Cardiorespiratory Adaptation to Short-Term Exposure to Altitude vs. Normobaric Hypoxia in Patients with Pulmonary Hypertension
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Design and Interventions
2.3. Assessments
2.4. Index and Reference Test
2.5. Data Analysis and Statistics
3. Results
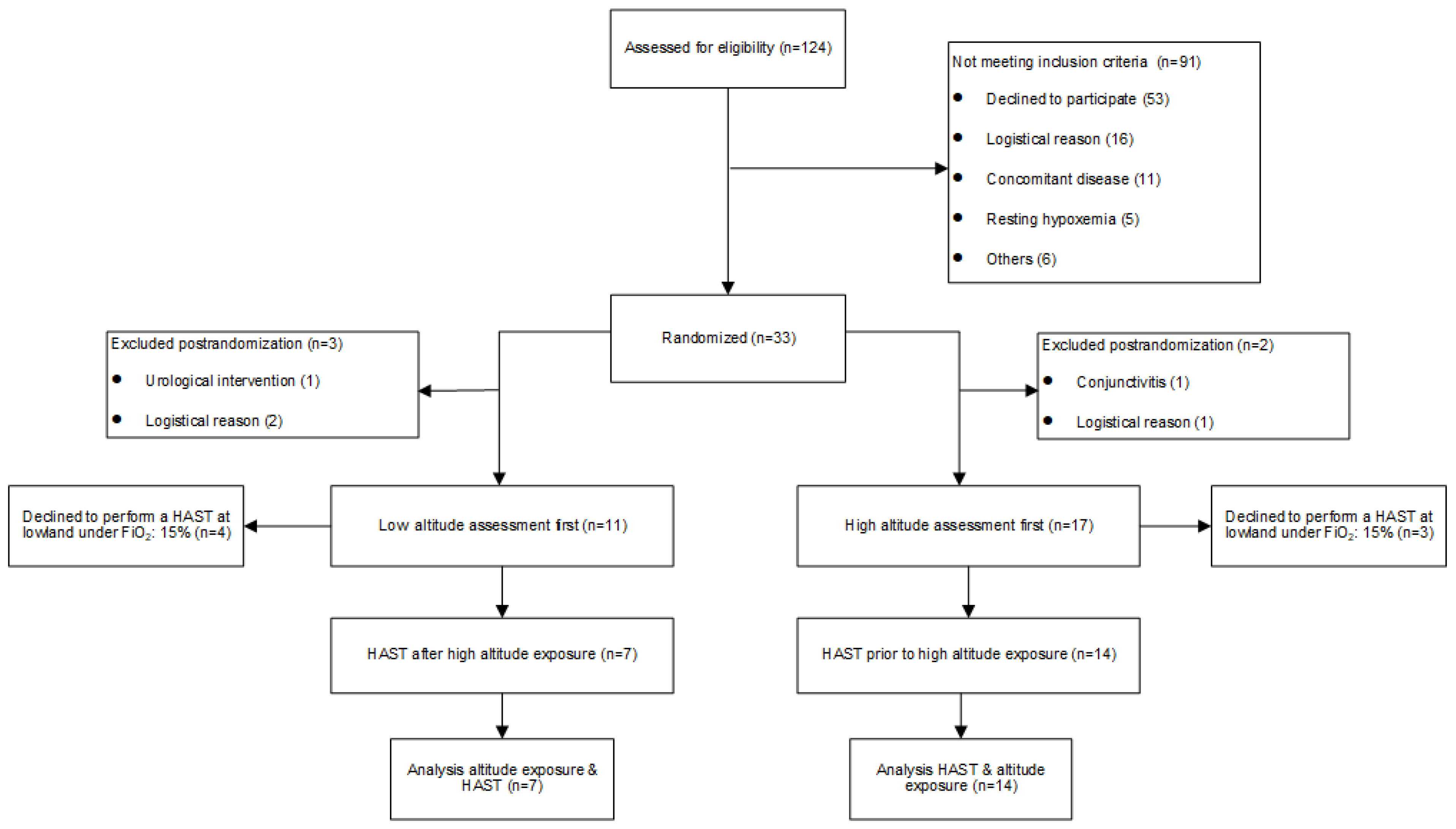
3.1. Study Population
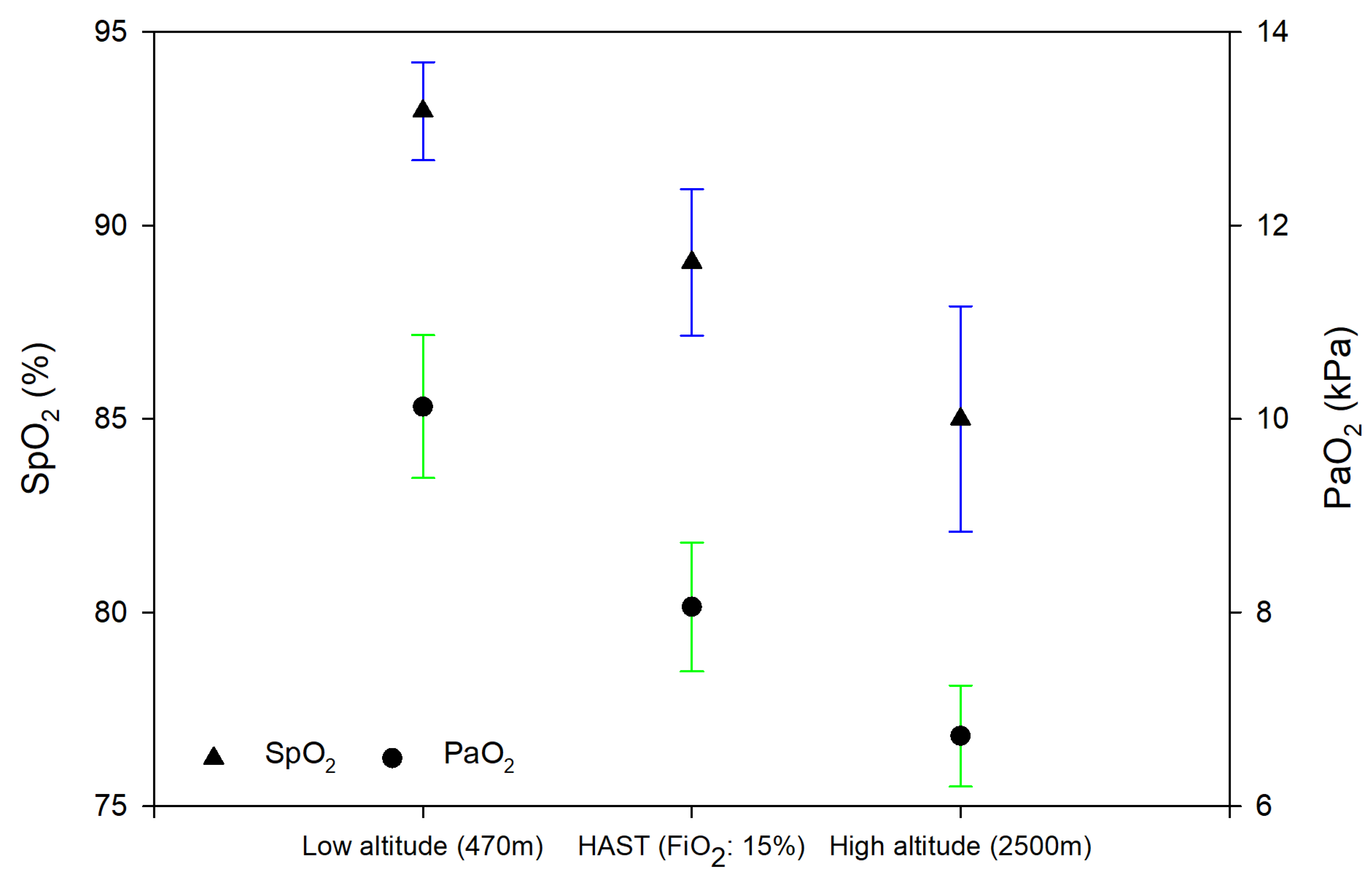
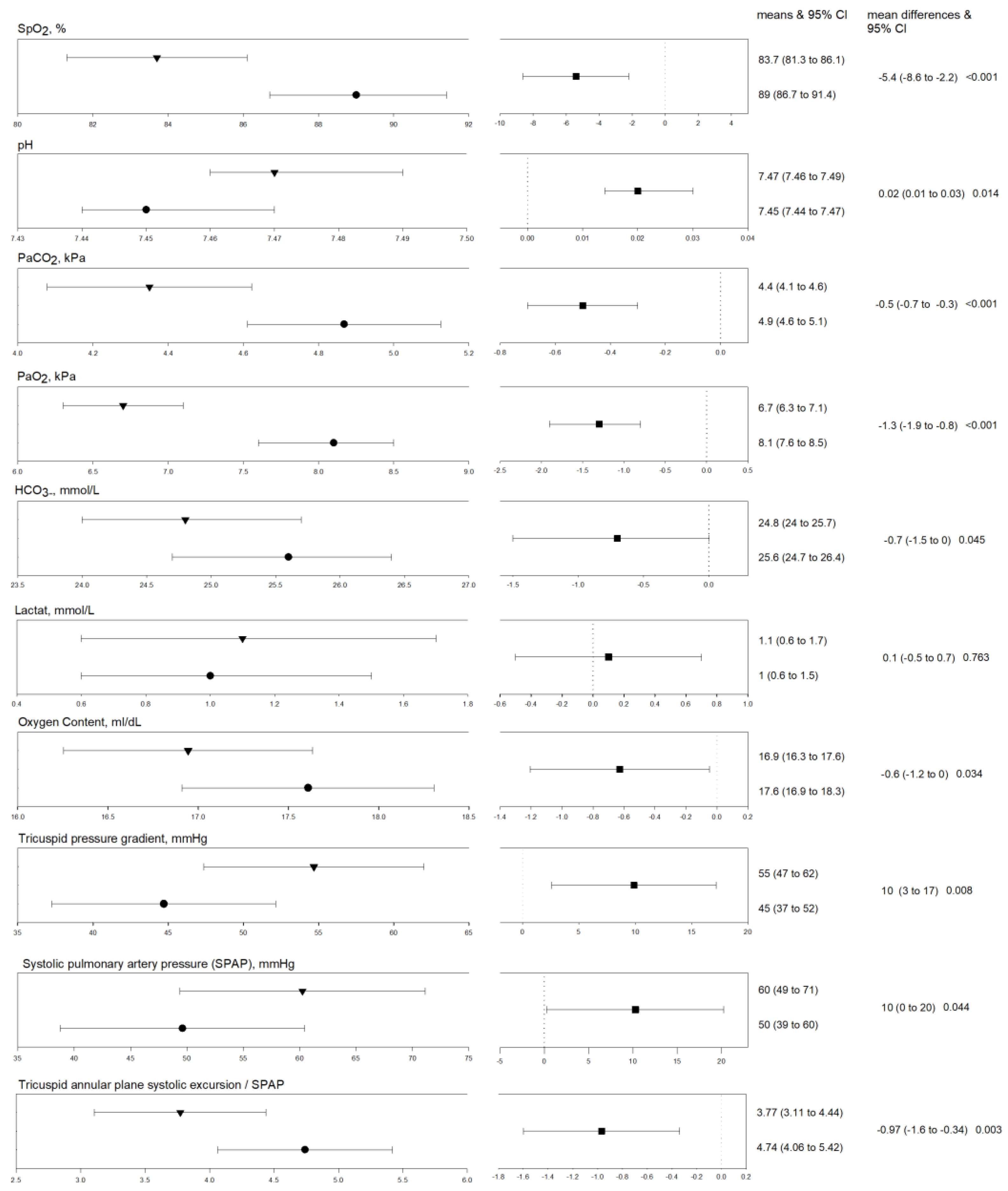
3.1.1. Differences between Exposure to Normobaric vs. Hypobaric Hypoxia
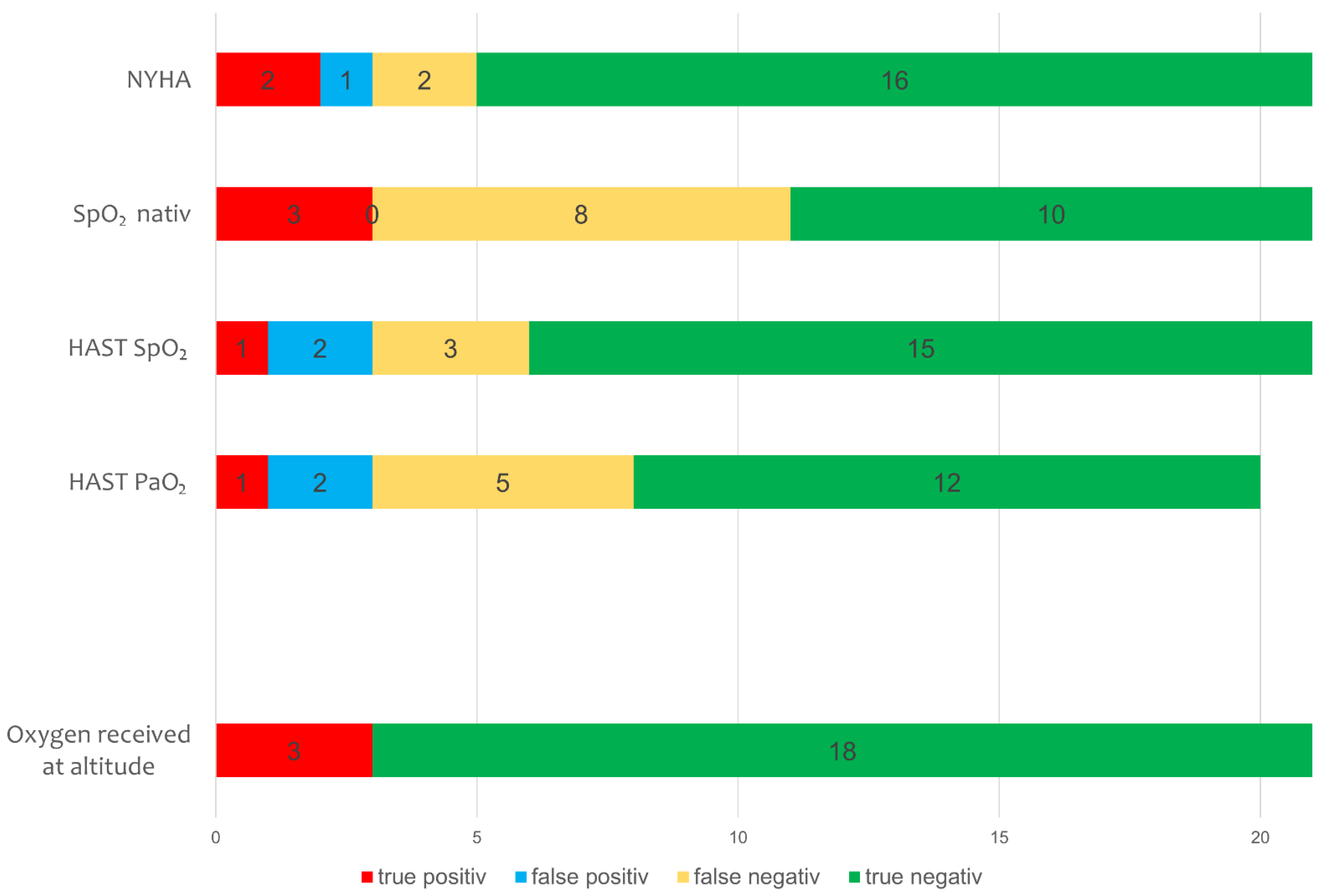
3.1.2. HAST and Baseline Measures to Predict Supplemental Oxygen at 2500 m
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tremblay, J.C.; Ainslie, P.N. Global and country-level estimates of human population at high altitude. Proc. Natl. Acad. Sci. USA 2021, 118, e2102463118. [Google Scholar] [CrossRef] [PubMed]
- Chatre, B.; Lanzinger, G.; Macaluso, M.; Mayrhofer, W.; Morandini, M.; Onida, M.; Polajnar, B. The Alps: People and Pressures in the Mountains, the Facts at a Glance: Vademecum; Permanent Sekretariat of the Alpine Convention: Innsbruck, Austria, 2010. [Google Scholar]
- Furian, M.; Buergin, A.; Scheiwiller, P.M.; Mayer, L.; Schneider, S.; Mademilov, M.; Emilov, B.; Lichtblau, M.; Muralt, L.; Sheraliev, U.; et al. Prevention of altitude-related illness in patients with COPD by acetazolamide. RCT. Eur. Respir. J. 2019, 54, PA3938. [Google Scholar] [CrossRef]
- Furian, M.; Lichtblau, M.; Aeschbacher, S.S.; Estebesova, B.; Emilov, B.; Sheraliev, U.; Marazhapov, N.H.; Mademilov, M.; Osmonov, B.; Bisang, M.; et al. Efficacy of Dexamethasone in Preventing Acute Mountain Sickness in COPD Patients: Randomized Trial. Chest 2018, 154, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Furian, M.; Flueck, D.; Latshang, T.D.; Scheiwiller, P.M.; Segitz, S.D.; Mueller-Mottet, S.; Murer, C.; Steiner, A.; Ulrich, S.; Rothe, T.; et al. Exercise performance and symptoms in lowlanders with COPD ascending to moderate altitude: Randomized trial. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 3529–3538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roach, R.C.; Hackett, P.H.; Oelz, O.; Bartsch, P.; Luks, A.M.; MacInnis, M.J.; Baillie, J.K.; Lake Louise, A.M.S.S.C.C. The 2018 Lake Louise Acute Mountain Sickness Score. High Alt. Med. Biol. 2018, 19, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Lichtblau, M.; Saxer, S.; Furian, M.; Mayer, L.; Bader, P.R.; Scheiwiller, P.; Mademilov, M.; Sheraliev, U.; Tanner, F.C.; Sooronbaev, T.M.; et al. Cardiac function and pulmonary hypertension in Central Asian highlanders at 3250 m. Eur. Respir. J. 2020, 56, 1902474. [Google Scholar] [CrossRef]
- Groth, A.; Saxer, S.; Bader, P.R.; Lichtblau, M.; Furian, M.; Schneider, S.R.; Schwarz, E.I.; Bloch, K.E.; Ulrich, S. Acute hemodynamic changes by breathing hypoxic and hyperoxic gas mixtures in pulmonary arterial and chronic thromboembolic pulmonary hypertension. Int. J. Cardiol. 2018, 270, 262–267. [Google Scholar] [CrossRef]
- Schneider, S.R.; Mayer, L.C.; Lichtblau, M.; Berlier, C.; Schwarz, E.I.; Saxer, S.; Furian, M.; Bloch, K.E.; Ulrich, S. Effect of Normobaric Hypoxia on Exercise Performance in Pulmonary Hypertension: Randomized Trial. Chest 2021, 159, 757–771. [Google Scholar] [CrossRef]
- Schneider, S.R.; Mayer, L.C.; Lichtblau, M.; Berlier, C.; Schwarz, E.I.; Saxer, S.; Tan, L.; Furian, M.; Bloch, K.E.; Ulrich, S. Effect of a daytrip to altitude (2500 m) on exercise performance in pulmonary hypertension–randomized cross-over trial. ERJ Open Res. 2021, 7, 00314–02021. [Google Scholar] [CrossRef]
- Edvardsen, A.; Ryg, M.; Akero, A.; Christensen, C.C.; Skjonsberg, O.H. COPD and air travel: Does hypoxia-altitude simulation testing predict in-flight respiratory symptoms? Eur. Respir. J. 2013, 42, 1216–1223. [Google Scholar] [CrossRef] [Green Version]
- Edvardsen, A.; Akero, A.; Christensen, C.C.; Ryg, M.; Skjonsberg, O.H. Air travel and chronic obstructive pulmonary disease: A new algorithm for pre-flight evaluation. Thorax 2012, 67, 964–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coker, R.K.; Armstrong, A.; Church, A.C.; Holmes, S.; Naylor, J.; Pike, K.; Saunders, P.; Spurling, K.J.; Vaughn, P. BTS Clinical Statement on air travel for passengers with respiratory disease. Thorax 2022, 77, 329–350. [Google Scholar] [CrossRef] [PubMed]
- Akero, A.; Edvardsen, A.; Christensen, C.C.; Owe, J.O.; Ryg, M.; Skjonsberg, O.H. COPD and air travel: Oxygen equipment and preflight titration of supplemental oxygen. Chest 2011, 140, 84–90. [Google Scholar] [CrossRef]
- Bradi, A.C.; Faughnan, M.E.; Stanbrook, M.B.; Deschenes-Leek, E.; Chapman, K.R. Predicting the need for supplemental oxygen during airline flight in patients with chronic pulmonary disease: A comparison of predictive equations and altitude simulation. Can. Respir. J. 2009, 16, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Howard, L.S. Last call for the flight simulation test? Eur. Respir. J. 2013, 42, 1175–1177. [Google Scholar] [CrossRef] [Green Version]
- Naeije, R. Preflight medical screening of patients. Eur. Respir. J. 2000, 16, 197–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef]
- Chemla, D.; Humbert, M.; Sitbon, O.; Montani, D.; Herve, P. Systolic and mean pulmonary artery pressures: Are they interchangeable in patients with pulmonary hypertension? Chest 2015, 147, 943–950. [Google Scholar] [CrossRef]
- Doutreleau, S.; Canuet, M.; Enache, I.; Di Marco, P.; Lonsdorfer, E.; Oswald-Mammoser, M.; Charloux, A. Right Heart Hemodynamics in Pulmonary Hypertension—An Echocardiography and Catheterization Study. Circ. J. 2016, 80, 2019–2025. [Google Scholar] [CrossRef] [Green Version]
- Huez, S.; Faoro, V.; Guenard, H.; Martinot, J.B.; Naeije, R. Echocardiographic and tissue Doppler imaging of cardiac adaptation to high altitude in native highlanders versus acclimatized lowlanders. Am. J. Cardiol. 2009, 103, 1605–1609. [Google Scholar] [CrossRef] [PubMed]
- Tello, K.; Wan, J.; Dalmer, A.; Vanderpool, R.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Mohajerani, E.; Seeger, W.; Herberg, U.; et al. Validation of the Tricuspid Annular Plane Systolic Excursion/Systolic Pulmonary Artery Pressure Ratio for the Assessment of Right Ventricular-Arterial Coupling in Severe Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2019, 12, e009047. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Khatana, S.; Gupta, P. Blood gas analysis for bedside diagnosis. Natl. J. Maxillofac. Surg. 2013, 4, 136–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hext, F.; Stubbings, A.; Bird, B.; Patey, S.; Wright, A.; the Birmingham Medical Research Expeditionary Society. Visual analogue scores in assessment of acute mountain sickness. High Alt. Med. Biol. 2011, 12, 329–333. [Google Scholar] [CrossRef] [PubMed]
- British Thoracic Society Standards of Care Committee. Managing passengers with respiratory disease planning air travel: British Thoracic Society recommendations. Thorax 2002, 57, 289–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Josephs, L.K.; Coker, R.K.; Thomas, M.; Group, B.T.S.A.T.W.; British Thoracic, S. Managing patients with stable respiratory disease planning air travel: A primary care summary of the British Thoracic Society recommendations. Prim. Care Respir. J. 2013, 22, 234–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmedzai, S.; Balfour-Lynn, I.M.; Bewick, T.; Buchdahl, R.; Coker, R.K.; Cummin, A.R.; Gradwell, D.P.; Howard, L.; Innes, J.A.; Johnson, A.O.; et al. Managing passengers with stable respiratory disease planning air travel: British Thoracic Society recommendations. Thorax 2011, 66 (Suppl. 1), i1–i30. [Google Scholar] [CrossRef] [Green Version]
- Bartsch, P.; Bailey, D.M.; Berger, M.M.; Knauth, M.; Baumgartner, R.W. Acute mountain sickness: Controversies and advances. High Alt. Med. Biol. 2004, 5, 110–124. [Google Scholar] [CrossRef]
- Brack, T. Into Thin Air: A Step Forward to Counsel Patients with Pulmonary Hypertension Travelling to High Altitude. Chest 2021, 159, 484–485. [Google Scholar] [CrossRef]
- Matthys, H. Fit for high altitude: Are hypoxic challenge tests useful? Multidiscip. Respir. Med. 2011, 6, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Conkin, J.; Wessel, J.H., 3rd. Critique of the equivalent air altitude model. Aviat. Space Environ. Med. 2008, 79, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, R.H. “La Pression barométrique”: Paul Bert’s hypoxia theory and its critics. Respir. Physiol. 1978, 34, 1–28. [Google Scholar] [CrossRef]
- Conkin, J. Equivalent Air Altitude and the Alveolar Gas Equation. Aerosp. Med. Hum. Perform. 2016, 87, 61–64. [Google Scholar] [CrossRef]
- Dine, C.J.; Kreider, M.E. Hypoxia altitude simulation test. Chest 2008, 133, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.O. Chronic obstructive pulmonary disease* 11: Fitness to fly with COPD. Thorax 2003, 58, 729–732. [Google Scholar] [CrossRef] [Green Version]
- Coker, R.K.; Shiner, R.J.; Partridge, M.R. Is air travel safe for those with lung disease? Eur. Respir. J. 2007, 30, 1057–1063. [Google Scholar] [CrossRef] [Green Version]
- Loeppky, J.A.; Icenogle, M.; Scotto, P.; Robergs, R.; Hinghofer-Szalkay, H.; Roach, R.C. Ventilation during simulated altitude, normobaric hypoxia and normoxic hypobaria. Respir. Physiol. 1997, 107, 231–239. [Google Scholar] [CrossRef]
- Savourey, G.; Launay, J.C.; Besnard, Y.; Guinet, A.; Travers, S. Normo- and hypobaric hypoxia: Are there any physiological differences? Eur. J. Appl. Physiol. 2003, 89, 122–126. [Google Scholar] [CrossRef]
- Dunham-Snary, K.J.; Wu, D.; Sykes, E.A.; Thakrar, A.; Parlow, L.R.G.; Mewburn, J.D.; Parlow, J.L.; Archer, S.L. Hypoxic Pulmonary Vasoconstriction: From Molecular Mechanisms to Medicine. Chest 2017, 151, 181–192. [Google Scholar] [CrossRef] [Green Version]

| Participants/women (%) | 21/10 (48) |
| Age, years | 64 ± 15 |
| Body mass index, kg/m2 | 25.6 ± 3.8 |
| Pulmonary hypertension classification | |
| 1. Pulmonary arterial hypertension | 12 (58) |
| 1.1. idiopathic | 10 (48) |
| 1.4.1. connective tissue disease | 1 (5) |
| 1.4.3. portopulmonary hypertension | 1 (5) |
| 4. Chronic thromboembolic pulmonary hypertension | 9 (43) |
| 6-min walk distance, m | 538 ± 94 |
| New York Heart Association functional class I, II, III | 8 (38), 9 (43), 4 (19) |
| N-terminal pro brain natriuretic peptide, ng/l | 427 ± 620 |
| Incremental ramp cycle exercise, Watt | 114 ± 36 |
| Maximal oxygen uptake, ml/min/kg | 18.2 ± 3.9 |
| Resting arterial partial pressure of oxygen, kPa | 10.1 ± 1.6 |
| Mean pulmonary arterial pressure, mmHg * | 42 ± 11 |
| Pulmonary vascular resistance, WU * | 6 ± 3 |
| PH targeted therapy | |
| Endothelin receptor antagonist | 14 (67) |
| Phosphodiesterase-5 inhibitor including Soluble guanylate cyclase stimulators | 9 (43) |
| Soluble guanylate cyclase stimulators | 2 (10) |
| Prostacyclin-receptor agonist or prostacyclin | 2 (10) |
| Combination therapy | 8 (38) |
| Parameter | FiO2: 15% (Mean ± SE) | Altitude (2500 m) (Mean ± SE) | Mean Difference (95% CI) | p-Value |
|---|---|---|---|---|
| Peripheral oxygen saturation, % | 89 ± 1.2 | 83.7 ± 1.2 | −5.4 (−8.6 to −2.2) | <0.001 |
| pH | 7.45 ± 0.01 | 7.47 ± 0.01 | 0.02 (0.00 to 0.03) | 0.014 |
| Partial pressure of carbon dioxide, kPa | 4.9 ± 0.1 | 4.4 ± 0.1 | −0.5 (−0.7 to −0.3) | <0.001 |
| Partial pressure of oxygen, kPa | 8.1 ± 0.2 | 6.7 ± 0.2 | −1.3 (−1.9 to −0.8) | <0.001 |
| Hydrogen carbonate, mmol/L | 25.6 ± 0.4 | 24.8 ± 0.4 | −0.7 (−1.5 to 0.0) | 0.045 |
| Lactate, mmol/L | 1 ± 0.2 | 1.1 ± 0.3 | 0.1 (−0.5 to 0.7) | 0.763 |
| Arterial oxygen saturation, % | 90.5 ± 1.2 | 83.7 ± 1.2 | −6.8 (−9.6 to −4.0) | <0.001 |
| Arterial oxygen content, mL/dL | 17.6 ± 0.4 | 16.9 ± 0.4 | −0.6 (−1.2 to 0.0) | 0.034 |
| Heart rate, min−1 | 71 ± 3 | 69 ± 3 | −1.2 (−9 to 6.6) | 0.762 |
| Breathing rate, min−1 | 16 ± 1 | 19 ± 1 | 3 (0 to 7) | 0.056 |
| Right atrial pressure, mmHg | 4 ± 1 | 5 ± 1 | 1 (0 to 1) | 0.160 |
| Tricuspid regurgitation pressure gradient (TRPG), mmHg | 45 ± 4 | 55 ± 4 | 10 (3 to 17) | 0.008 |
| Systolic pulmonary arterial pressure (SPAP), mmHg | 50 ± 6 | 60 ± 6 | 10 (0 to 20) | 0.044 |
| Stroke volume, mL | 73 ± 4.4 | 74.3 ± 4.3 | 0.8 (−9.4 to 11) | 0.876 |
| Cardiac output (CO), L/min | 5.1 ± 0.4 | 5.1 ± 0.4 | 0 (−0.9 to 0.8) | 0.920 |
| Oxygen delivery, mL/min | 906.6 ± 66.9 | 854.6 ± 65.6 | −44.2 (−192.9 to 104.6) | 0.560 |
| Pulmonary vascular resistance, WU | 4.3 ± 2.9 | 5.4 ± 2.6 | 1.4 (−6.3 to 9.1) | 0.718 |
| TRPG/CO, WU | 8.4 ± 2 | 11.3 ± 2 | 2.8 (−2.5 to 8.2) | 0.302 |
| Tricuspid annular plane systolic excursion (TAPSE), cm | 2 ± 0.1 | 2 ± 0.1 | 0 (−0.2 to 0.1) | 0.760 |
| TAPSE/SPAP ratio | 4.74 ± 0.35 | 3.77 ± 0.34 | −0.97 (−1.6 to −0.34) | 0.003 |
| Fractional area change, % | 32 ± 2 | 30 ± 2 | −2 (−6 to 2) | 0.422 |
| Visual analog scale (general wellbeing), cm | 8.5 ± 0.4 | 8.7 ± 0.4 | 0.17 (−0.78 to 1.12) | 0.730 |
| Visual analog scale (dyspnea), cm | 8.2 ± 0.5 | 8.4 ± 0.5 | 0.21 (−0.83 to 1.24) | 0.694 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider, S.R.; Lichtblau, M.; Furian, M.; Mayer, L.C.; Berlier, C.; Müller, J.; Saxer, S.; Schwarz, E.I.; Bloch, K.E.; Ulrich, S. Cardiorespiratory Adaptation to Short-Term Exposure to Altitude vs. Normobaric Hypoxia in Patients with Pulmonary Hypertension. J. Clin. Med. 2022, 11, 2769. https://doi.org/10.3390/jcm11102769
Schneider SR, Lichtblau M, Furian M, Mayer LC, Berlier C, Müller J, Saxer S, Schwarz EI, Bloch KE, Ulrich S. Cardiorespiratory Adaptation to Short-Term Exposure to Altitude vs. Normobaric Hypoxia in Patients with Pulmonary Hypertension. Journal of Clinical Medicine. 2022; 11(10):2769. https://doi.org/10.3390/jcm11102769
Chicago/Turabian StyleSchneider, Simon R., Mona Lichtblau, Michael Furian, Laura C. Mayer, Charlotte Berlier, Julian Müller, Stéphanie Saxer, Esther I. Schwarz, Konrad E. Bloch, and Silvia Ulrich. 2022. "Cardiorespiratory Adaptation to Short-Term Exposure to Altitude vs. Normobaric Hypoxia in Patients with Pulmonary Hypertension" Journal of Clinical Medicine 11, no. 10: 2769. https://doi.org/10.3390/jcm11102769
APA StyleSchneider, S. R., Lichtblau, M., Furian, M., Mayer, L. C., Berlier, C., Müller, J., Saxer, S., Schwarz, E. I., Bloch, K. E., & Ulrich, S. (2022). Cardiorespiratory Adaptation to Short-Term Exposure to Altitude vs. Normobaric Hypoxia in Patients with Pulmonary Hypertension. Journal of Clinical Medicine, 11(10), 2769. https://doi.org/10.3390/jcm11102769





