Predictors of Kidney Delayed Graft Function and Its Prognostic Impact following Combined Liver–Kidney Transplantation: A Recent Single-Center Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Endpoints
2.2. Hypothermic Pulsatile Machine Perfusion
2.3. Transplant Protocol
2.4. Statistical Analysis
3. Results
3.1. Distributions of Baseline Characteristics
3.2. Distributions of Outcome Variables
3.3. Univariable Comparisons of Baseline Variables between kIGF and kDGF
3.4. Univariable Comparisons of Outcome Variables between kIGF and kDGF
3.5. Multivariable Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiesner, R.H.; McDiarmid, S.V.; Kamath, P.S.; Edwards, E.B.; Malinchoc, M.; Kremers, W.K.; Krom, R.A.; Kim, W.R. MELD and PELD: Application of survival models to liver allocation. Liver Transplant. 2001, 7, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Ekser, B.; Contreras, A.G.; Andraus, W.; Taner, T. Current status of combined liver-kidney transplantation. Int. J. Surg. 2020, 82, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Simpson, N.; Cho, Y.W.; Cicciarelli, J.C.; Selby, R.R.; Fong, T.-L. Comparison of Renal Allograft Outcomes in Combined Liver-Kidney Transplantation Versus Subsequent Kidney Transplantation in Liver Transplant Recipients: Analysis of UNOS Database. Transplantation 2006, 82, 1298–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fong, T.-L.; Khemichian, S.; Shah, T.; Hutchinson, I.V.; Cho, Y.W. Combined Liver-Kidney Transplantation Is Preferable to Liver Transplant Alone for Cirrhotic Patients with Renal Failure. Transplantation 2012, 94, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Creput, C.; Durrbach, A.; Samuel, D.; Eschwege, P.; Amor, M.; Kriaa, F.; Kreis, H.; Benoit, G.; Bismuth, H.; Charpentier, B. Incidence of Renal and Liver Rejection and Patient Survival Rate Following Combined Liver and Kidney Transplantation. Am. J. Transplant. 2003, 3, 348–356. [Google Scholar] [CrossRef]
- Taner, T.; Heimbach, J.K.; Rosen, C.B.; Nyberg, S.L.; Park, W.; Stegall, M.D. Decreased chronic cellular and antibody-mediated injury in the kidney following simultaneous liver-kidney transplantation. Kidney Int. 2016, 89, 909–917. [Google Scholar] [CrossRef] [Green Version]
- Ekser, B.; Mangus, R.; Fridell, J.A.; Kubal, C.; Nagai, S.; Kinsella, S.B.; Bayt, D.R.; Bell, T.; Powelson, J.A.; Goggins, W.C.; et al. A Novel Approach in Combined Liver and Kidney Transplantation With Long-term Outcomes. Ann. Surg. 2017, 265, 1000–1008. [Google Scholar] [CrossRef]
- Korayem, I.M.; Agopian, V.G.; Lunsford, K.; Gritsch, H.A.; Veale, J.L.; Lipshutz, G.S.; Yersiz, H.; Serrone, C.L.; Kaldas, F.M.; Farmer, U.G.; et al. Factors predicting kidney delayed graft function among recipients of simultaneous liver-kidney transplantation: A single-center experience. Clin. Transplant. 2019, 33, e13569. [Google Scholar] [CrossRef]
- Lunsford, K.E.; Bodzin, A.S.; Markovic, D.; Zarrinpar, A.; Kaldas, F.M.; Gritsch, H.A.; Xia, V.; Farmer, D.G.; Danovitch, G.M.; Hiatt, J.R.; et al. Avoiding Futility in Simultaneous Liver-kidney Transplantation. Ann. Surg. 2017, 265, 1016–1024. [Google Scholar] [CrossRef]
- Weeks, S.R.; Luo, X.; Haugen, C.E.; Ottmann, S.E.; Gurakar, A.O.; Naqvi, F.F.; Alqahtani, S.A.; Philosophe, B.; Cameron, A.M.; Desai, N.M.; et al. Delayed Graft Function in Simultaneous Liver Kidney Transplantation. Transplantation 2020, 104, 542–550. [Google Scholar] [CrossRef]
- Mannon, R.B. Delayed Graft Function: The AKI of Kidney Transplantation. Nephron 2018, 140, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Wadei, H.M.; Bulatao, I.G.; Gonwa, T.A.; Mai, M.L.; Prendergast, M.; Keaveny, A.P.; Rosser, B.G.; Taner, C.B. Inferior long-term outcomes of liver-kidney transplantation using donation after cardiac death donors: Single-center and organ procurement and transplantation network analyses. Liver Transplant. 2014, 20, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Lunsford, K.; Agopian, V.G.; Yi, S.G.; Nguyen, D.T.; Graviss, E.A.; Harlander-Locke, M.P.; Saharia, A.; Kaldas, F.M.; Mobley, C.M.; Zarrinpar, A.; et al. Delayed Implantation of Pumped Kidneys Decreases Renal Allograft Futility in Combined Liver–Kidney Transplantation. Transplantation 2020, 104, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Jay, C.; Pugh, J.; Halff, G.; Abrahamian, G.; Cigarroa, F.; Washburn, K. Graft quality matters: Survival after simultaneous liver-kidney transplant according to KDPI. Clin. Transplant. 2017, 31, e12933. [Google Scholar] [CrossRef] [PubMed]
- Bronzatto, E.; Quadros, K.D.S.; Santos, R.; Alves-Filho, G.; Mazzali, M. Delayed Graft Function in Renal Transplant Recipients: Risk Factors and Impact on 1-Year Graft Function: A Single Center Analysis. Transplant. Proc. 2009, 41, 849–851. [Google Scholar] [CrossRef] [PubMed]
- De Sandes-Freitas, T.V.; Felipe, C.R.; Aguiar, W.; Cristelli, M.P.; Tedesco-Silva, H.; Pestana, J.M. Prolonged Delayed Graft Function Is Associated with Inferior Patient and Kidney Allograft Survivals. PLoS ONE 2015, 10, e0144188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedesco-Silva, H.; Offerni, J.C.M.; Carneiro, V.A.; De Paula, M.I.; Neto, E.D.; Lemos, F.B.C.; Moura, L.R.R.; Filho, A.P.E.S.; Cunha, M.D.F.D.M.; Da Silva, E.F.; et al. Randomized Trial of Machine Perfusion Versus Cold Storage in Recipients of Deceased Donor Kidney Transplants With High Incidence of Delayed Graft Function. Transplant. Direct 2017, 3, e155. [Google Scholar] [CrossRef]
- Irish, W.D.; Ilsley, J.N.; Schnitzler, M.A.; Feng, S.; Brennan, D.C. A Risk Prediction Model for Delayed Graft Function in the Current Era of Deceased Donor Renal Transplantation. Am. J. Transplant. 2010, 10, 2279–2286. [Google Scholar] [CrossRef]
- Tingle, S.J.; Figueiredo, R.S.; Moir, J.A.; Goodfellow, M.; Talbot, D.; Wilson, C.H. Machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst. Rev. 2019, 3, CD011671. [Google Scholar] [CrossRef]
- Hameed, A.M.; Pleass, H.C.; Wong, G.; Hawthorne, W.J. Maximizing kidneys for transplantation using machine perfusion: From the past to the future A comprehensive systematic review and meta-analysis. Medicine 2016, 95, e5083. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Roufosse, C.; Simmonds, N.; Groningen, M.C.-V.; Haas, M.; Henriksen, K.J.; Horsfield, C.; Loupy, A.; Mengel, M.; Perkowska-Ptasińska, A.; Rabant, M.; et al. A 2018 Reference Guide to the Banff Classification of Renal Allograft Pathology. Transplantation 2018, 102, 1795–1814. [Google Scholar] [CrossRef] [PubMed]
- Demetris, A.J.; Batts, K.P.; Dhillon, A.P.; Ferrell, L.; Fung, J.; Geller, S.A.; Hart, J.; Hayry, P.; Hofmann, W.J.; Hubscher, S.; et al. Banff schema for grading liver allograft rejection: An international consensus document. Hepatology 1997, 25, 658–663. [Google Scholar] [CrossRef]
- Reed, D.; Kemmerly, S.A. Infection Control and prevention: A review of hospital-acquired infections and the economic impli-cations. Ochsner J. 2009, 9, 27–31. [Google Scholar] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Slankamenac, K.; Graf, R.; Barkun, J.; Puhan, M.A.; Clavien, P.-A. The Comprehensive Complication Index. Ann. Surg. 2013, 258, 1–7. [Google Scholar] [CrossRef] [Green Version]
- EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: Long-term management of the transplant recipient. IV.13 Analysis of patient and graft survival. Nephrol. Dial. Transplant. 2002, 17 (Suppl. 4), 60–67. [Google Scholar]
- Angeli, P.; Gines, P.; Wong, F.; Bernardi, M.; Boyer, T.D.; Gerbes, A.L.; Moreau, R.; Jalan, R.; Sarin, S.K.; Piano, S.; et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites. Gut 2015, 64, 531–537. [Google Scholar] [CrossRef] [Green Version]
- Organ Recovery Systems Home Page. Available online: http://www.organ-recovery.com (accessed on 1 April 2022).
- Ciancio, G.; Gaynor, J.J.; Sageshima, J.; Roth, D.; Kupin, W.; Guerra, G.; Tueros, L.; Zarak, A.; Hanson, L.; Ganz, S.; et al. Machine perfusion following static cold storage preservation in kidney transplantation: Donor-matched pair analysis of the prognostic impact of longer pump time. Transpl. Int. 2011, 25, 34–40. [Google Scholar] [CrossRef]
- Ciancio, G.; Gaynor, J.J.; Sageshima, J.; Chen, L.; Roth, D.; Kupin, W.; Guerra, G.; Tueros, L.; Zarak, A.; Hanson, L.; et al. Favorable Outcomes With Machine Perfusion and Longer Pump Times in Kidney Transplantation: A Single-Center, Observational Study. Transplantation 2010, 90, 882–890. [Google Scholar] [CrossRef]
- Mangus, R.S.; Fridell, J.A.; Vianna, R.M.; Kwo, P.Y.; Chen, J.; Tector, A.J. Immunosuppression induction with rabbit anti-thymocyte globulin with or without rituximab in 1000 liver transplant patients with long-term follow-up. Liver Transplant. 2012, 18, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Ciancio, G.; Farag, A.; Gonzalez, J.; Vincenzi, P.; Gaynor, J.J. Results of a previously unreported extravesical ureteroneocystostomy technique without ureteral stenting in 500 consecutive kidney transplant recipients. PLoS ONE 2021, 16, e0244248. [Google Scholar] [CrossRef] [PubMed]
- Sageshima, J.; Ciancio, G.; Gaynor, J.J.; Chen, L.; Guerra, G.; Kupin, W.; Roth, D.; Ruiz, P.; Burke, G.W. Addition of anti-CD25 to thymoglobulin for induction therapy: Delayed return of peripheral blood CD25-positive population. Clin. Transplant. 2010, 25, E132–E135. [Google Scholar] [CrossRef] [PubMed]
- Sageshima, J.; Ciancio, G.; Guerra, G.; Gaynor, J.J.; Cova, D.; Zarak, A.; Chen, L.; Mattiazzi, A.; Kupin, W.; Roth, D.; et al. Prolonged lymphocyte depletion by single-dose rabbit anti-thymocyte globulin and alemtuzumab in kidney transplantation. Transpl. Immunol. 2011, 25, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Ciancio, G.; Gaynor, J.J.; Guerra, G.; Sageshima, J.; Roth, D.; Chen, L.; Kupin, W.; Mattiazzi, A.; Tueros, L.; Flores, S.; et al. Randomized trial of rATg/Daclizumab vs. rATg/Alemtuzumab as dual induction therapy in renal transplantation: Results at 8 years of follow-up. Transpl. Immunol. 2017, 40, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Spagnoletti, G.; Salerno, M.P.; Calia, R.; Romagnoli, J.; Citterio, F. Thymoglobuline plus basiliximab a mixed cocktail to start? Transpl. Immunol. 2017, 43–44, 1–2. [Google Scholar] [CrossRef]
- Li, J.; Koch, M.; Kramer, K.; Kloth, K.; Abu Ganim, A.R.; Scheidat, S.; Rinninger, F.; Thaiss, F.; Gulati, A.; Herden, U.; et al. Dual antibody induction and de novo use of everolimus enable low-dose tacrolimus with early corticosteroid withdrawal in simultaneous pancreas-kidney transplantation. Transpl. Immunol. 2018, 50, 26–33. [Google Scholar] [CrossRef]
- Ruggenenti, P.; Codreanu, I.; Cravedi, P.; Perna, A.; Gotti, E.; Remuzzi, G. Basiliximab Combined with Low-Dose Rabbit Anti-Human Thymocyte Globulin: A Possible Further Step toward Effective and Minimally Toxic T Cell–Targeted Therapy in Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2006, 1, 546–554. [Google Scholar] [CrossRef] [Green Version]
- Rao, P.S.; Schaubel, D.E.; Guidinger, M.K.; Andreoni, K.A.; Wolfe, R.A.; Merion, R.M.; Port, F.K.; Sung, R.S. A Comprehensive Risk Quantification Score for Deceased Donor Kidneys: The Kidney Donor Risk Index. Transplantation 2009, 88, 231–236. [Google Scholar] [CrossRef]
- Feng, S.; Goodrich, N.; Bragg-Gresham, J.; Dykstra, D.; Punch, J.; DebRoy, M.; Greenstein, S.; Merion, R. Characteristics Associated with Liver Graft Failure: The Concept of a Donor Risk Index. Am. J. Transplant. 2006, 6, 783–790, Erratum in 2018, 18, 3085. [Google Scholar] [CrossRef] [Green Version]
- Kox, J.; Moers, C.; Monbaliu, D.; Strelniece, A.; Treckmann, J.; Jochmans, I.; Leuvenink, H.; Van Heurn, E.; Pirenne, J.; Paul, A.; et al. The Benefits of Hypothermic Machine Preservation and Short Cold Ischemia Times in Deceased Donor Kidneys. Transplantation 2018, 102, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Debout, A.; Foucher, Y.; Trébern-Launay, K.; Legendre, C.; Kreis, H.; Mourad, G.; Garrigue, V.; Morelon, E.; Buron, F.; Rostaing, L.; et al. Each additional hour of cold ischemia time significantly increases the risk of graft failure and mortality following renal transplantation. Kidney Int. 2015, 87, 343–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Vliet, J.A.; Warlé, M.C. The need to reduce cold ischemia time in kidney transplantation. Curr. Opin. Organ Transplant. 2013, 18, 174–178. [Google Scholar] [CrossRef]
- van der Vliet, J.A.; Warlé, M.C.; Cheung, C.L.S.; Teerenstra, S.; Hoitsma, A.J. Influence of prolonged cold ischemia in renal transplantation. Clin. Transplant. 2011, 25, E612–E616. [Google Scholar] [CrossRef]
- Port, F.K.; Bragg-Gresham, J.L.; Metzger, R.A.; Dykstra, D.M.; Gillespie, B.W.; Young, E.W.; Delmonico, F.L.; Wynn, J.J.; Merion, R.M.; Wolfe, R.A.; et al. Donor characteristics associated with reduced graft survival: An approach to expanding the pool of kidney donors. Transplantation 2002, 74, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Ekser, B.; Mangus, R.; Kubal, C.; Powelson, J.A.; Fridell, J.A.; Goggins, W.C. Excellent outcomes in combined liver-kidney transplantation: Impact of kidney donor profile index and delayed kidney transplantation. Liver Transplant. 2018, 24, 222–232. [Google Scholar] [CrossRef]
- Sharma, P.; Sui, Z.; Zhang, M.; Magee, J.C.; Barman, P.; Patel, Y.; Schluger, A.; Walter, K.; Biggins, S.W.; Cullaro, G.; et al. Renal Outcomes After Simultaneous Liver-Kidney Transplantation: Results from the US Multicenter Simultaneous Liver-Kidney Transplantation Consortium. Liver Transplant. 2021, 27, 1144–1153. [Google Scholar] [CrossRef]
- Nascimento, P.R.C.D.; Poitras, S.; Bilodeau, M. How do we define and measure sarcopenia? Protocol for a systematic review. Syst. Rev. 2018, 7, 51. [Google Scholar] [CrossRef] [Green Version]
- Willicombe, M.; Rizzello, A.; Goodall, D.; Papalois, V.; McLean, A.G.; Taube, D. Risk factors and outcomes of delayed graft function in renal transplant recipients receiving a steroid sparing immunosuppression protocol. World J. Transplant. 2017, 7, 34–42. [Google Scholar] [CrossRef]
- Miglinas, M.; Supranaviciene, L.; Mateikaite, K.; Skebas, K.; Kubiliene, A. Delayed Graft Function: Risk Factors and the Effects of Early Function and Graft Survival. Transplant. Proc. 2013, 45, 1363–1367. [Google Scholar] [CrossRef]
- Hibi, T.; Sageshima, J.; Molina, E.; Ciancio, G.; Nishida, S.; Chen, L.; Arosemena, L.; Mattiazzi, A.; Guerra, G.; Kupin, W.; et al. Predisposing Factors of Diminished Survival in Simultaneous Liver/Kidney Transplantation. Am. J. Transplant. 2012, 12, 2966–2973. [Google Scholar] [CrossRef] [PubMed]

| kIGF Group (n = 72) | kDGF Group (n = 43) | p Value | |
|---|---|---|---|
| Recipient data | |||
| Age (years), mean ± SE | 60.4 ± 1.1 | 58.4 ± 1.6 | 0.31 |
| Male, % (n) | 54.2 (39) | 69.8 (30) | 0.10 |
| BMI (kg/m2), mean ± SE | 28.9 ± 0.6 | 25.8 ± 0.6 | 0.002 |
| Race, % (n) | 0.48 | ||
| Afro-American | 12.5 (9) | 16.3 (7) | |
| Hispanic | 29.2 (21) | 34.9 (15) | |
| Caucasian | 56.9 (41) | 44.2 (19) | |
| Other | 1.4 (1) | 4.7 (2) | |
| MELD score at listing, mean ± SE | 28.8 ± 0.8 | 28.6 ± 1.1 | 0.83 |
| MELD score at transplant, mean ± SE | 28.4 ± 0.9 | 28.7 ± 1.1 | 0.80 |
| Waitlist time(days), geometric mean */SE | 65.1 */1.2 | 61.6 */1.2 | 0.84 |
| DM, % (n) | 58.3 (42) | 69.8 (30) | 0.22 |
| HTN, % (n) | 61.1 (44) | 60.5 (26) | 0.95 |
| Liver cirrhosis etiology, % (n) | |||
| Alcoholic | 18.1 (13) | 23.3 (10) | 0.50 |
| NASH | 33.3 (24) | 34.9 (15) | 0.87 |
| Hepatitis C | 26.4 (19) | 14.0 (6) | 0.12 |
| Cryptogenic/Autoimmune | 18.1 (13) | 16.2 (7) | 0.81 |
| HCC | 15.3 (11) | 9.3 (4) | 0.36 |
| Other ° | 12.5 (9) | 14.0 (6) | 0.82 |
| Combination of ≥ 2 etiologic agents | 6.9 (5) | 4.6 (2) | 0.61 |
| ESKD etiology, % (n) | |||
| HRS | 41.7 (30) | 39.5 (17) | 0.82 |
| Metabolic (DM and/or HTN) | 52.8 (38) | 55.8 (24) | 0.75 |
| ADPKD | 12.5 (9) | 11.6 (5) | 0.89 |
| Glomerulonephritis | 4.2 (3) | 11.6 (5) | 0.13 |
| Other °° | 23.6 (17) | 16.3 (7) | 0.35 |
| Combination of ≥ 2 etiologic agents | 37.5 (27) | 30.2 (13) | 0.42 |
| Timing of Kidney Failure, % (n) | |||
| AKI | 9.7 (7) | 11.6 (5) | 0.75 |
| AKI on CKD | 30.6 (22) | 27.9 (12) | 0.76 |
| CKD | 59.7 (43) | 60.5 (26) | 0.94 |
| Dialysis modality | |||
| Pre-emptive, % (n) | 36.1 (26) | 7.0 (3) | 0.0005 |
| Hemodialysis, % (n) | 63.9 (46) | 93.0 (40) | 0.0005 |
| HD duration (days), mean ± SE | 268.4 ± 68.1 | 585.9 ± 124.6 | 0.02 |
| Pre-transplant CVVHD, % (n) | 13.9 (10) | 27.9 (12) | 0.06 |
| Liver re-transplant, % (n) | 5.6 (4) | 4.7 (2) | 0.83 |
| Kidney re-transplant, % (n) | 1.4 (1) | 9.3 (4) | 0.04 |
| Preoperative hospitalization, % (n) | 26.4 (19) | 39.5 (17) | 0.14 |
| Hospitalization duration(days), mean ± SE | 6.4 ± 1.9 | 9.0 ± 2.7 | 0.42 |
| Preoperative ICU stay, % (n) | 19.4 (14) | 32.6 (14) | 0.11 |
| ICU stay duration (days), mean ± SE | 2.4 ± 0.8 | 4.4 ± 1.7 | 0.22 |
| RRI score, geometric mean */SE | 7.4 */1.1 | 9.3 */1.1 | 0.08 |
| cPRA ≥ 40%, % (n) | 33.3 (24) | 25.6 (11) | 0.38 |
| Preformed DSAs (class I or II), % (n) | 16.7 (12) | 20.9 (9) | 0.57 |
| Positive Crossmatch, % (n) | 13.9 (10) | 20.9 (9) | 0.33 |
| kIGF Group (n = 72) | kDGF Group (n = 43) | p-Value | |
|---|---|---|---|
| Donor data | |||
| Age (years), mean ± SE | 36.6 ± 1.8 | 41.4 ± 2.1 | 0.10 |
| BMI (kg/m2), mean ± SE | 25.3 ± 0.5 | 26.6 ± 0.9 | 0.17 |
| Race, % (n) | 0.41 | ||
| Afro-American | 16.7 (12) | 18.6 (8) | |
| Hispanic | 27.8 (20) | 34.9 (15) | |
| Caucasian | 55.6 (40) | 44.2 (19) | |
| Other | 0 (0) | 2.3 (1) | |
| DCD, % (n) | 8.3 (6) | 30.2 (13) | 0.002 |
| KDPI (%), mean ± SE | 37.7 ± 3.0 | 46.2 ± 4.2 | 0.10 |
| ECD, % (n) | 9.7 (7) | 18.6 (8) | 0.17 |
| DRI, mean ± SE | 1.37 ± 0.04 | 1.55 ± 0.06 | 0.01 |
| DM, % (n) | 2.8 (2) | 0.0 (0) | 0.27 |
| HTN, % (n) | 11.1 (8) | 25.6 (11) | 0.04 |
| Cause of death, % (n) | |||
| Anoxia | 38.9 (28) | 41.9 (18) | 0.75 |
| Stroke | 18.1 (13) | 23.3 (10) | 0.50 |
| Head Trauma | 41.7 (30) | 34.9 (15) | 0.47 |
| Other ° | 1.3 (1) | 0.0 (0) | 0.44 |
| Vasopressor support (n), % (n) | 0.03 | ||
| 0 | 6.9 (5) | 25.6 (11) | |
| 1 | 33.3 (24) | 18.6 (8) | |
| 2 | 45.8 (33) | 37.2 (16) | |
| 3 | 13.9 (10) | 18.6 (8) | |
| Terminal UO (mL/min), mean ± SE | 146.3 ± 16.3 | 136.6 ± 11.2 | 0.67 |
| Terminal sCr (mg/dL), geometric mean */SE | 0.77 */1.05 | 0.89 */1.09 | 0.12 |
| Pre-implant kidney biopsy, % (n) | 83.3 (60) | 90.7 (39) | 0.27 |
| (1) Glomerulosclerosis, mean ± SE | 5.4 ± 0.7 | 4.2 ± 0.6 | 0.23 |
| (2) Chronic Tubulo-Interstitial Injury | 0.46 | ||
| None, % (n) | 31.7 (19) | 20.5 (8) | |
| Minimal, % (n) | 16.7 (10) | 15.4 (6) | |
| Mild, % (n) | 50.0 (30) | 64.1 (25) | |
| Moderate, % (n) | 1.7 (1) | 0.0 (0) | |
| (3) Arteriolosclerosis | 0.13 | ||
| None, % (n) | 38.3 (23) | 25.6 (10) | |
| Minimal, % (n) | 5.0 (3) | 17.9 (7) | |
| Mild, % (n) | 55.0 (33) | 56.4 (22) | |
| Moderate, % (n) | 1.7 (1) | 0.0 (0) | |
| (4) Acute Tubular Necrosis | 0.41 | ||
| None, % (n) | 3.3 (2) | 0.0 (0) | |
| Minimal, % (n) | 1.7 (1) | 2.6 (1) | |
| Mild, % (n) | 86.7 (52) | 94.9 (37) | |
| Moderate, % (n) | 8.3 (5) | 2.6 (1) | |
| HPMP data | |||
| Timing of initiation (hr), mean ± SE | 3.93 ± 0.31 | 3.95 ± 0.29 | 0.96 |
| Duration of perfusion (hr), mean ± SE | 16.83 ± 1.23 | 24.88 ± 1.77 | 0.0002 |
| Initial Flows (mL/min), mean ± SE | 52.3 ± 4.7 | 60.2 ± 7.5 | 0.35 |
| Initial Resistance (mmHg/mL/min), mean ± SE | 0.74 ± 0.05 | 0.62 ± 0.05 | 0.15 |
| Final Flows (mL/min), mean ± SE | 153.1 ± 2.9 | 150.3 ± 4.4 | 0.59 |
| Final Resistance (mmHg/mL/min), mean ± SE | 0.20 ± 0.01 | 0.23 ± 0.01 | 0.02 |
| kIGF Group (n = 72) | kDGF Group (n = 43) | p-Value | |
|---|---|---|---|
| Time from LT to KT (hr), mean ± SE | 11.4 ± 1.2 | 19.5 ± 1.8 | 0.0002 |
| Delayed KT, % (n) | 62.5 (45) | 81.4 (35) | 0.03 |
| CIT liver (hr), mean ± SE | 5.4 ± 0.1 | 5.1 ± 0.2 | 0.38 |
| WIT liver (min), mean ± SE | 26.3 ± 0.7 | 27.7 ± 0.7 | 0.17 |
| CIT kidney (hr), mean ± SE | 20.7 ± 1.2 | 28.8 ± 1.8 | 0.0002 |
| WIT kidney (min), mean ± SE | 29.8 ± 1.0 | 29.9 ± 1.6 | 0.95 |
| PRBC units during LT, mean ± SE | 10.5 ± 1.1 | 19.2 ± 3.6 | 0.006 |
| PRBC units during KT, mean ± SE | 2.4 ± 0.2 | 3.3 ± 0.4 | 0.04 |
| Vasopressor support, n (%) | 59.7 (43) | 65.1 (28) | 0.56 |
| kIGF Group (n = 72) | kDGF Group (n = 43) | p-Value | |
|---|---|---|---|
| Vasopressor support, % (n) | 52.8 (38) | 60.5 (26) | 0.42 |
| sCr (mg/dL), geometric mean */SE ° | |||
| at 1 mo post-transplant | 0.88 */1.04 (n = 72) | 1.72 */1.13 (n = 39) | <0.000001 |
| at 3 mo post-transplant | 0.95 */1.04 (n = 72) | 1.35 */1.12 (n = 36) | 0.0004 |
| at 6 mo post-transplant | 1.01 */1.03 (n = 70) | 1.31 */1.08 (n = 32) | 0.0005 |
| at 12 mo post-transplant | 1.05 */1.03 (n = 69) | 1.35 */1.08 (n = 23) | 0.0006 |
| MDRD-eGFR (mL/min/1.73 m2), mean ± SE ° | |||
| at 1 mo post-transplant | 84.2 ± 3.1 | 50.4 ± 6.0 | <0.000001 |
| at 3 mo post-transplant | 79.2 ± 3.1 | 61.8 ± 5.6 | 0.004 |
| at 6 mo post-transplant | 73.3 ± 2.7 | 61.9 ± 5.2 | 0.03 |
| at 12 mo post-transplant | 69.1 ± 2.4 | 57.9 ± 4.6 | 0.03 |
| CKD stage ≥ 3B (eGFR < 45 mL/min/1.73 m2), % (n) ° | |||
| at 1 mo post-transplant | 6.9 (5/72) | 66.7 (26/39) | <0.000001 |
| at 3 mo post-transplant | 6.9 (5/72) | 36.1 (13/36) | 0.0001 |
| at 6 mo post-transplant | 10.0 (7/70) | 31.3 (10/32) | 0.008 |
| at 12 mo post-transplant | 10.1 (7/69) | 30.4 (7/23) | 0.02 |
| RAF, % (n) | 0.0 (0) | 27.9 (12) | 0.000002 |
| kPNF, % (n) | 0.0 (0) | 18.6 (8) | 0.0001 |
| Kidney BPAR, % (n) | 4.2 (3) | 9.3 (4) | 0.27 |
| Induction treatment | 0.002 | ||
| Anti-thymocyte globulin doses, % (n) | |||
| 1 | 4.2 (3) | 25.6 (11) | |
| 2 | 5.6 (4) | 9.3 (4) | |
| 3 | 90.3 (65) | 65.1 (28) | 0.0009 |
| Death-censored renal allograft failure, % (n) | 4.2 (3) | 25.6 (11) | 0.00004 2 |
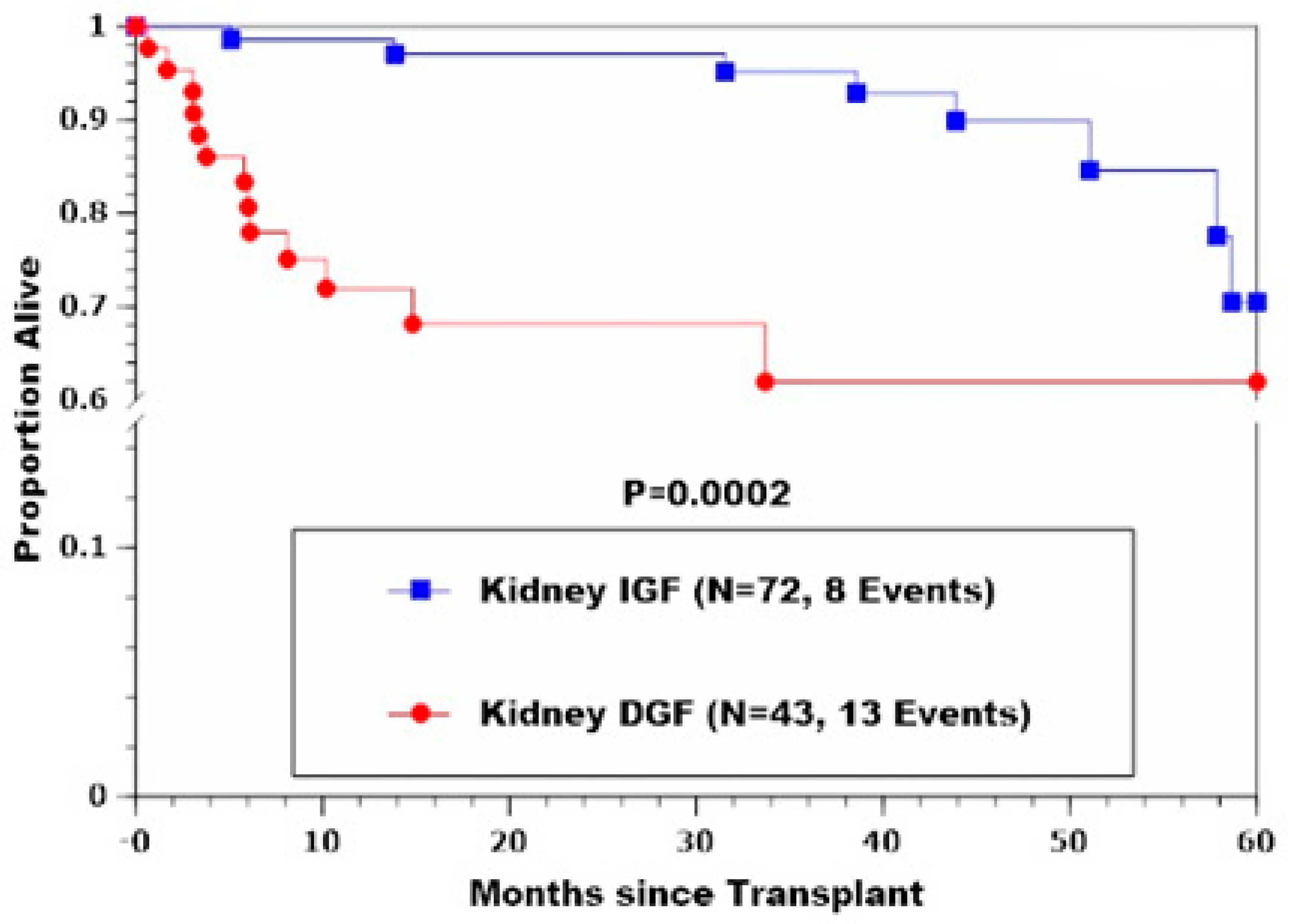
| Death, % (n) | 11.1 (8) | 30.2 (13) | 0.0002 2 |
| Morbidity | |||
| Clavien-Dindo grade | <0.000001 | ||
| I, % (n) | 6.9 (5) | 0.0 (0) | |
| II, % (n) | 51.4 (37) | 14.0 (6) | |
| IIIA, % (n) | 6.9 (5) | 7.0 (3) | |
| IIIB, % (n) | 22.2 (16) | 9.3 (4) | |
| IVA, % (n) | 9.7 (7) | 32.6 (14) | |
| IVB, % (n) | 2.8 (2) | 18.6 (8) | |
| V, % (n) | 0.0 (0) | 18.6 (8) | |
| Clavien-Dindo grade ≥ III, % (n) | 41.7 (30) | 86.0 (37) | 0.000003 |
| CCI, mean ± SE | 39.2 ± 2.2 | 71.9 ± 3.7 | <0.000001 |
| Hospital-acquired infections, % (n) | 37.5 (27) | 67.4 (29) | 0.002 |
| Length of hospital stay (days), geometric mean */SE | 16.5 */1.09 | 45.1 */1.14 | <0.000001 |
| p-Value | Logistic Regression Model Coefficient ± SE |
| Recipient BMI | 0.006 | −0.142 ± 0.055 |
| Pre-transplant HD | 0.0003 | 2.489 ± 0.766 |
| Time from LT to KT | 0.0003 | 0.084 ± 0.025 |
| DCD graft | 0.007 | 1.829 ± 0.709 |
| Donor age | 0.003 | 0.052 ± 0.019 |
| p-value | Linear Regression Model Coefficient ± SE |
| Donor age | 0.007 | −0.108 ± 0.039 |
| Delayed KT | <0.000001 | 18.876 ± 1.329 |
| PRBC units during LT | 0.007 | 0.102 ± 0.037 |
| Postoperative vasopressor support | 0.01 | 3.102 ± 1.218 |
| p-value | Linear Regression Model Coefficient ± SE |
| Pre-transplant HD | 0.002 | 14.914 ± 4.701 |
| Time from LT to KT | 0.004 | 0.580 ± 0.195 |
| PRBC units during LT | 0.02 | 0.314 ± 0.134 |
| Postoperative vasopressor support | 0.006 | 12.249 ± 4.370 |
| Positive Association of kDGF with CCI ° | <0.000001 | |
| p-value | Logistic Regression Model Coefficient ± SE |
| Pre-transplant ICU stay | 0.004 | 1.373 ± 0.493 |
| Time from LT to KT | 0.003 | 0.052 ± 0.018 |
| Positive Association of kDGF with hospital-acquired infections °° | 0.05 | |
| p-value | Logistic Regression Model Coefficient ± SE |
| DCD graft | 0.01 | 1.319 ± 0.551 |
| RPM final RIs | 0.0002 | 13.268 ± 4.150 |
| Positive Association of kDGF with CKD stage ≥ 3B at 1 mo °°° | <0.000001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vincenzi, P.; Gaynor, J.J.; Vianna, R.; Ciancio, G. Predictors of Kidney Delayed Graft Function and Its Prognostic Impact following Combined Liver–Kidney Transplantation: A Recent Single-Center Experience. J. Clin. Med. 2022, 11, 2724. https://doi.org/10.3390/jcm11102724
Vincenzi P, Gaynor JJ, Vianna R, Ciancio G. Predictors of Kidney Delayed Graft Function and Its Prognostic Impact following Combined Liver–Kidney Transplantation: A Recent Single-Center Experience. Journal of Clinical Medicine. 2022; 11(10):2724. https://doi.org/10.3390/jcm11102724
Chicago/Turabian StyleVincenzi, Paolo, Jeffrey J. Gaynor, Rodrigo Vianna, and Gaetano Ciancio. 2022. "Predictors of Kidney Delayed Graft Function and Its Prognostic Impact following Combined Liver–Kidney Transplantation: A Recent Single-Center Experience" Journal of Clinical Medicine 11, no. 10: 2724. https://doi.org/10.3390/jcm11102724







