Brain Extract of Subacute Traumatic Brain Injury Promotes the Neuronal Differentiation of Human Neural Stem Cells via Autophagy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Controlled Cortical Impact (CCI) Model and Brain Extract
2.2. Human NSCs (hNSCs) Culture and Treatment of Brain Extract
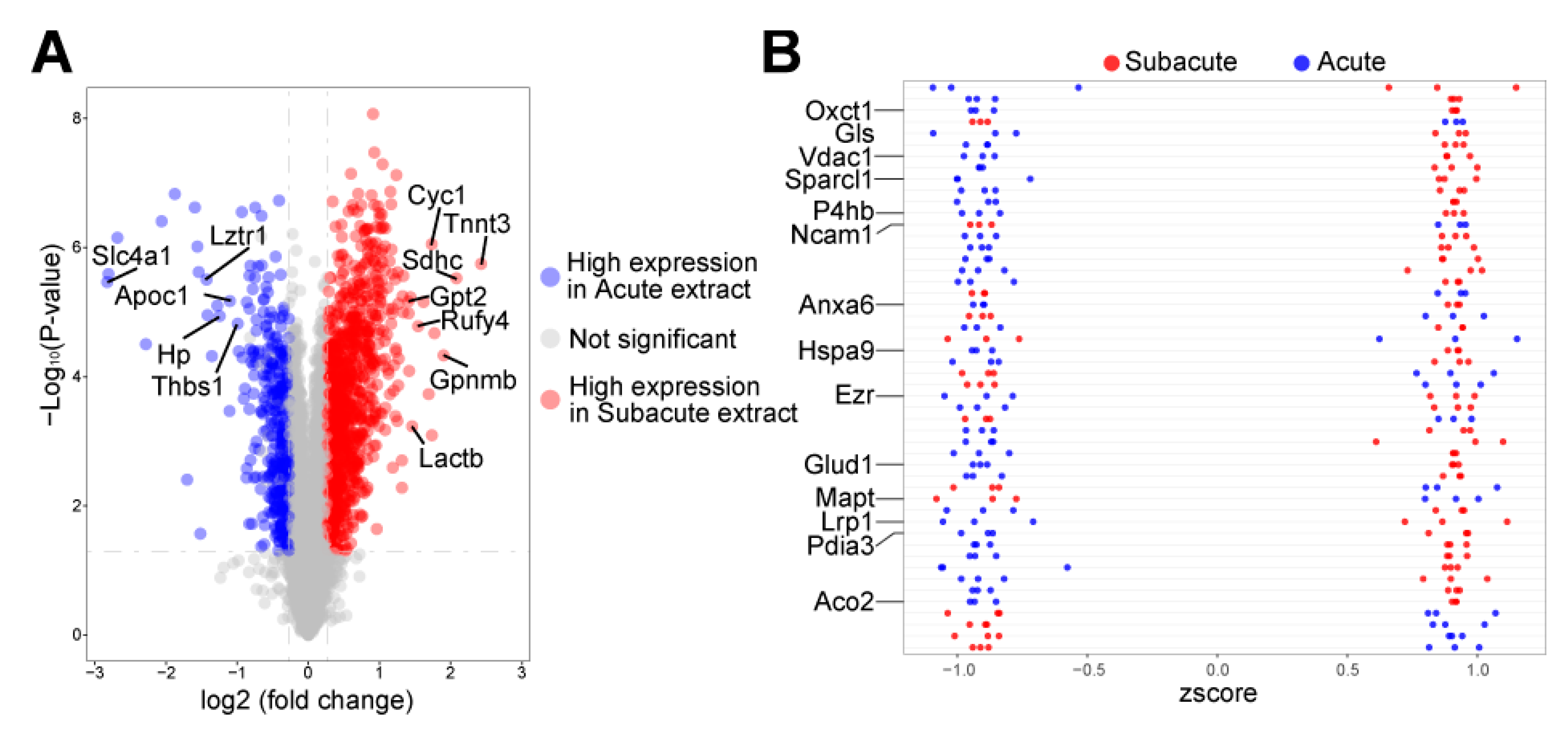
2.3. Protein Profile Analysis of Brain Extract
2.4. Immunofluorescence
2.5. 5-Ethynyl-2-Deoxyuridine (EdU) Labeling
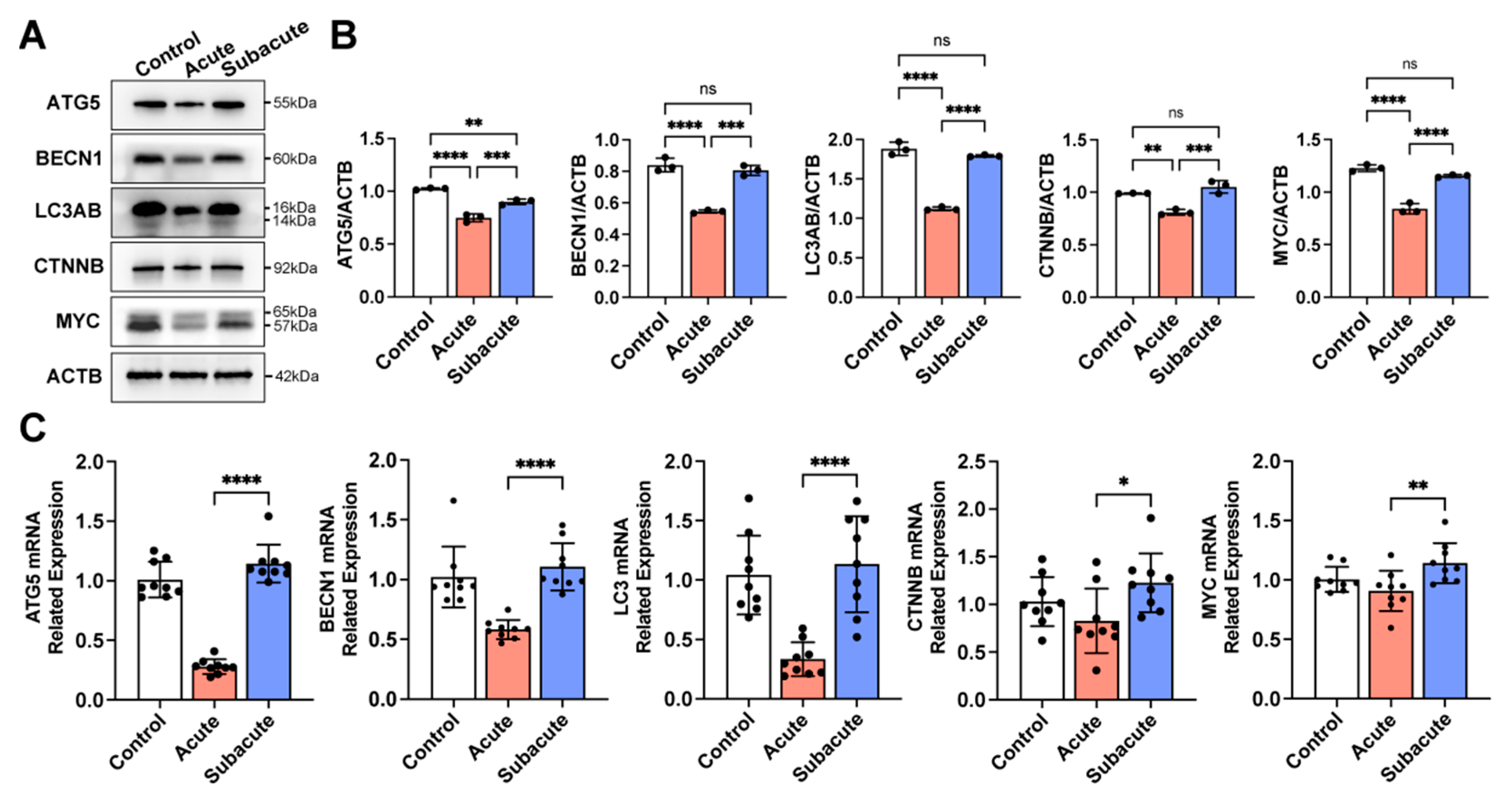
2.6. Western Blot
2.7. RNA Extraction and Quantitative Real-Time PCR Analysis
2.8. Statistical Analyses
3. Results
3.1. Human NSCs Differentiated a Higher Ratio of Neurons in Subacute/Chronic TBI Brain Extracts
3.2. Autophagy-Related Proteins Differentially Expressed in Acute and Subacute TBI Brain Extracts
3.3. Subacute TBI Brain Extract Promoted Neuronal Differentiation through the Activation of Autophagy in hNSCs
3.4. Activating Autophagy Rescued the Neuronal Differentiation within Acute TBI Brain Extract
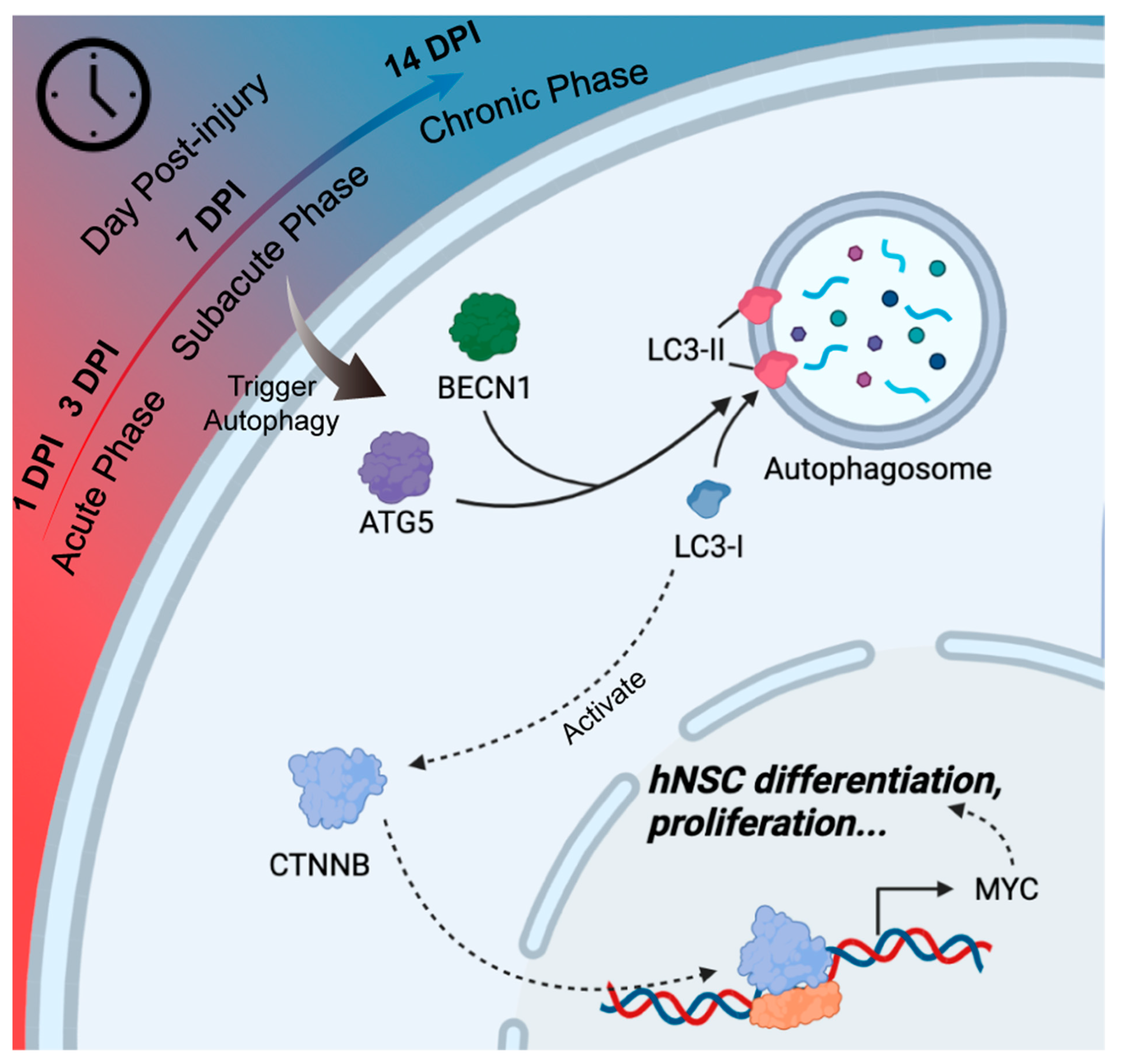
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashina, H.; Eigenbrodt, A.K.; Seifert, T.; Sinclair, A.J.; Scher, A.I.; Schytz, H.W.; Lee, M.J.; De Icco, R.; Finkel, A.G.; Ashina, M. Post-traumatic headache attributed to traumatic brain injury: Classification, clinical characteristics, and treatment. Lancet Neurol. 2021, 20, 460–469. [Google Scholar] [CrossRef]
- Levin, H.S.; Diaz-Arrastia, R.R. Diagnosis, prognosis, and clinical management of mild traumatic brain injury. Lancet Neurol. 2015, 14, 506–517. [Google Scholar] [CrossRef]
- Rosenfeld, J.V.; McFarlane, A.C.; Bragge, P.; Armonda, R.A.; Grimes, J.B.; Ling, G.S. Blast-related traumatic brain injury. Lancet Neurol. 2013, 12, 882–893. [Google Scholar] [CrossRef]
- Jiang, J.Y.; Gao, G.Y.; Feng, J.F.; Mao, Q.; Chen, L.G.; Yang, X.F.; Liu, J.F.; Wang, Y.H.; Qiu, B.H.; Huang, X.J. Traumatic brain injury in China. Lancet Neurol. 2019, 18, 286–295. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, H.; Guo, X.; Pluimer, B.; Zhao, Z. Blood-Brain Barrier Dysfunction in Mild Traumatic Brain Injury: Evidence From Preclinical Murine Models. Front. Physiol. 2020, 11, 1030. [Google Scholar] [CrossRef] [PubMed]
- Frankowski, J.C.; Foik, A.T.; Tierno, A.; Machhor, J.R.; Lyon, D.C.; Hunt, R.F. Traumatic brain injury to primary visual cortex produces long-lasting circuit dysfunction. Commun. Biol. 2021, 4, 1297. [Google Scholar] [CrossRef]
- Zou, Z.; Li, L.; Schäfer, N.; Huang, Q.; Maegele, M.; Gu, Z. Endothelial glycocalyx in traumatic brain injury associated coagulopathy: Potential mechanisms and impact. J. Neuroinflamm. 2021, 18, 134. [Google Scholar] [CrossRef]
- Weston, N.M.; Sun, D. The Potential of Stem Cells in Treatment of Traumatic Brain Injury. Curr. Neurol. Neurosci. Rep. 2018, 18, 1. [Google Scholar] [CrossRef]
- Lee, J.Y.; Acosta, S.; Tuazon, J.P.; Xu, K.; Nguyen, H.; Lippert, T.; Liska, M.G.; Semechkin, A.; Garitaonandia, I.; Gonzalez, R.; et al. Human parthenogenetic neural stem cell grafts promote multiple regenerative processes in a traumatic brain injury model. Theranostics 2019, 9, 1029–1046. [Google Scholar] [CrossRef]
- Xiong, L.L.; Hu, Y.; Zhang, P.; Zhang, Z.; Li, L.H.; Gao, G.D.; Zhou, X.F.; Wang, T.H. Neural Stem Cell Transplantation Promotes Functional Recovery from Traumatic Brain Injury via Brain Derived Neurotrophic Factor-Mediated Neuroplasticity. Mol. Neurobiol. 2018, 55, 2696–2711. [Google Scholar] [CrossRef]
- Beretta, S.; Cunningham, K.M.; Haus, D.L.; Gold, E.M.; Perez, H.; López-Velázquez, L.; Cummings, B.J. Effects of Human ES-Derived Neural Stem Cell Transplantation and Kindling in a Rat Model of Traumatic Brain Injury. Cell Transpl. 2017, 26, 1247–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Gajavelli, S.; Spurlock, M.S.; Mahavadi, A.; Quesada, L.S.; Gajavelli, G.R.; Andreoni, C.B.; Di, L.; Janecki, J.; Lee, S.W.; et al. Human neural stem cell transplant location-dependent neuroprotection and motor deficit amelioration in rats with penetrating traumatic brain injury. J. Trauma Acute Care Surg. 2020, 88, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Nie, D.; Jin, G.; Chen, W.; Xia, L.; Wu, X.; Su, X.; Xu, X.; Ni, L.; Zhang, X.; et al. BDNF blended chitosan scaffolds for human umbilical cord MSC transplants in traumatic brain injury therapy. Biomaterials 2012, 33, 3119–3126. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Prough, D.S.; McAdoo, D.J.; Grady, J.J.; Parsley, M.O.; Ma, L.; Tarensenko, Y.I.; Wu, P. Transplantation of primed human fetal neural stem cells improves cognitive function in rats after traumatic brain injury. Exp. Neurol. 2006, 201, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Li, X.; Wang, C.; Hao, P.; Song, W.; Li, M.; Zhao, W.; Gao, Y.; Yang, Z. Functional hyaluronate collagen scaffolds induce NSCs differentiation into functional neurons in repairing the traumatic brain injury. Acta Biomater. 2016, 45, 182–195. [Google Scholar] [CrossRef]
- Jha, R.M.; Kochanek, P.M.; Simard, J.M. Pathophysiology and treatment of cerebral edema in traumatic brain injury. Neuropharmacology 2019, 145, 230–246. [Google Scholar] [CrossRef]
- Simon, D.W.; McGeachy, M.J.; Bayır, H.; Clark, R.S.; Loane, D.J.; Kochanek, P.M. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 2017, 13, 171–191. [Google Scholar] [CrossRef] [Green Version]
- Dinet, V.; Petry, K.G.; Badaut, J. Brain-Immune Interactions and Neuroinflammation After Traumatic Brain Injury. Front. Neurosci. 2019, 13, 1178. [Google Scholar] [CrossRef] [Green Version]
- Ismail, H.; Shakkour, Z.; Tabet, M.; Abdelhady, S.; Kobaisi, A.; Abedi, R.; Nasrallah, L.; Pintus, G.; Al-Dhaheri, Y.; Mondello, S.; et al. Traumatic Brain Injury: Oxidative Stress and Novel Anti-Oxidants Such as Mitoquinone and Edaravone. Antioxidants 2020, 9, 943. [Google Scholar] [CrossRef]
- Turtzo, L.C.; Luby, M.; Jikaria, N.; Griffin, A.D.; Greenman, D.; Bokkers, R.P.H.; Parikh, G.; Peterkin, N.; Whiting, M.; Latour, L.L. Cytotoxic Edema Associated with Hemorrhage Predicts Poor Outcome after Traumatic Brain Injury. J. Neurotrauma 2021, 38, 3107–3118. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wei, M.G.; Liang, J.; Xu, H.H.; Wang, J.J.; Wang, J.; Yang, X.P.; Lv, F.F.; Wang, K.Q.; Duan, J.H.; et al. Injury-preconditioning secretome of umbilical cord mesenchymal stem cells amplified the neurogenesis and cognitive recovery after severe traumatic brain injury in rats. J. Neurochem. 2020, 153, 230–251. [Google Scholar] [CrossRef] [PubMed]
- Chudickova, M.; Bruza, P.; Zajicova, A.; Trosan, P.; Svobodova, L.; Javorkova, E.; Kubinova, S.; Holan, V. Targeted neural differentiation of murine mesenchymal stem cells by a protocol simulating the inflammatory site of neural injury. J. Tissue Eng. Regen. Med. 2017, 11, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romine, J.; Gao, X.; Chen, J. Controlled cortical impact model for traumatic brain injury. J. Vis. Exp. 2014, 90, e51781. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.; Zhang, Y.; Jiang, W.; He, L.; Qu, H. Modulation of autophagy in traumatic brain injury. J. Cell Physiol. 2020, 235, 1973–1985. [Google Scholar] [CrossRef]
- Fleming, A.; Rubinsztein, D.C. Autophagy in Neuronal Development and Plasticity. Trends Neurosci. 2020, 43, 767–779. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Liu, S.; Li, F.; Wang, B.; Wang, J.; Cao, L.; Xia, T.; Yao, Q.; Chen, H.; et al. Melatonin Enhances Proliferation and Modulates Differentiation of Neural Stem Cells Via Autophagy in Hyperglycemia. Stem Cells 2019, 37, 504–515. [Google Scholar] [CrossRef]
- Littlejohn, E.L.; Scott, D.; Saatman, K.E. Insulin-like growth factor-1 overexpression increases long-term survival of posttrauma-born hippocampal neurons while inhibiting ectopic migration following traumatic brain injury. Acta Neuropathol. Commun. 2020, 8, 46. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.; Hu, W.; Wang, X.; Gao, X.; He, C.; Chen, J. Traumatic Brain Injury Causes Aberrant Migration of Adult-Born Neurons in the Hippocampus. Sci. Rep. 2016, 6, 21793. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Kang, Y.; Huang, L.; Wu, L.; Peng, Y. TIMP1 preserves the blood-brain barrier through interacting with CD63/integrin β 1 complex and regulating downstream FAK/RhoA signaling. Acta Pharm. Sin. B 2020, 10, 987–1003. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Liu, H.; Zhang, Y.; Chen, G.; Li, Z.; Wang, Q. An Activatable NIR-II Nanoprobe for In Vivo Early Real-Time Diagnosis of Traumatic Brain Injury. Angew. Chem. Int. Ed. Engl. 2020, 59, 247–252. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, E.A.; Ndode-Ekane, X.E.; Lehto, L.J.; Gorter, J.A.; Andrade, P.; Aronica, E.; Gröhn, O.; Pitkänen, A. Long-lasting blood-brain barrier dysfunction and neuroinflammation after traumatic brain injury. Neurobiol. Dis. 2020, 145, 105080. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yu, Z.; Liu, Y.; Bai, Y.; Jiang, Y.; van Leyen, K.; Yang, Y.G.; Lok, J.M.; Whalen, M.J.; Lo, E.H.; et al. CD47 deficiency improves neurological outcomes of traumatic brain injury in mice. Neurosci. Lett. 2017, 643, 125–130. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Q.; Hu, E.; Wang, Y.; Lu, H. Quantitative Mass Spectrometry Imaging of Metabolomes and Lipidomes for Tracking Changes and Therapeutic Response in Traumatic Brain Injury Surrounding Injured Area at Chronic Phase. ACS Chem. Neurosci. 2021, 12, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Hachem, L.D.; Mothe, A.J.; Tator, C.H. Glutamate Increases In Vitro Survival and Proliferation and Attenuates Oxidative Stress-Induced Cell Death in Adult Spinal Cord-Derived Neural Stem/Progenitor Cells via Non-NMDA Ionotropic Glutamate Receptors. Stem Cells Dev. 2016, 25, 1223–1233. [Google Scholar] [CrossRef]
- Hwang, I.; Tang, D.; Paik, J. Oxidative stress sensing and response in neural stem cell fate. Free Radic. Biol. Med. 2021, 169, 74–83. [Google Scholar] [CrossRef]
- Bankston, A.N.; Forston, M.D.; Howard, R.M.; Andres, K.R.; Smith, A.E.; Ohri, S.S.; Bates, M.L.; Bunge, M.B.; Whittemore, S.R. Autophagy is essential for oligodendrocyte differentiation, survival, and proper myelination. Glia 2019, 67, 1745–1759. [Google Scholar] [CrossRef]
- Ha, S.; Jeong, S.H.; Yi, K.; Chu, J.J.; Kim, S.; Kim, E.K.; Yu, S.W. Autophagy Mediates Astrogenesis in Adult Hippocampal Neural Stem Cells. Exp. Neurobiol. 2019, 28, 229–246. [Google Scholar] [CrossRef]
- Fan, Q.; Yang, L.; Zhang, X.; Ma, Y.; Li, Y.; Dong, L.; Zong, Z.; Hua, X.; Su, D.; Li, H.; et al. Autophagy promotes metastasis and glycolysis by upregulating MCT1 expression and Wnt/β-catenin signaling pathway activation in hepatocellular carcinoma cells. J. Exp. Clin. Cancer Res. 2018, 37, 9. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Liang, Y.; Zhou, L.; Yan, Y.; Liu, N.; Zhang, R.; Huang, Y.; Wang, M.; Tang, Y.; Ali, D.W.; et al. TSPAN1 promotes autophagy flux and mediates cooperation between WNT-CTNNB1 signaling and autophagy via the MIR454-FAM83A-TSPAN1 axis in pancreatic cancer. Autophagy 2021, 17, 3175–3195. [Google Scholar] [CrossRef]
- Nicklas, S.; Hillje, A.L.; Okawa, S.; Rudolph, I.M.; Collmann, F.M.; van Wuellen, T.; Del Sol, A.; Schwamborn, J.C. A complex of the ubiquitin ligase TRIM32 and the deubiquitinase USP7 balances the level of c-Myc ubiquitination and thereby determines neural stem cell fate specification. Cell Death Differ. 2019, 26, 728–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Wang, S.S.; Shan, B.Q.; Qin, J.B.; Zhao, H.Y.; Tian, M.L.; He, H.; Cheng, X.; Zhang, X.H.; Jin, G.H. miR-103-3p targets Ndel1 to regulate neural stem cell proliferation and differentiation. Neural. Regen. Res. 2022, 17, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fang, F.; Song, R.; Gao, X.; Jiang, M.; Cang, J. Sevoflurane affects neurogenesis through cell cycle arrest via inhibiting wnt/β-catenin signaling pathway in mouse neural stem cells. Life Sci. 2018, 209, 34–42. [Google Scholar] [CrossRef] [PubMed]



| Gene | Direction | Primer Sequence (5′-3′) |
|---|---|---|
| GAPDH | Forward | GGAGTCCACTGGCGTCTTCA |
| Reverse | GTCATGAGTCCTTCCACGATACC | |
| ACTB | Forward | CATGTACGTTGCTATCCAGGC |
| Reverse | CTCCTTAATGTCACGCACGAT | |
| ATG5 | Forward | AGAAGCTGTTTCGTCCTGTGG |
| Reverse | AGGTGTTTCCAACATTGGCTC | |
| BECN1 | Forward | GGTGTCTCTCGCAGATTCATC |
| Reverse | TCAGTCTTCGGCTGAGGTTCT | |
| LC3 | Forward | AACATGAGCGAGTTGGTCAAG |
| Reverse | GCTCGTAGATGTCCGCGAT | |
| CTNNB | Forward | CATCTACACAGTTTGATGCTGCT |
| Reverse | GCAGTTTTGTCAGTTCAGGGA | |
| MYC | Forward | TCCCTCCACTCGGAAGGAC |
| Reverse | CTGGTGCATTTTCGGTTGTTG | |
| NES | Forward | CTGCTACCCTTGAGACACCTG |
| Reverse | GGGCTCTGATCTCTGCATCTAC | |
| SOX2 | Forward | GCCGAGTGGAAACTTTTGTCG |
| Reverse | GGCAGCGTGTACTTATCCTTCT | |
| SOX1 | Forward | CAGTACAGCCCCATCTCCAAC |
| Reverse | GCGGGCAAGTACATGCTGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Lang, L.; Hui, J.; Ma, Y.; Yang, C.; Weng, W.; Huang, J.; Zhao, X.; Zhang, X.; Liang, Q.; et al. Brain Extract of Subacute Traumatic Brain Injury Promotes the Neuronal Differentiation of Human Neural Stem Cells via Autophagy. J. Clin. Med. 2022, 11, 2709. https://doi.org/10.3390/jcm11102709
He Z, Lang L, Hui J, Ma Y, Yang C, Weng W, Huang J, Zhao X, Zhang X, Liang Q, et al. Brain Extract of Subacute Traumatic Brain Injury Promotes the Neuronal Differentiation of Human Neural Stem Cells via Autophagy. Journal of Clinical Medicine. 2022; 11(10):2709. https://doi.org/10.3390/jcm11102709
Chicago/Turabian StyleHe, Zhenghui, Lijian Lang, Jiyuan Hui, Yuxiao Ma, Chun Yang, Weiji Weng, Jialin Huang, Xiongfei Zhao, Xiaoqi Zhang, Qian Liang, and et al. 2022. "Brain Extract of Subacute Traumatic Brain Injury Promotes the Neuronal Differentiation of Human Neural Stem Cells via Autophagy" Journal of Clinical Medicine 11, no. 10: 2709. https://doi.org/10.3390/jcm11102709






