, J.; Trotti, A.; Garden, A.S.; Weber, R.S.; Cooper, J.S.; Forastiere, A.; Ang, K.K. Factors Associated with Severe Late Toxicity After Concurrent Chemoradiation for Locally Advanced Head and Neck Cancer: An RTOG Analysis. J. Clin. Oncol. 2008, 26, 3582–3589. [Google Scholar] [CrossRef]
- Li, P.; Constantinescu, G.C.; Nguyen, N.A.; Jeffery, C.C. Trends in Reporting of Swallowing Outcomes in Oropharyngeal Cancer Studies: A Systematic Review. Dysphagia 2020, 35, 18–23. [Google Scholar] [CrossRef]
- Ihara, Y.; Crary, M.A.; Madhavan, A.; Gregorio, D.C.; Im, I.; Ross, S.E.; Carnaby, G.D. Dysphagia and Oral Morbidities in Chemoradiation-Treated Head and Neck Cancer Patients. Dysphagia 2018, 33, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Erovic, B.M.; Pelzmann, M.; Grasl, M.; Pammer, J.; Kornek, G.; Brannath, W.; Selzer, E.; Thurnher, D. Mcl-1, vascular endothelial growth factor-R2, and 14-3-3sigma expression might predict primary response against radiotherapy and chemotherapy in patients with locally advanced squamous cell carcinomas of the head and neck. Clin. Cancer Res. 2005, 11, 8632–8636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enzenhofer, E.; Parzefall, T.; Haymerle, G.; Schneider, S.; Kadletz, L.; Heiduschka, G.; Pammer, J.; Oberndorfer, F.; Wrba, F.; Loader, B.; et al. Impact of Sonic Hedgehog Pathway Expression on Outcome in HPV Negative Head and Neck Carcinoma Patients after Surgery and Adjuvant Radiotherapy. PLoS ONE 2016, 11, e0167665. [Google Scholar] [CrossRef] [PubMed]
- Vyskocil, E.; Pammer, J.; Altorjai, G.; Grasl, M.C.; Parzefall, T.; Haymerle, G.; Janik, S.; Perisanidis, C.; Erovic, B.M. Dysregulation of ss-catenin, WISP1 and TCF21 predicts disease-specific survival and primary response against radio(chemo)therapy in patients with locally advanced squamous cell carcinomas of the head and neck. Clin. Otolaryngol. 2019, 44, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Danan, D.; Shonka, D.C., Jr.; Selman, Y.; Chow, Z.; Smolkin, M.E.; Jameson, M.J. Prognostic value of albumin in patients with head and neck cancer. Laryngoscope 2016, 126, 1567–1571. [Google Scholar] [CrossRef]
- Sroussi, H.Y.; Epstein, J.B.; Bensadoun, R.J.; Saunders, D.P.; Lalla, R.V.; Migliorati, C.A.; Heaivilin, N.; Zumsteg, Z.S. Common oral complications of head and neck cancer radiation therapy: Mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017, 6, 2918–2931. [Google Scholar] [CrossRef]
- Hedstrom, J.; Tuomi, L.; Finizia, C.; Olsson, C. Correlations Between Patient-Reported Dysphagia Screening and Penetration-Aspiration Scores in Head and Neck Cancer Patients Post-oncological Treatment. Dysphagia 2018, 33, 206–215. [Google Scholar] [CrossRef] [Green Version]
- Jensen, K.; Lambertsen, K.; Torkov, P.; Dahl, M.; Jensen, A.B.; Grau, C. Patient assessed symptoms are poor predictors of objective findings. Results from a cross sectional study in patients treated with radiotherapy for pharyngeal cancer. Acta Oncol. 2007, 46, 1159–1168. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.S.; Jen, Y.M.; Lin, J.C. Radiation-related cranial nerve palsy in patients with nasopharyngeal carcinoma. Cancer 2002, 95, 404–409. [Google Scholar] [CrossRef]
- Dixon, L.; Ramasamy, S.; Cardale, K.; Dyker, K.; Garcez, K.; Lee, L.W.; McPartlin, A.; Murray, P.; Sen, M.; Slevin, N.; et al. Long term patient reported swallowing function following chemoradiotherapy for oropharyngeal carcinoma. Radiother. Oncol. 2018, 128, 452–458. [Google Scholar] [CrossRef]
- Morgan, H.E.; Sher, D.J. Adaptive radiotherapy for head and neck cancer. Cancers Head Neck 2020, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Chambers, M.S.; Garden, A.S.; Kies, M.S.; Martin, J.W. Radiation-induced xerostomia in patients with head and neck cancer: Pathogenesis, impact on quality of life, and management. Head Neck 2004, 26, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Dirix, P.; Nuyts, S.; Van den Bogaert, W. Radiation-induced xerostomia in patients with head and neck cancer: A literature review. Cancer 2006, 107, 2525–2534. [Google Scholar] [CrossRef]
- Kraaijenga, S.A.; van der Molen, L.; van den Brekel, M.W.; Hilgers, F.J. Current assessment and treatment strategies of dysphagia in head and neck cancer patients: A systematic review of the 2012/13 literature. Curr. Opin. Support Palliat. Care 2014, 8, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Denk, D.M.; Swoboda, H.; Schima, W.; Eibenberger, K. Prognostic factors for swallowing rehabilitation following head and neck cancer surgery. Acta Otolaryngol. 1997, 117, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Grasl, S.; Schmid, E.; Heiduschka, G.; Brunner, M.; Marijic, B.; Grasl, M.C.; Faisal, M.; Erovic, B.M.; Janik, S. A New Classification System to Predict Functional Outcome after Laryngectomy and Laryngopharyngectomy. Cancers 2021, 13, 1474. [Google Scholar] [CrossRef]
- Lahtinen, S.; Koivunen, P.; Ala-Kokko, T.; Kaarela, O.; Laurila, P.; Liisanantti, J.H. Swallowing-related quality of life after free flap surgery due to cancer of the head and neck. Eur. Arch. Otorhinolaryngol. 2019, 276, 821–826. [Google Scholar] [CrossRef] [Green Version]
- Harris, A.; Lyu, L.; Wasserman-Winko, T.; George, S.; Johnson, J.T.; Nilsen, M.L. Neck Disability and Swallowing Function in Posttreatment Head and Neck Cancer Patients. Otolaryngol. Head Neck Surg. 2020, 163, 763–770. [Google Scholar] [CrossRef]
- Krebbers, I.; Simon, S.R.; Pilz, W.; Kremer, B.; Winkens, B.; Baijens, L.W.J. Patients with Head-and-Neck Cancer: Dysphagia and Affective Symptoms. Folia Phoniatr. Logop. 2020, 73, 308–315. [Google Scholar] [CrossRef]


| VARIABLES | Total | Pre-Treatment Dysphagia | Follow-Up Dysphagia | ||
|---|---|---|---|---|---|
| n (%) | n (%) | p-Value | n (%) | p-Value | |
| Sex | 50 (100) | 24 (48) | 12 (24) | ||
| Female | 12 (24) | 4 (33) | 3 (25) | ||
| Male | 38 (76) | 20 (53) | 0.333 a | 9 (24) | 1.000 a |
| Age (median ± SD) | 73 ± 10.5 | ||||
| ≤73 years | 27 (54) | 14 (52) | 5 (19) | ||
| >73 years | 23 (46) | 10 (43) | 0.584 a | 7 (30) | 0.508 a |
| BMI (mean ± SD) | 25.2 ± 4.3 | ||||
| <25 | 23 (46) | 10 (43) | 7 (30) | ||
| ≥25 | 27 (54) | 14 (52) | 0.567 a | 5 (19) | 0.508 a |
| Tumor-Stage | |||||
| Stage III | 10 (20) | 8 (80) | 0 (0) | ||
| Stage IV | 40 (80) | 14 (35) | 0.035 a | 12 (30) | 0.092 a |
| T-Classification | |||||
| T1–T2 | 20 (40) | 6 (30) | 2 (20) | ||
| T3–T4a | 30 (60) | 18 (60) | 0.080 a | 10 (33) | 0.091 a |
| N-Classification | |||||
| N0 | 6 (12) | 3 (50) | 1 (17) | ||
| N1 | 9 (18) | 7 (78) | 1 (11) | ||
| N2 | 33 (66) | 13 (39) | 10 (30) | ||
| N3 | 2 (4) | 0 (0) | 0.655 a | 0 (0) | 1.000 a |
| Tumor Site | |||||
| Oral Cavity | 23 (46) | 7 (30) | 6 (26) | ||
| Oropharynx | 16 (32) | 9 (56) | 4 (25) | ||
| Hypopharynx | 8 (16) | 6 (75) | 2 (25) | ||
| Larynx | 3 (6) | 2 (67) | 0.124 a | 0 (0) | 0.798 a |
| Therapy | Total | Follow-Up Dysphagia |
|---|---|---|
| n (%) | n (%) | |
| Treatment | ||
| Surgery + PORT | 17 (34) | 6 (35) |
| CRT | 14 (28) | 4 (29) |
| RT | 6 (12) | 0 (0) |
| Surgery + CRT | 13 (26) | 2 (15) |
| Radiation dose | ||
| Total irradiation | 65.51 ± 7.3 Gy (n = 50, 100%) | - |
| Neck irradiation | 52.2 ± 15.7 Gy (n = 34, 68%) | - |
| Neck-Dissection | ||
| Yes | 28 (56) | 6 (21) |
| No | 22 (44) | 6 (27) |
| Tracheostomy | ||
| Yes | 17 (34) | 6 (35) |
| No | 33 (66) | 6 (18) |
| Free Flap | ||
| Yes | 9 (18) | 5 (56) |
| No | 41 (82) | 7 (17) |
| CLINICAL VARIABLES | UNIVARIATE ANALYSIS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-Treatment Dysphagia | Post-Treatment Dysphagia | Follow-Up Dysphagia | |||||||
| OR | p | 95% CI | OR | p | 95% CI | OR | p | 95% CI | |
| Sex (Female) | 0.47 | 0.282 | 0.12–1.85 | 0.94 | 0.961 | 0.09–10.0 | 1.07 | 0.926 | 0.24–4.84 |
| Age (≤73 y) | 1.3 | 0.648 | 0.42–4.01 | 3.90 | 0.254 | 0.38–40.37 | 0.52 | 0.329 | 0.14–1.94 |
| T3-T4a | 3.30 | 0.053 | 0.99–11.1 | 0.47 | 0.531 | 0.05–4.90 | 4.50 | 0.073 | 0.87–23.3 |
| N pos. | 0.56 | 0.541 | 0.05–3.66 | 10.5 | 0.037 | 1.15–100 | 1.67 | 0.657 | 1.76–15.9 |
| OPSCC + HPXSCC | 2.92 | 0.073 | 0.91–9.43 | * | 1.38 | 0.632 | 0.37–5.05 | ||
| T3-T4a AND OPSCC + HPXSCC | 9.26 | 0.009 | 1.75–47.6 | * | 4.42 | 0.037 | 1.10–17.9 | ||
| G-tube during irradiation (YES) | - | - | - | 0.94 | 0.961 | 0.09–10.0 | 3.59 | 0.228 | 0.45–28.6 |
| Free Flap (YES) | - | - | - | 0.63 | 0.706 | 0.06–6.90 | 6.10 | 0.022 | 1.29–28.6 |
| Tracheostomy (YES) | - | - | - | 0.48 | 0.489 | 0.06–3.77 | 2.46 | 0.186 | 0.65–9.26 |
| Neck-Dissection (YES) | - | - | - | 1.30 | 0.801 | 0.17–10.0 | 0.73 | 0.632 | 0.20–2.67 |
| Total | Follow-Up Dysphagia | |||
|---|---|---|---|---|
| VARIABLES | n (%) | OR | p | 95% CI |
| Radiation Side-Effects | ||||
| Soor | 8 (16) | 0.40 | 0.419 | 0.04–3.65 |
| Dysgeusia | 19 (38) | 9.9 | 0.036 | 1.16–84.47 |
| Erythema | 31 (62) | 1.30 | 0.767 | 0.33–5.10 |
| Xerostomia | 28 (56) | 5.77 | 0.019 | 1.33–25.05 |
| Mucositis | 36 (72) | 1.22 | 0.791 | 0.28–5.38 |
| PAS-Score | ||||
| Retention | 13 (26) | 2.68 | 0.164 | 0.67–10.75 |
| Penetration | 13 (26) | 4.42 | 0.037 | 1.10–17.86 |
| Aspiration | 12 (24) | 1.88 | 0.389 | 0.45–7.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yildiz, E.; Grasl, S.; Denk-Linnert, D.-M.; Altorjai, G.; Herrmann, H.; Grasl, M.C.; Erovic, B.M.; Janik, S. Long-Term Swallowing Outcome and Dysphagia in Advanced Staged Head and Neck Squamous Cell Carcinomas after Radiotherapy. J. Clin. Med. 2022, 11, 2688. https://doi.org/10.3390/jcm11102688
Yildiz E, Grasl S, Denk-Linnert D-M, Altorjai G, Herrmann H, Grasl MC, Erovic BM, Janik S. Long-Term Swallowing Outcome and Dysphagia in Advanced Staged Head and Neck Squamous Cell Carcinomas after Radiotherapy. Journal of Clinical Medicine. 2022; 11(10):2688. https://doi.org/10.3390/jcm11102688
Chicago/Turabian StyleYildiz, Erdem, Stefan Grasl, Doris-Maria Denk-Linnert, Gabriela Altorjai, Harald Herrmann, Matthaeus C. Grasl, Boban M. Erovic, and Stefan Janik. 2022. "Long-Term Swallowing Outcome and Dysphagia in Advanced Staged Head and Neck Squamous Cell Carcinomas after Radiotherapy" Journal of Clinical Medicine 11, no. 10: 2688. https://doi.org/10.3390/jcm11102688
APA StyleYildiz, E., Grasl, S., Denk-Linnert, D.-M., Altorjai, G., Herrmann, H., Grasl, M. C., Erovic, B. M., & Janik, S. (2022). Long-Term Swallowing Outcome and Dysphagia in Advanced Staged Head and Neck Squamous Cell Carcinomas after Radiotherapy. Journal of Clinical Medicine, 11(10), 2688. https://doi.org/10.3390/jcm11102688







