Brain Tissue Damage Induced by Multimodal Neuromonitoring In Situ during MRI after Severe Traumatic Brain Injury: Incidence and Clinical Relevance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Intracranial Probes
2.3. Magnetic Resonance Imaging
2.4. Statistical Analysis
3. Results
3.1. Patients
3.2. Intracranial Probes
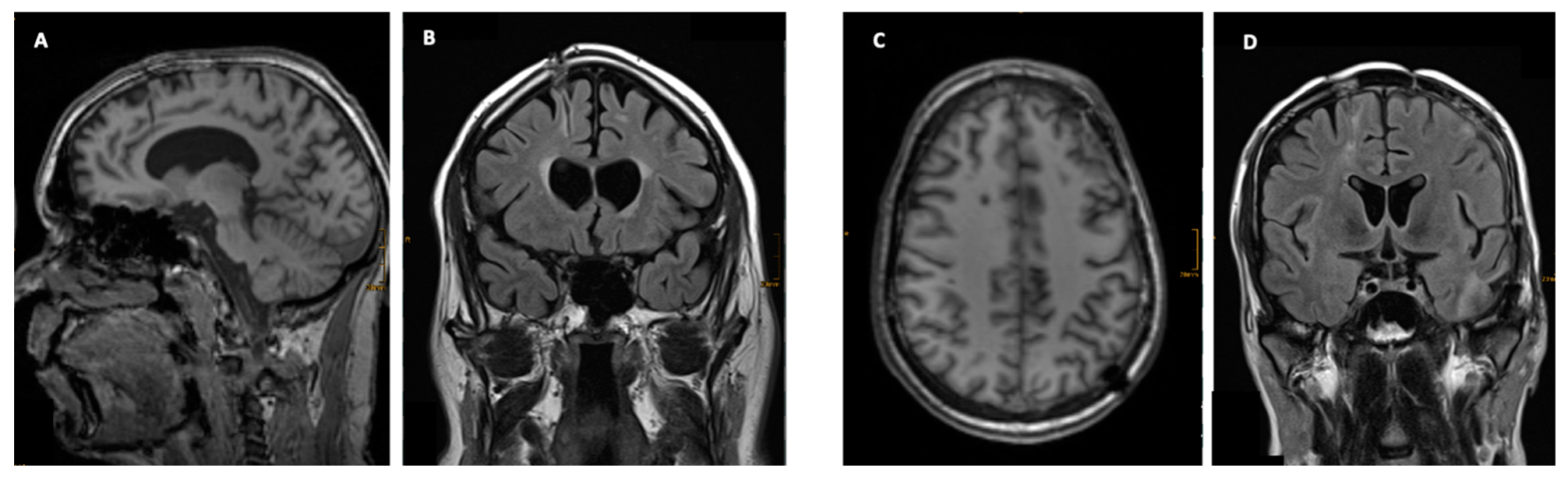
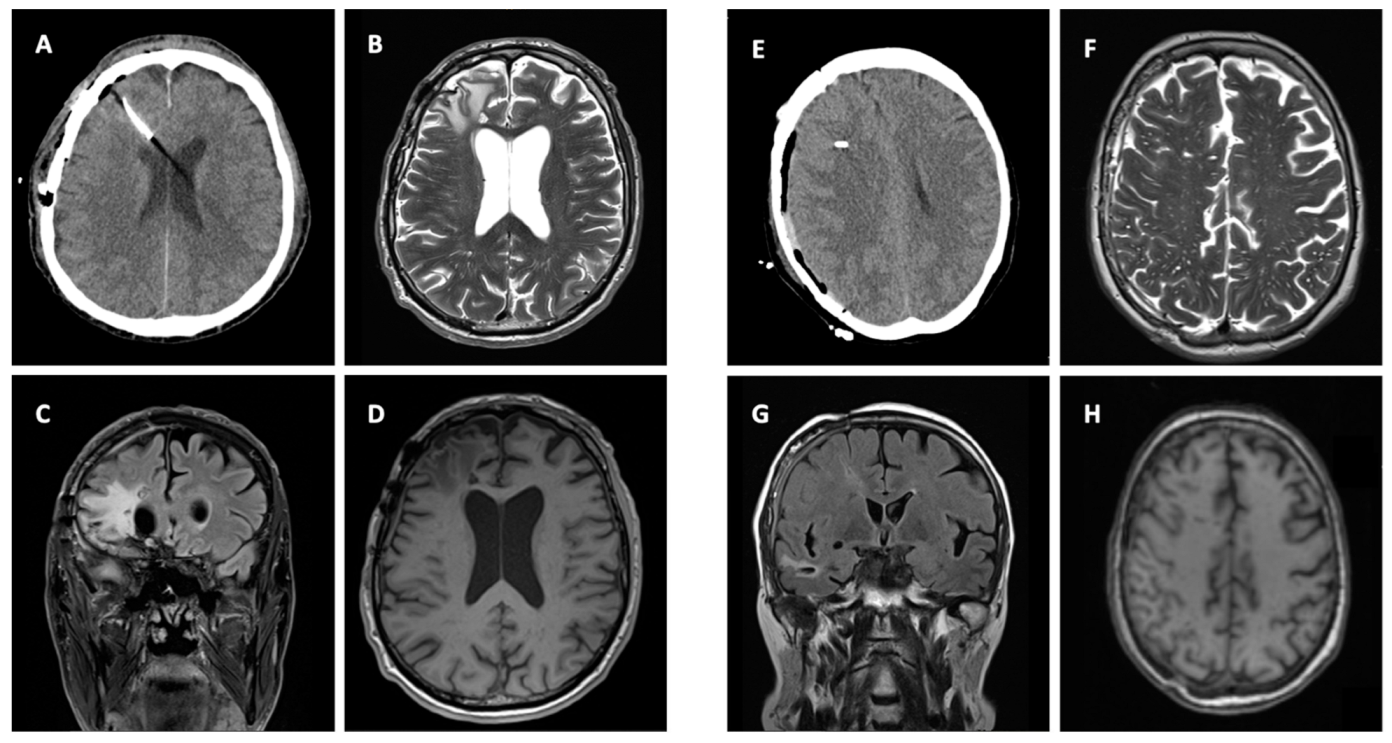
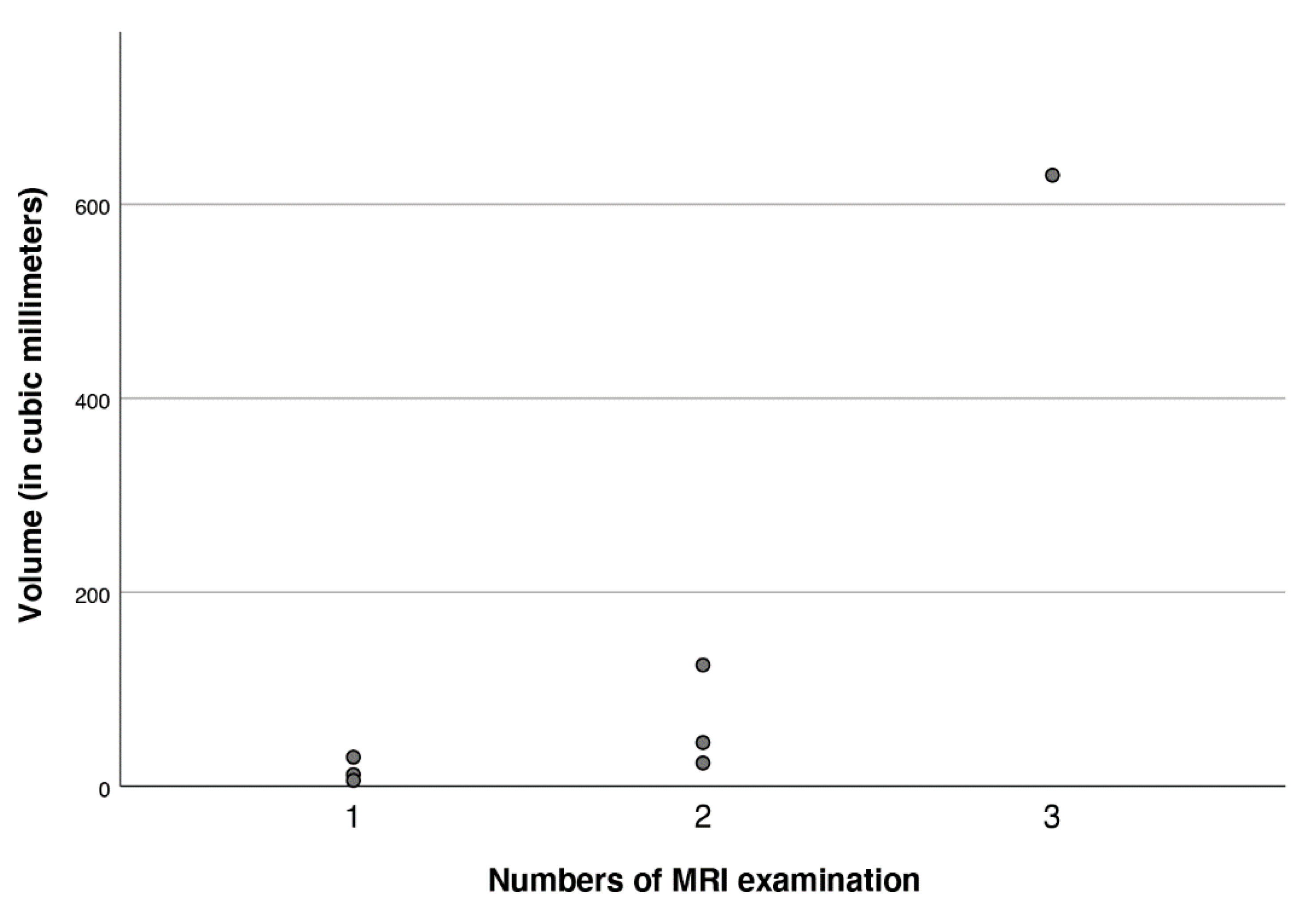
3.3. Imaging
3.4. Clinical Signicance
4. Discussion
- Stringent indications for all MRI examinations in neurointensive care patients in a close coordination between neuroradiologists and neurointensivists/neurosurgeons.
- Early cerebral CT scan to determine the accurate location of neuromonitoring probes, detecting malposition (i.e., eloquent areas), and/or neuromonitoring-related surgical complications.
- MRI protocol adjustments (i.e., reduced number of applied MR sequences depending on underlying questions, e.g., only DWI, 3D SWI, T2, T1; reduction in specific absorption rate; greater slice thickness, etc.).
- Pre-imaging removal or reduction of neuromonitoring probes to avoid all potential enhancing effects due to the proximity of multiple probes. Currently, we perform MRI only with one ICP probe in situ.
- No use of bolt systems in patients with severe TBI to avoid artefacts and problems with patient positioning in the head coil.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stiefel, M.F.; Spiotta, A.; Gracias, V.H.; Garuffe, A.M.; Guillamondegui, O.; Maloney-Wilensky, E.; Bloom, S.; Grady, M.S.; Leroux, P.D. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J. Neurosurg. 2005, 103, 805–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawoos, U.; McCarron, R.M.; Auker, C.R.; Chavko, M. Advances in Intracranial Pressure Monitoring and Its Significance in Managing Traumatic Brain Injury. Int. J. Mol. Sci. 2015, 16, 28979–28997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccinini, A.; Lewis, M.; Benjamin, E.; Aiolfi, A.; Inaba, K.; Demetriades, D. Intracranial pressure monitoring in severe traumatic brain injuries: A closer look at level 1 trauma centers in the United States. Injury 2017, 48, 1944–1950. [Google Scholar] [CrossRef]
- Hawryluk, G.W.J.; Aguilera, S.; Buki, A.; Bulger, E.; Citerio, G.; Cooper, D.J.; Arrastia, R.D.; Diringer, M.; Figaji, A.; Gao, G.; et al. A management algorithm for patients with intracranial pressure monitoring: The Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2019, 45, 1783–1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakhit, S.; Nordness, M.F.; Lombardo, S.R.; Cook, M.; Smith, L.; Patel, M.B. Management and Challenges of Severe Traumatic Brain Injury. Semin. Respir. Crit. Care Med. 2020, 42, 127–144. [Google Scholar] [CrossRef]
- Carney, N.; Totten, A.M.; O'Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.D.; Hannawi, Y.; Puybasset, L. MRI for coma emergence and recovery. Curr. Opin. Crit. Care 2014, 20, 168–173. [Google Scholar] [CrossRef]
- Humble, S.S.; Wilson, L.D.; Wang, L.; Long, D.A.; Smith, M.A.; Siktberg, J.C.; Mirhoseini, M.F.; Bhatia, A.; Pruthi, S.; Day, M.A.; et al. Prognosis of diffuse axonal injury with traumatic brain injury. J. Trauma Acute Care Surg. 2018, 85, 155–159. [Google Scholar] [CrossRef]
- Haghbayan, H.; Boutin, A.; Laflamme, M.; Lauzier, F.; Shemilt, M.; Moore, L.; Zarychanski, R.; Douville, V.; Fergusson, D.; Turgeon, A.F. The Prognostic Value of MRI in Moderate and Severe Traumatic Brain Injury: A Sys-tematic Review and Meta-Analysis. Crit. Care Med. 2017, 45, e1280–e1288. [Google Scholar] [CrossRef]
- Betz, J.; Zhuo, J.; Roy, A.; Shanmuganathan, K.; Gullapalli, R.P. Prognostic Value of Diffusion Tensor Imaging Parameters in Severe Traumatic Brain Injury. J. Neurotrauma 2012, 29, 1292–1305. [Google Scholar] [CrossRef]
- Newcombe, V.; Hawkes, R.C.; Harding, S.G.; Willcox, R.; Brock, S.; Hutchinson, P.J.; Menon, D.K.; Carpenter, T.A.; Coles, J.P. Potential heating caused by intraparenchymal intracranial pressure transducers in a 3-tesla magnetic resonance imaging system using a body radiofrequency resonator: Assessment of the Codman MicroSensor Transducer. J. Neurosurg. 2008, 109, 159–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dempsey, M.F.; Condon, B. Thermal Injuries Associated with MRI. Clin. Radiol. 2001, 56, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Rezai, A.R.; Finelli, D.; Nyenhuis, J.A.; Hrdlicka, G.; Tkach, J.; Sharan, A.; Rugieri, P.; Stypulkowski, P.H.; Shellock, F.G. Neurostimulation systems for deep brain stimulation: In vitro evaluation of magnetic resonance imaging-related heating at 1.5 tesla. J. Magn. Reson. Imaging 2002, 15, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Sharan, A.; Rezai, A.R.; Nyenhuis, J.A.; Hrdlicka, G.; Tkach, J.; Baker, K.; Turbay, M.; Rugieri, P.; Phillips, M.; Shellock, F.G. MR safety in patients with implanted deep brain stimulation systems (DBS). In Neurosurgical Re-Engineering of the Damaged Brain and Spinal Cord; Katayama, Y., Ed.; Springer: Vienna, Austria, 2003; Volume 87, pp. 141–145. [Google Scholar] [CrossRef]
- Rezai, A.R.; Phillips, M.; Baker, K.B.; Sharan, A.D.; Nyenhuis, J.; Tkach, J.; Henderson, J.; Shellock, F.G. Neurostimulation System Used for Deep Brain Stimulation (DBS): MR safety issues and implications of failing to follow safety recommendations. Investig. Radiol. 2004, 39, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.B.; Nyenhuis, J.A.; Hrdlicka, G.; Rezai, A.R.; Tkach, J.A.; Shellock, F.G. Neurostimulation systems: Assessment of magnetic field interactions associated with 1.5- and 3-Tesla MR systems. J. Magn. Reson. Imaging 2004, 21, 72–77. [Google Scholar] [CrossRef]
- Tanaka, R.; Yumoto, T.; Shiba, N.; Okawa, M.; Yasuhara, T.; Ichikawa, T.; Tokunaga, K.; Date, I.; Ujike, Y. Overheated and melted intracranial pressure transducer as cause of thermal brain injury during magnetic resonance imaging: Case report. J. Neurosurg. 2012, 117, 1100–1109. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, S.; Peitz, G.; Ares, W.; Hafeez, S.; Grandhi, R. Complications of invasive intracranial pressure monitoring devices in neurocritical care. Neurosurg. Focus 2017, 43, E6. [Google Scholar] [CrossRef] [Green Version]
- Gelabert-González, M.; Ginesta-Galan, V.; Sernamito-García, R.; Allut, A.G.; Bandin-Diéguez, J.; Rumbo, R.M. The Camino intracranial pressure device in clinical practice. Assessment in a 1000 cases. Acta Neurochir. 2005, 148, 435–441. [Google Scholar] [CrossRef]
- Pinggera, D.; Steiger, R.; Bauer, M.; Kerschbaumer, J.; Luger, M.; Beer, R.; Rietzler, A.; Grams, A.E.; Gizewski, E.R.; Thomé, C.; et al. Cerebral Energy Status and Altered Metabolism in Early Severe TBI: First Results of a Prospective 31P-MRS Feasibility Study. Neurocritical Care 2020, 34, 432–440. [Google Scholar] [CrossRef]
- Pinggera, D.; Steiger, R.; Bauer, M.; Kerschbaumer, J.; Beer, R.; Rietzler, A.; Grams, A.E.; Gizewski, E.R.; Thomé, C.; Petr, O. Repeated 31P-Magnetic Resonance Spectroscopy in Severe Traumatic Brain Injury: Insights into Cerebral Energy Status and Altered Metabolism. J. Neurotrauma 2021, 38, 2822–2830. [Google Scholar] [CrossRef]
- Pinggera, D.; Luger, M.; Bürgler, I.; Bauer, M.; Thomé, C.; Petr, O. Safety of Early MRI Examinations in Severe TBI: A Test Battery for Proper Patient Selection. Front. Neurol. 2020, 11, 219. [Google Scholar] [CrossRef]
- Pinggera, D.; Petr, O.; Putzer, G.; Thomé, C. Adjustable and Rigid Fixation of Brain Tissue Oxygenation Probe (Licox) in Neurosurgery: From Bench to Bedside. World Neurosurg. 2018, 117, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Wu, X.; Yu, J.; Sun, Y.; Li, Z.; Du, Z.; Mao, Y.; Zhou, L.; Hu, J. Effects and Clinical Characteristics of Intracranial Pressure Monitoring–Targeted Management for Subsets of Traumatic Brain Injury: An Observational Multicenter Study. Crit. Care Med. 2015, 43, 1405–1414. [Google Scholar] [CrossRef]
- Yuan, Q.; Wu, X.; Sun, Y.; Yu, J.; Li, Z.; Du, Z.; Mao, Y.; Zhou, L.; Hu, J. Impact of intracranial pressure monitoring on mortality in patients with traumatic brain injury: A systematic review and meta-analysis. J. Neurosurg. 2015, 122, 574–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okonkwo, D.O.; Shutter, L.; Moore, C.; Temkin, N.R.; Puccio, A.M.; Madden, C.J.; Andaluz, N.; Chesnut, R.; Bullock, M.R.; Grant, G.A.; et al. Brain Oxygen Optimization in Severe Traumatic Brain Injury Phase-II: A Phase II Randomized Trial. Crit. Care Med. 2017, 45, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Narotam, P.K.; Morrison, J.F.; Nathoo, N. Brain tissue oxygen monitoring in traumatic brain injury and major trauma: Outcome analysis of a brain tissue oxygen–directed therapy. J. Neurosurg. 2009, 111, 672–682. [Google Scholar] [CrossRef]
- Lin, C.-M.; Lin, M.-C.; Huang, S.-J.; Chang, C.-K.; Chao, D.-P.; Lui, T.-N.; Ma, H.-I.; Liu, M.-Y.; Chung, W.-Y.; Shih, Y.-H.; et al. A Prospective Randomized Study of Brain Tissue Oxygen Pressure-Guided Management in Moderate and Severe Traumatic Brain Injury Patients. BioMed Res. Int. 2015, 2015, 529580. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishna, R.; Stiefel, M.; Udoteuk, J.; Spiotta, A.; Levine, J.M.; Kofke, W.A.; Zager, E.; Yang, W.; Leroux, P. Brain oxygen tension and outcome in patients with aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2008, 109, 1075–1082. [Google Scholar] [CrossRef]
- Väth, A.; Kunze, E.; Roosen, K.; Meixensberger, J. Therapeutic Aspects of Brain Tissue pO2 Monitoring after Subarachnoid Hemorrhage. Acta Neurochir. Suppl. 2002, 81, 307–309. [Google Scholar]
- Oddo, M.; Bösel, J.; the Participants in the International Multidisciplinary Consensus Conference on Multimodality Monitoring. Monitoring of Brain and Systemic Oxygenation in Neurocritical Care Patients. Neurocritical Care 2014, 21, 103–120. [Google Scholar] [CrossRef]
- Lawrence, T.P.; Steel, A.; Ezra, M.; Speirs, M.; Pretorius, P.M.; Douaud, G.; Sotiropoulos, S.; Cadoux-Hudson, T.; Emir, U.E.; Voets, N.L. MRS and DTI evidence of progressive posterior cingulate cortex and corpus callosum injury in the hyper-acute phase after Traumatic Brain Injury. Brain Inj. 2019, 33, 854–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, A.; Xiao, X.H.; Liu, Z.H.; Wan, Z.L.; Wang, Z.Y. A Multimodal Magnetic Resonance Imaging Study of Recovery of Consciousness in Severe Traumatic Brain Injury: Preliminary Results. J. Neurotrauma 2018, 35, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.P.; Pretorius, P.M.; Ezra, M.; Cadoux-Hudson, T.; Voets, N.L. Early detection of cerebral microbleeds following traumatic brain injury using MRI in the hyper-acute phase. Neurosci. Lett. 2017, 655, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Shakir, A.; Aksoy, D.; Mlynash, M.; Harris, O.A.; Albers, G.W.; Hirsch, K.G. Prognostic Value of Quantitative Diffusion-Weighted MRI in Patients with Traumatic Brain Injury. J. Neuroimaging 2015, 26, 103–108. [Google Scholar] [CrossRef]
- De Marchis, G.M.; Filippi, C.G.; Guo, X.; Pugin, D.; Gaffney, C.D.; Dangayach, N.S.; Suwatcharangkoon, S.; Falo, M.C.; Schmidt, J.M.; Agarwal, S.; et al. Brain Injury Visible on Early MRI After Subarachnoid Hemorrhage Might Predict Neurological Impairment and Functional Outcome. Neurocritical Care 2014, 22, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Coles, J.P.; Steiner, L.A.; Martin, J.; Donovan, T.; Hutchinson, P.J.; Carpenter, T.A.; Menon, D.K. Assessment of the Ventrix parenchymal intracranial pressure monitoring probe (NL950-P) and Monitor (NL950-100) in a 3 Tesla magnetic resonance scanner. Anaesthesia 2003, 58, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Scheel, M.; Dreier, J.P.; Bohner, G. Evaluation of Intracranial Electrocorticography Recording Strips and Tissue Partial Pressure of Oxygen and Temperature Probes for Radio-Frequency-Induced Heating. Acta Neurochir. Suppl. 2012, 115, 149–152. [Google Scholar] [CrossRef]
- Ercole, A. Radiofrequency properties of two different Licox® parenchymal brain tissue oxygen probe designs. Magn. Reson. Imaging 2018, 49, 159–160. [Google Scholar] [CrossRef]
- Davis, L.M.; Spencer, D.D.; Spencer, S.S.; A Bronen, R. MR imaging of implanted depth and subdural electrodes: Is it safe? Epilepsy Res. 1999, 35, 95–98. [Google Scholar] [CrossRef]
- Carmichael, D.W.; Thornton, J.; Rodionov, R.; Thornton, R.; McEvoy, A.; Allen, P.J.; Lemieux, L. Safety of localizing epilepsy monitoring intracranial electroencephalograph electrodes using MRI: Radiofrequency-induced heating. J. Magn. Reson. Imaging 2008, 28, 1233–1244. [Google Scholar] [CrossRef] [Green Version]

| Patient-# | Sex | Age at Trauma | ICP | PbtO2 | EVD | Surgery | Lesion | Number of MRIs with Probes | Probes Localization | Size of Lesion (Length × with × Height, in mm) | GOS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | male | 35 | yes | yes | no | Open/tunneled | No Lesion | 1 | Frontal, left | 4 | |
| 2 | male | 27 | yes | no | no | Bolt | Lesion | 3 | Frontal, right | 9 × 10 × 7 | 4 |
| 3 | male | 49 | yes | yes | no | Open/tunneled | Lesion | 2 | Frontal, left | 5 × 3 × 3 | 5 |
| 4 | female | 47 | yes | yes | no | Open/tunneled | No Lesion | 1 | Frontal, right | 5 | |
| 5 | male | 60 | yes | yes | no | Open/tunneled | Probe trajectory | 1 | Frontal, right | 5 | |
| 6 | male | 20 | yes | no | no | Bolt | Lesion | 1 | Frontal, right | 5 × 3 × 2 | 5 |
| 7 | male | 41 | yes | yes | no | Open/tunneled | Probe trajectory | 1 | Frontal, right | 4 | |
| 8 | male | 56 | yes | yes | no | Open/tunneled | No Lesion | 1 | Frontal, left | 5 | |
| 9 | male | 55 | yes | yes | no | Bolt | Lesion | 1 | Frontal, right | 3 × 2 × 2 | 1 |
| 10 | male | 74 | yes | yes | no | Open/tunneled | Lesion | 2 | Frontal, right | 5 × 5 × 5 | 5 |
| 11 | female | 49 | yes | no | no | Open/tunneled | No Lesion | 2 | Frontal, right | 4 | |
| 12 | male | 32 | yes | no | no | Open/tunneled | No Lesion | 1 | Frontal, left | 5 | |
| 13 | male | 46 | yes | yes | no | Open/tunneled | No Lesion | 2 | Frontal, right | 3 | |
| 14 | male | 34 | yes | yes | no | Open/tunneled | Lesion | 2 | Frontal, right | 4 × 3 × 2 | 4 |
| 15 | male | 29 | yes | yes | yes | Open/tunneled | Probe trajectory | 1 | Frontal, right | 5 | |
| 16 | male | 21 | yes | yes | no | Open/tunneled | Probe trajectory | 1 | Frontal, right | 5 | |
| 17 | female | 69 | yes | yes | no | Open/tunneled | Probe trajectory | 2 | Frontal, right | 4 | |
| 18 | male | 30 | yes | no | no | Open/tunneled | Lesion | 1 | Frontal, left | 3 × 2 × 1 | 4 |
| 19 | male | 40 | yes | no | no | Open/tunneled | Probe trajectory | 1 | Frontal, right | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinggera, D.; Rhomberg, P.; Beer, R.; Thomé, C.; Petr, O. Brain Tissue Damage Induced by Multimodal Neuromonitoring In Situ during MRI after Severe Traumatic Brain Injury: Incidence and Clinical Relevance. J. Clin. Med. 2022, 11, 3169. https://doi.org/10.3390/jcm11113169
Pinggera D, Rhomberg P, Beer R, Thomé C, Petr O. Brain Tissue Damage Induced by Multimodal Neuromonitoring In Situ during MRI after Severe Traumatic Brain Injury: Incidence and Clinical Relevance. Journal of Clinical Medicine. 2022; 11(11):3169. https://doi.org/10.3390/jcm11113169
Chicago/Turabian StylePinggera, Daniel, Paul Rhomberg, Ronny Beer, Claudius Thomé, and Ondra Petr. 2022. "Brain Tissue Damage Induced by Multimodal Neuromonitoring In Situ during MRI after Severe Traumatic Brain Injury: Incidence and Clinical Relevance" Journal of Clinical Medicine 11, no. 11: 3169. https://doi.org/10.3390/jcm11113169
APA StylePinggera, D., Rhomberg, P., Beer, R., Thomé, C., & Petr, O. (2022). Brain Tissue Damage Induced by Multimodal Neuromonitoring In Situ during MRI after Severe Traumatic Brain Injury: Incidence and Clinical Relevance. Journal of Clinical Medicine, 11(11), 3169. https://doi.org/10.3390/jcm11113169






