Factors Influencing the Outcome of Head and Neck Cancer of Unknown Primary (HNCUP)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Objectives
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Treatment Characteristics
3.3. Oncological Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grau, C.; Johansen, L.V.; Jakobsen, J.; Geertsen, P.; Andersen, E.; Jensen, B.B. Cervical lymph node metastases from unknown primary tumours. Results from a national survey by the Danish Society for Head and Neck Oncology. Radiother. Oncol. 2000, 55, 121–129. [Google Scholar] [CrossRef]
- Motz, K.; Qualliotine, J.R.; Rettig, E.; Richmon, J.D.; Eisele, D.W.; Fakhry, C. Changes in Unknown Primary Squamous Cell Carcinoma of the Head and Neck at Initial Presentation in the Era of Human Papillomavirus. JAMA Otolaryngol.-Head Neck Surg. 2016, 142, 223–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galloway, T.J.; Ridge, J.A. Management of Squamous Cancer Metastatic to Cervical Nodes with an Unknown Primary Site. J. Clin. Oncol. 2015, 33, 3328–3337. [Google Scholar] [CrossRef] [PubMed]
- Strojan, P.; Ferlito, A.; Medina, J.E.; Woolgar, J.A.; Rinaldo, A.; Robbins, K.T.; Fagan, J.J.; Mendenhall, W.M.; Paleri, V.; Silver, C.E.; et al. Contemporary management of lymph node metastases from an unknown primary to the neck: I. A review of diagnostic approaches. Head Neck 2013, 35, 123–132. [Google Scholar] [CrossRef]
- Jensen, D.H.; Hedback, N.; Specht, L.; Hogdall, E.; Andersen, E.; Therkildsen, M.H.; Friis-Hansen, L.; Norrild, B.; von Buchwald, C. Human papillomavirus in head and neck squamous cell carcinoma of unknown primary is a common event and a strong predictor of survival. PLoS ONE 2014, 9, e110456. [Google Scholar] [CrossRef]
- Keller, L.M.; Galloway, T.J.; Holdbrook, T.; Ruth, K.; Yang, D.; Dubyk, C.; Flieder, D.; Lango, M.N.; Mehra, R.; Burtness, B.; et al. p16 status, pathologic and clinical characteristics, biomolecular signature, and long-term outcomes in head and neck squamous cell carcinomas of unknown primary. Head Neck 2014, 36, 1677–1684. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Saito, Y.; Omura, G.; Ando, M.; Sakamoto, T.; Yamasoba, T.; Asakage, T. Clinical features of human papilloma virus-related head and neck squamous cell carcinoma of an unknown primary site. ORL 2014, 76, 137–146. [Google Scholar] [CrossRef]
- Axelsson, L.; Nyman, J.; Haugen-Cange, H.; Bove, M.; Johansson, L.; De Lara, S.; Kovacs, A.; Hammerlid, E. Prognostic factors for head and neck cancer of unknown primary including the impact of human papilloma virus infection. J. Otolaryngol.-Head Neck Surg. 2017, 46, 45. [Google Scholar] [CrossRef]
- Balk, M.; Rupp, R.; Mantsopoulos, K.; Allner, M.; Grundtner, P.; Mueller, S.K.; Traxdorf, M.; Eckstein, M.; Speer, S.; Semrau, S.; et al. Relevance of the time interval between surgery and adjuvant radio (chemo) therapy in HPV-negative and advanced head and neck carcinoma of unknown primary (CUP). BMC Cancer 2021, 21, 1236. [Google Scholar] [CrossRef]
- Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Anzai, Y.; Brizel, D.M.; Bruce, J.Y.; Busse, P.M.; Caudell, J.J.; Cmelak, A.J.; et al. Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2020, 18, 873–898. [Google Scholar] [CrossRef]
- Kirschner, M.J.; Fietkau, R.; Waldfahrer, F.; Iro, H.; Sauer, R. Therapy pf cervical lymph node metastases of unknown primary tumor. Strahlenther. Und Onkol. Organ. Der Dtsch. Rontgenges. [Et Al] 1997, 173, 362–368. [Google Scholar] [CrossRef] [PubMed]
- de Braud, F.; Heilbrun, L.K.; Ahmed, K.; Sakr, W.; Ensley, J.F.; Kish, J.A.; Tapazoglou, E.; al-Sarraf, M. Metastatic squamous cell carcinoma of an unknown primary localized to the neck. Advantages of an aggressive treatment. Cancer 1989, 64, 510–515. [Google Scholar] [CrossRef]
- Haussmann, J.; Tamaskovics, B.; Bolke, E.; Djiepmo-Njanang, F.J.; Kammers, K.; Corradini, S.; Hautmann, M.; Ghadjar, P.; Maas, K.; Schuler, P.J.; et al. Addition of chemotherapy to hyperfractionated radiotherapy in advanced head and neck cancer-a meta-analysis. Strahlenther. Und Onkol. Organ. Der Dtsch. Rontgenges. [Et Al] 2019, 195, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, M.M.; Temming, S.; Kocher, M.; Kunze, S.; Semrau, R. Low risk of contralateral lymph node recurrence in lateralized head and neck carcinoma after postoperative ipsilateral radiotherapy. Strahlenther. Und Onkol. Organ. Der Dtsch. Rontgenges. [Et Al] 2020, 196, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Balaker, A.E.; Abemayor, E.; Elashoff, D.; St John, M.A. Cancer of unknown primary: Does treatment modality make a difference? Laryngoscope 2012, 122, 1279–1282. [Google Scholar] [CrossRef] [PubMed]
- Maghami, E.; Ismaila, N.; Alvarez, A.; Chernock, R.; Duvvuri, U.; Geiger, J.; Gross, N.; Haughey, B.; Paul, D.; Rodriguez, C.; et al. Diagnosis and Management of Squamous Cell Carcinoma of Unknown Primary in the Head and Neck: ASCO Guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 2570–2596. [Google Scholar] [CrossRef] [PubMed]
- Robbins, K.T.; Medina, J.E.; Wolfe, G.T.; Levine, P.A.; Sessions, R.B.; Pruet, C.W. Standardizing neck dissection terminology. Official report of the Academy’s Committee for Head and Neck Surgery and Oncology. Arch. Otolaryngol.-Head Neck Surg. 1991, 117, 601–605. [Google Scholar] [CrossRef]
- Stein, J.P.; Cai, J.; Groshen, S.; Skinner, D.G. Risk factors for patients with pelvic lymph node metastases following radical cystectomy with en bloc pelvic lymphadenectomy: Concept of lymph node density. J. Urol. 2003, 170, 35–41. [Google Scholar] [CrossRef]
- Gil, Z.; Carlson, D.L.; Boyle, J.O.; Kraus, D.H.; Shah, J.P.; Shaha, A.R.; Singh, B.; Wong, R.J.; Patel, S.G. Lymph node density is a significant predictor of outcome in patients with oral cancer. Cancer 2009, 115, 5700–5710. [Google Scholar] [CrossRef]
- Brierley, J.D.G.M.; Wittekind, C. TNM Classification of Malignant Tumors, 8th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2016. [Google Scholar]
- Therneau, T.; Atkinson, B. Rpart: Recursive Partitioning and Regression Trees. R Package, Version 4.1.16. 2022. Available online: https://cran.r-project.org/package=rpart (accessed on 9 March 2022).
- Aslani, M.; Sultanem, K.; Voung, T.; Hier, M.; Niazi, T.; Shenouda, G. Metastatic carcinoma to the cervical nodes from an unknown head and neck primary site: Is there a need for neck dissection? Head Neck 2007, 29, 585–590. [Google Scholar] [CrossRef]
- Rodel, R.M.; Matthias, C.; Blomeyer, B.D.; Wolff, H.A.; Jung, K.; Christiansen, H. Impact of distant metastasis in patients with cervical lymph node metastases from cancer of an unknown primary site. Ann. Otol. Rhinol. Laryngol. 2009, 118, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Beldi, D.; Jereczek-Fossa, B.A.; D’Onofrio, A.; Gambaro, G.; Fiore, M.R.; Pia, F.; Chiesa, F.; Orecchia, R.; Krengli, M. Role of radiotherapy in the treatment of cervical lymph node metastases from an unknown primary site: Retrospective analysis of 113 patients. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.; Richards, G.M.; Harari, P.M.; Kirwan, J.M.; Morris, C.G.; Katakam, H.; Mendenhall, W.M. Head and neck squamous cell carcinoma from an unknown primary site. Am. J. Otolaryngol. 2011, 32, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Wang, S.; Wang, K.; Chen, C.; Zhao, J.; Guo, L. Squamous cell carcinoma of cervical lymph nodes from an unknown primary site: The impact of neck dissection. J. Cancer Res. Ther. 2015, 11 (Suppl. 2), C161–C167. [Google Scholar] [CrossRef] [PubMed]
- Amsbaugh, M.J.; Yusuf, M.; Gaskins, J.; Silverman, C.; Potts, K.; Bumpous, J.; Redman, R.; Perez, C.; Dunlap, N. Neck dissection for unknown cancer of the head and neck in the era of chemoradiation. Am. J. Otolaryngol. 2017, 38, 588–592. [Google Scholar] [CrossRef]
- Cooper, J.S.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Campbell, B.H.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2004, 350, 1937–1944. [Google Scholar] [CrossRef] [Green Version]
- Bernier, J.; Cooper, J.S.; Pajak, T.F.; van Glabbeke, M.; Bourhis, J.; Forastiere, A.; Ozsahin, E.M.; Jacobs, J.R.; Jassem, J.; Ang, K.K.; et al. Defining risk levels in locally advanced head and neck cancers: A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 2005, 27, 843–850. [Google Scholar] [CrossRef]
- Argiris, A.; Smith, S.M.; Stenson, K.; Mittal, B.B.; Pelzer, H.J.; Kies, M.S.; Haraf, D.J.; Vokes, E.E. Concurrent chemoradiotherapy for N2 or N3 squamous cell carcinoma of the head and neck from an occult primary. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol./ESMO 2003, 14, 1306–1311. [Google Scholar] [CrossRef]
- Sher, D.J.; Balboni, T.A.; Haddad, R.I.; Norris, C.M., Jr.; Posner, M.R.; Wirth, L.J.; Goguen, L.A.; Annino, D.; Tishler, R.B. Efficacy and toxicity of chemoradiotherapy using intensity-modulated radiotherapy for unknown primary of head and neck. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 1405–1411. [Google Scholar] [CrossRef]
- Eldeeb, H.; Hamed, R.H. Squamous cell carcinoma metastatic to cervical lymph nodes from unknown primary origin: The impact of chemoradiotherapy. Chin. J. Cancer 2012, 31, 484–490. [Google Scholar] [CrossRef] [Green Version]
- Kaizu, H.; Ogino, I.; Hata, M.; Oba, M.S.; Shiono, O.; Komatsu, M.; Inoue, T. Chemoradiation as a definitive treatment for cervical lymph node metastases from unknown primary cancer. Anticancer. Res. 2013, 33, 5187–5192. [Google Scholar] [PubMed]
- Mourad, W.F.; Hu, K.S.; Shasha, D.; Concert, C.; Ishihara, D.; Lin, W.; Shourbaji, R.A.; Ryniak, M.; Gamez, M.E.; Lukens, J.N.; et al. Initial experience with oropharynx-targeted radiation therapy for metastatic squamous cell carcinoma of unknown primary of the head and neck. Anticancer. Res. 2014, 34, 243–248. [Google Scholar]
- Dalianis, T. Human papillomavirus and oropharyngeal cancer, the epidemics, and significance of additional clinical biomarkers for prediction of response to therapy (Review). Int. J. Oncol. 2014, 44, 1799–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirghani, H.; Amen, F.; Blanchard, P.; Moreau, F.; Guigay, J.; Hartl, D.M.; Lacau St Guily, J. Treatment de-escalation in HPV-positive oropharyngeal carcinoma: Ongoing trials, critical issues and perspectives. Int. J. Cancer. J. Int. Du Cancer 2015, 136, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Sivars, L.; Tani, E.; Nasman, A.; Ramqvist, T.; Munck-Wikland, E.; Dalianis, T. Human Papillomavirus as a Diagnostic and Prognostic Tool in Cancer of Unknown Primary in the Head and Neck Region. Anticancer. Res. 2016, 36, 487–493. [Google Scholar] [PubMed]
- Cheraghlou, S.; Torabi, S.J.; Husain, Z.A.; Otremba, M.D.; Osborn, H.A.; Mehra, S.; Yarbrough, W.G.; Burtness, B.A.; Judson, B.L. HPV status in unknown primary head and neck cancer: Prognosis and treatment outcomes. Laryngoscope 2019, 129, 684–691. [Google Scholar] [CrossRef]
- Boeker, R.; Stromberger, C.; Heiland, M.; Beck-Broichsitter, B.; Hofmann, V.M.; Neumann, K.; Ochsenreither, S.; Olze, H.; Dommerich, S.; Piwonski, I.; et al. Carcinoma of Unknown Primary and the 8th Edition TNM Classification for Head and Neck Cancer. Laryngoscope 2021, 131, E2534–E2542. [Google Scholar] [CrossRef]
- Tribius, S.; Hoffmann, A.S.; Bastrop, S.; Gorogh, T.; Haag, J.; Rocken, C.; Clauditz, T.; Grob, T.; Wilczak, W.; Tennstedt, P.; et al. HPV status in patients with head and neck of carcinoma of unknown primary site: HPV, tobacco smoking, and outcome. Oral Oncol. 2012, 48, 1178–1184. [Google Scholar] [CrossRef]
- Park, G.C.; Jung, J.H.; Roh, J.L.; Lee, J.H.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Prognostic value of metastatic nodal volume and lymph node ratio in patients with cervical lymph node metastases from an unknown primary tumor. Oncology 2014, 86, 170–176. [Google Scholar] [CrossRef]
- Kim, S.Y.; Nam, S.Y.; Choi, S.H.; Cho, K.J.; Roh, J.L. Prognostic value of lymph node density in node-positive patients with oral squamous cell carcinoma. Ann. Surg. Oncol. 2011, 18, 2310–2317. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Clark, J.R.; Zhang, W.J.; Elliott, M.S.; Gao, K.; Milross, C.G.; Shannon, K.F. Lymph node ratio as an independent prognostic factor in oral squamous cell carcinoma. Head Neck 2011, 33, 1245–1251. [Google Scholar] [CrossRef]

| All Patients (n = 80) | |
|---|---|
| Gender (n, %) | |
| Male | 66/80 (82.5%) |
| Female | 14/80 (17.5%) |
| Age (mean years ± SD) | 60.6 ± 10.3 |
| Nodal stage (n, %) | Total (N = 80/100%) |
| p16 status +/− (n, %) | 59 (73.8%)/21 (26.2 %) |
| pN1 | 23 (28.7%) |
| p16+/− | 5 (8.5%)/18 (85.7%) |
| pN2 | 3 (3.7%) |
| p16+/− | 0 (0%)/3 (14.3%) |
| pN2a | 5 (6.3%) |
| p16+/− | 5 (8.5%)/0 (0%) |
| pN2b | 9 (11.3%) |
| p16+/− | 9 (15.3%)/0 (0%) |
| pN3b | 40 (50.0%) |
| p16+/− | 40 (67.7%)/0 (0%) |
| UICC stage (n, %) | |
| I | 18/80 (22.5%) |
| II | 3/80 (3.7%) |
| II | 4/80 (5.0%) |
| IVA | 27/80 (33.8%) |
| IVB | 28/80 (35.0%) |
| Extranodal extension (n, %) | |
| Yes | 55/80 (68.8%) |
| No | 25/80 (31.3%) |
| Noxious agents | |
| Smoking | 55/80 (68.8%) |
| Alcohol consumption | 60/75 (80.0%) |
| ASA score | |
| 1 | 6/80 (7.5%) |
| 2 | 61/80 (76.3%) |
| 3 | 13/80 (16.2%) |
| All Patients | |
|---|---|
| Time span | |
| Neck dissection—adjuvant therapy (mean d ± SD) | 54.3 ± 21.7 |
| Surgical treatment modality (n, %) | |
| Selective neck dissection | 43/80 (53.8%) |
| Modified radical neck dissection | 14/80 (17.5%) |
| Radical neck dissection | 23/80 (28.7%) |
| Adjuvant treatment modality (n, %) | |
| Radiation therapy | 8/80 (10.0%) |
| Chemoradiation therapy | 72/80 (90.0%) |
| Radiation dose in Gy (mean ± SD) | 70.05 ± 8.26 |
| Chemotherapy (n, %) | |
| Cisplatin/carboplatin + 5-FU | 69/72 (95.8%) |
| Other | 3/72 (4.2%) |
| Number of removed lymph nodes | 20.8 ± 10.5 |
| Lymph node ratio (LNR; mean ± SD) | 0.20 ± 0.22 |
| All Patients (n = 80) | |
|---|---|
| Recurrence of disease: | |
| Distant metastases | 6 (7.5%) |
| locoregional recurrence | 13 (16.3%) |
| 5-year OS rate | |
| Events (death) | 22 (27.5%) |
| KM estimate (95% CI) | 67.7% (54.2–81.2%) |
| 5-year DSS rate | |
| Events (death) | 12 (15.0%) |
| KM estimate (95% CI) | 82.3 % (72.1–92.5%) |
| 5-year PFS rate | |
| Events (death) | 19 (23.8%) |
| KM estimate (95% CI) | 72.8% (61.8–83.8%) |
| Univariate Cox Regression | Multiple Cox Regression | |||||
|---|---|---|---|---|---|---|
| HR (95%-CI) | Wald Statistic | N (%) | HR (95%-CI) | Wald Statistic | ||
| Age | 1.05 (0.99–1.09) | p = 0.063 | 80 | |||
| Nodal stage (pN) | ||||||
| pN1 (REF) | 23 (28.8%) | |||||
| pN2 | 2.73 (0.26–29.01) | p = 0.406 | 3 (3.8%) | |||
| pN2a | 0 (0–) * | p = 0.985 | 5 (6.3%) | |||
| pN2b | 0.85 (0.09–8.21) | p = 0.891 | 9 (11.3%) | |||
| pN3b | 2.91 (0.83–10.17) | p = 0.095 | 40 (50.0%) | |||
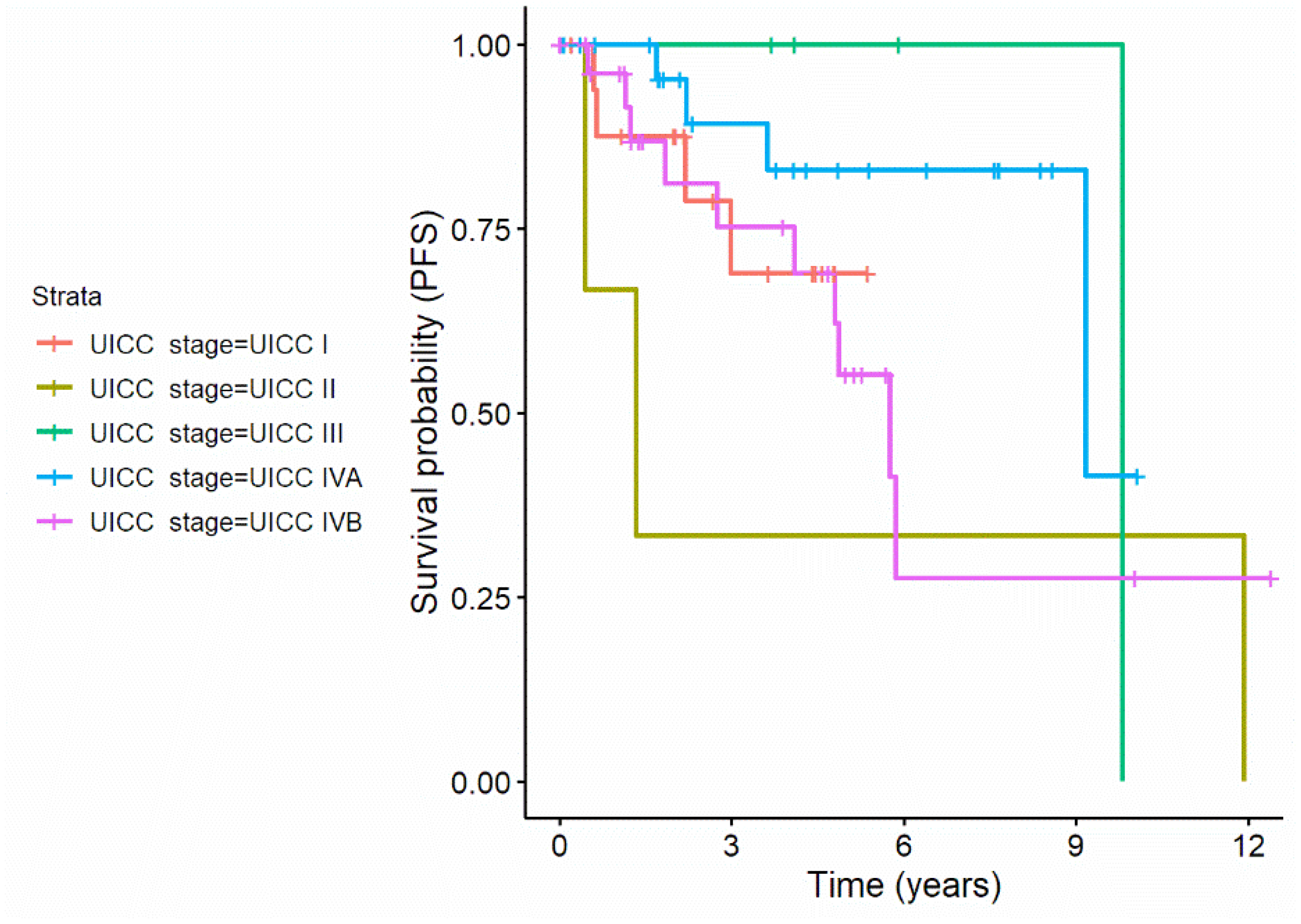
| UICC-stage | ||||||
| UICC 1 (REF) | 18 (22.5%) | |||||
| UICC 2 | 2.12 (0.21–21.95) | p = 0.528 | 3 (3.8%) | |||
| UICC 3 | 0 (0–) * | p = 0.985 | 4 (5.0%) | |||
| UICC IVa | 0.42 (0.07–2.51) | p = 0.342 | 15 (18.8%) | |||
| UICC IVb | 2.87 (0.81–10.14) | p = 0.102 | 40 (50.0%) | |||
| ENE(+) | 1.87 (0.68–5.13) | p = 0.226 | 55 (68.8%) | |||
| Surgical treatment modality | ||||||
| Selective neck dissection (REF) | 43 (53.8%) | |||||
| Modified-radical neck dissection | 3.77 (1.25–11.40) | p = 0.019 | 14 (17.5%) | |||
| Radical neck dissection | 2.24 (0.83–6.04) | p = 0.110 | 23 (28.8%) | |||
| Radiotherapy vs. chemoradiotherapy | 3.54 (1.16–10.81) | p = 0.027 | 7 (8.8%) | 4.45 (1.40–14.17) | p = 0.012 | 7 (8.8%) |
| No. of removed lymph nodes | 0.98 (0.93–1.02) | p = 0.303 | 80 | |||
| LNR | 5.01 (0.83–30.31) | p = 0.080 | 80 | |||
| Locoregional metastases (yes) | 2.70 (0.61–11.85) | p = 0.189 | 6 (7.5%) | |||
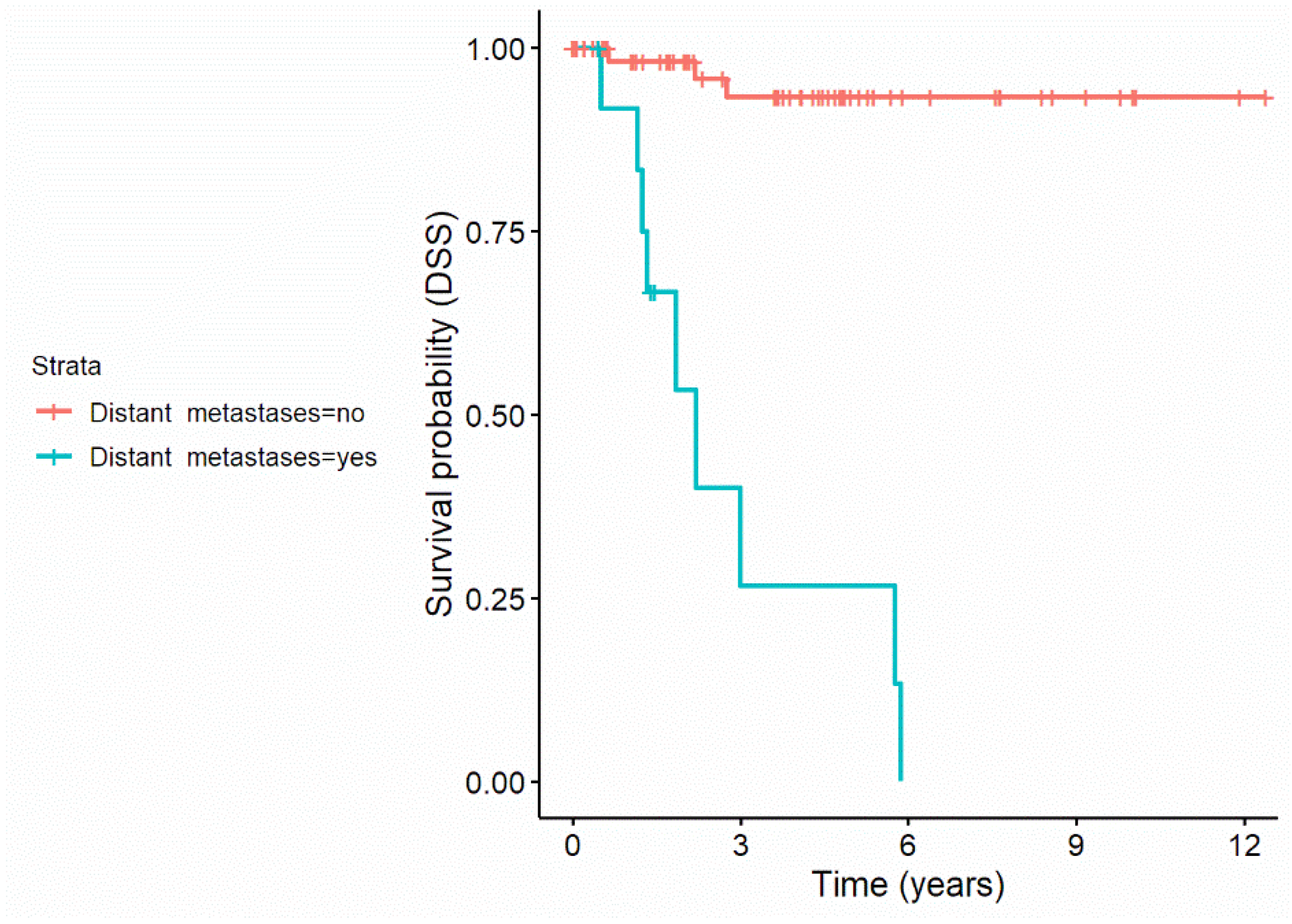
| Distant metastases (yes) | 7.41 (2.95–18.62) | p < 0.001 | 13 (16.3%) | 8.24 (3.21–21.15) | p < 0.001 | 13 (16.3%) |
| p16 status (positive) | 1.70 (0.67–4.29) | p = 0.261 | 21 (26.3%) | |||
| Time span ND—adjuvant therapy | 1.00 (0.98–1.02) | p = 0.999 | 80 | |||
| Univariate Cox Regression | Multiple Cox Regression | |||||
|---|---|---|---|---|---|---|
| HR (95%-CI) | Wald Statistic | N (%) | HR (95%-CI) | Wald Statistic | N (%) | |
| Age | 0.99 (0.92-1.05) | p = 0.646 | 80 | |||
| Nodal stage (pN) | ||||||
| pN1 (REF) | 23 (28.8%) | |||||
| pN2 | 2.52 (0.25–25.58) | p = 0.433 | 3 (3.8%) | |||
| pN2a | 0 (0–) * | p = 0.988 | 5 (6.3%) | |||
| pN2b | 0 (0–) * | p = 0.981 | 9 (11.3%) | |||
| pN3b | 1.68 (0.44–6.34) | p = 0.446 | 40 (50.0%) | |||
| UICC stage | ||||||
| UICC 1 (REF) | 18 (22.5%) | |||||
| UICC 2 | 1.52 (0.14–16.28) | p = 0.778 | 3 (3.8%) | |||
| UICC 3 | 0 (0–) * | p = 0.986 | 4 (5.0%) | |||
| UICC IVa | 0.14 (0.01–1.44) | p = 0.098 | 15 (18.8%) | |||
| UICC IVb | 1.28 (0.32–5.10) | p = 0.729 | 40 (50.0%) | |||
| ENE (+) | 42.90 (0.31–5891.45) | p = 0.134 | 55 (68.8%) | |||
| Surgical treatment modality | ||||||
| Selective neck dissection (REF) | 43 (53.8%) | |||||
| Modified radical neck dissection | 5.35 (1.06–27.09) | p = 0.043 | 14 (17.5%) | |||
| Radical neck dissection | 4.52 (1.23–18.14) | p = 0.033 | 23 (28.8%) | |||
| Radio- vs. chemoradiotherapy | 1.59 (0.21–12.36) | p = 0.657 | 7 (8.8%) | |||
| No. of removed lymph nodes | 0.97 (0.91–1.03) | p = 0.369 | 80 | |||
| LNR | 12.93 (1.54–108.45) | p = 0.018 | 80 | |||
| Recurrent locoregional disease (yes) | 4.89 (1.03–23.15) | p = 0.046 | 6 (7.5%) | |||
| Distant metastases (yes) | 23.79 (6.32–89.56) | p < 0.001 | 13 (16.3%) | 23.79 (6.32–89.56) | p < 0.001 | 13 (16.3%) |
| p16 status (positive) | 1.86 (0.54–6.32) | p = 0.324 | 21 (26.3%) | |||
| Time span ND—adjuvant therapy | 1.001 (0.98–1.03) | p = 0.930 | 80 | |||
| Univariate Cox Regression | Multiple Cox-Regression | |||||
|---|---|---|---|---|---|---|
| HR (95%-CI) | Wald Statistic | N (%) | HR (95%-CI) | Wald Statistic | N (%) | |
| Age | 0.99 (0.92–1.05) | p = 0.646 | 80 | |||
| Nodal stage (pN) | ||||||
| pN1 (REF) | 23 (28.8%) | |||||
| pN2 | 2.52 (0.25–25.58) | p = 0.433 | 3 (3.8%) | |||
| pN2a | 0 (0–) * | p = 0.988 | 5 (6.3%) | |||
| pN2b | 0 (0–) * | p = 0.981 | 9 (11.3%) | |||
| pN3b | 1.68 (0.44–6.34) | p = 0.446 | 40 (50.0%) | |||
| UICC stage | ||||||
| UICC 1 (REF) | 18 (22.5%) | |||||
| UICC 2 | 1.52 (0.14–16.28) | p = 0.778 | 3 (3.8%) | |||
| UICC 3 | 0 (0–) * | p = 0.986 | 4 (5.0%) | |||
| UICC IVa | 0.14 (0.01–1.44) | p = 0.098 | 15 (18.8%) | |||
| UICC IVb | 1.28 (0.32–5.10) | p = 0.729 | 40 (50.0%) | |||
| ENE(+) | 8.69 (1.16–65.37) | p = 0.036 | 2.10 ± 1.03 | 8.13 (1.08–61.34) | p = 0.042 | |
| Surgical treatment modality | ||||||
| Selective neck dissection (REF) | ||||||
| Modified-radical neck dissection | 5.42 (1.69–17.40) | p = 0.004 | ||||
| Radical neck dissection | 2.51 (0.79–7.98) | p = 0.119 | ||||
| Radio- vs. chemoradiotherapy | 1.95 (0.45; 8.48) | p = 0.375 | ||||
| No. of removed lymph nodes | 1.01 (0.97; 1.06) | p = 0.554 | ||||
| LNR | 7.93 (1.38–45.45) | p = 0.020 | 1.82 ± 0.87 | 6.17 (1.13–33.76) | p = 0.036 | |
| p16 status (positive) | 0.78 (0.26–2.34) | p = 0.654 | ||||
| Time span ND—adjuvant therapy | 1.001 (0.98–1.02) | p = 0.946 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balk, M.; Rupp, R.; Mantsopoulos, K.; Sievert, M.; Gostian, M.; Allner, M.; Grundtner, P.; Eckstein, M.; Iro, H.; Hecht, M.; et al. Factors Influencing the Outcome of Head and Neck Cancer of Unknown Primary (HNCUP). J. Clin. Med. 2022, 11, 2689. https://doi.org/10.3390/jcm11102689
Balk M, Rupp R, Mantsopoulos K, Sievert M, Gostian M, Allner M, Grundtner P, Eckstein M, Iro H, Hecht M, et al. Factors Influencing the Outcome of Head and Neck Cancer of Unknown Primary (HNCUP). Journal of Clinical Medicine. 2022; 11(10):2689. https://doi.org/10.3390/jcm11102689
Chicago/Turabian StyleBalk, Matthias, Robin Rupp, Konstantinos Mantsopoulos, Matti Sievert, Magdalena Gostian, Moritz Allner, Philipp Grundtner, Markus Eckstein, Heinrich Iro, Markus Hecht, and et al. 2022. "Factors Influencing the Outcome of Head and Neck Cancer of Unknown Primary (HNCUP)" Journal of Clinical Medicine 11, no. 10: 2689. https://doi.org/10.3390/jcm11102689
APA StyleBalk, M., Rupp, R., Mantsopoulos, K., Sievert, M., Gostian, M., Allner, M., Grundtner, P., Eckstein, M., Iro, H., Hecht, M., & Gostian, A.-O. (2022). Factors Influencing the Outcome of Head and Neck Cancer of Unknown Primary (HNCUP). Journal of Clinical Medicine, 11(10), 2689. https://doi.org/10.3390/jcm11102689









