Novel Computed Tomography Variables for Assessing Tricuspid Valve Morphology: Results from the TRIMA (Tricuspid Regurgitation IMAging) Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. CT Acquisition
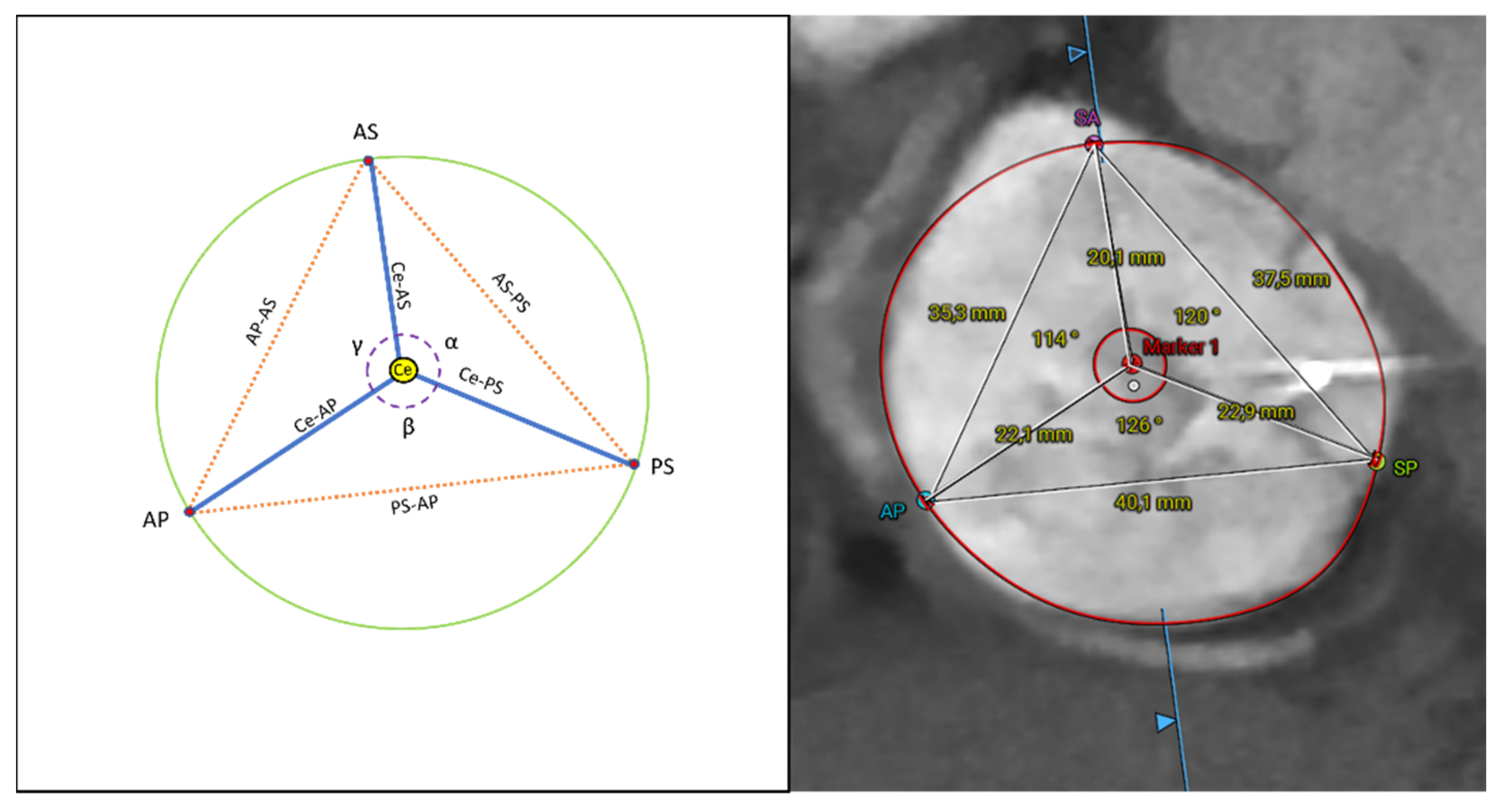
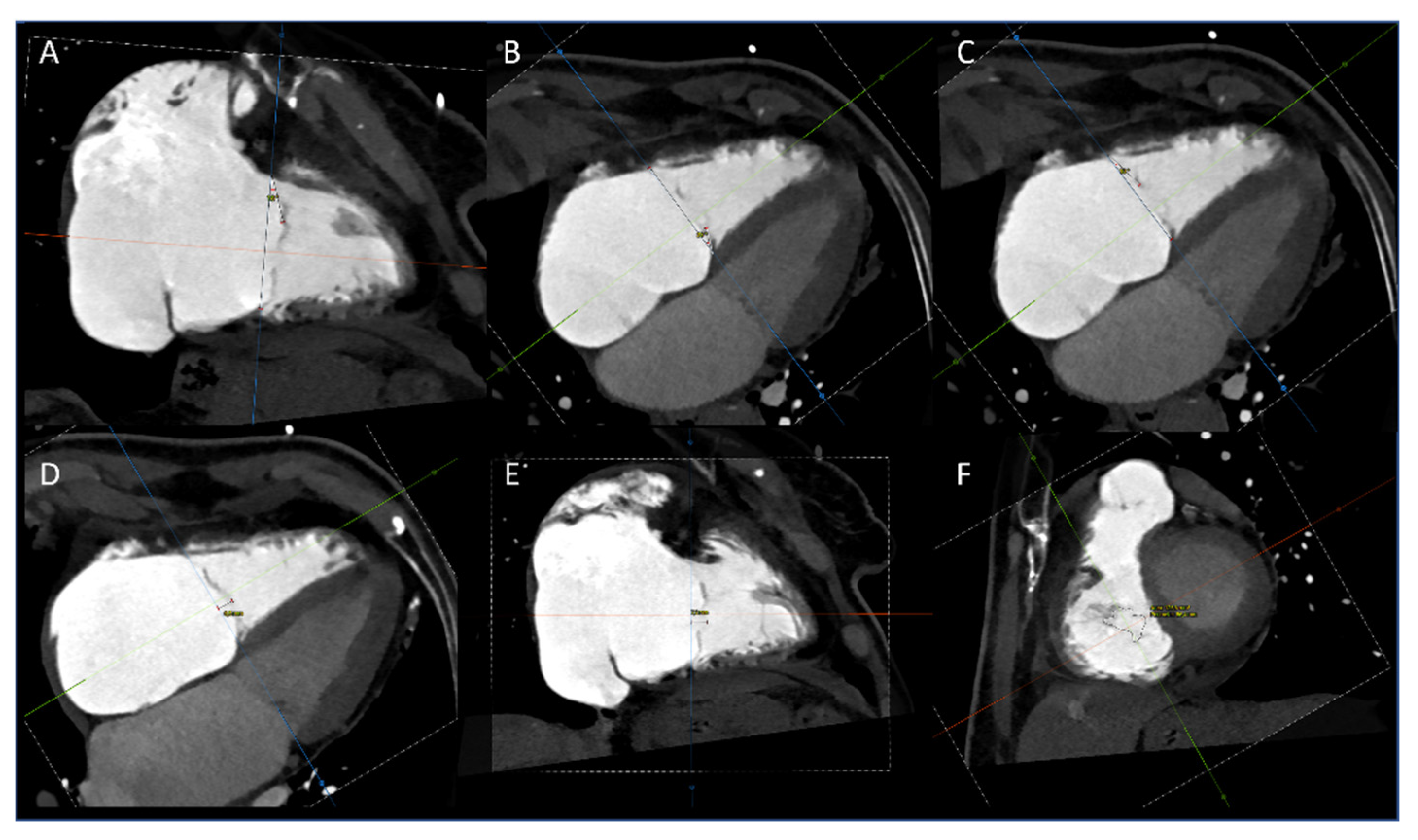
2.3. CT Data Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Annulus Sizing
4.2. Novel Annular Parameters
4.3. Leaflet Coaptation
4.4. Future Directions
4.5. Study Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beyersdorf, F.; Vahanian, A.; Milojevic, M.; Praz, F.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. J. Cardiothorac. Surg. 2021, 60, 727–800. [Google Scholar] [CrossRef] [PubMed]
- Khalique, O.K.; Cavalcante, J.L.; Shah, D.; Guta, A.C.; Zhan, Y.; Piazza, N.; Muraru, D. Multimodality Imaging of the Tricuspid Valve and Right Heart Anatomy. JACC Cardiovasc. Imaging 2019, 12, 516–531. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Thomas, J.D.; Khalique, O.K.; Cavalcante, J.L.; Praz, F.; Zoghbi, W.A. Imaging Assessment of Tricuspid Regurgitation Severity. JACC Cardiovasc. Imaging 2019, 12, 469–490. [Google Scholar] [CrossRef] [PubMed]
- Hell, M.M.; Emrich, T.; Kreidel, F.; Kreitner, K.-F.; Schoepf, U.J.; Münzel, T.; von Bardeleben, R.S. Computed Tomography Imaging Needs for Novel Transcatheter Tricuspid Valve Repair and Replacement Therapies. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Schlossbauer, S.A.; Faletra, F.F.; Paiocchi, V.L.; Leo, L.A.; Franciosi, G.; Bonanni, M.; Angelini, G.; Pavon, A.G.; Ferrari, E.; Ho, S.Y.; et al. Multimodality Imaging of the Anatomy of Tricuspid Valve. J. Cardiovasc. Dev. Dis. 2021, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Cammalleri, V.; Mega, S.; Ussia, G.P.; Grigioni, F. Mitral and Tricuspid Valves Percutaneous Repair in Patients with Advanced Heart Failure. Heart Fail. Clin. 2021, 17, 607–618. [Google Scholar] [CrossRef]
- Cammalleri, V.; Carpenito, M.; Bono, M.C.; Mega, S.; Ussia, G.P.; Grigioni, F. Transcatheter Tricuspid Valve Therapy: From Anatomy to Intervention. Front. Cardiovasc. Med. 2021, 8, 778445. [Google Scholar] [CrossRef]
- Addetia, K.; Muraru, D.; Veronesi, F.; Jenei, C.; Cavalli, G.; Besser, S.A.; Mor-Avi, V.; Lang, R.M.; Badano, L.P. 3-Dimensional Echocardiographic Analysis of the Tricuspid Annulus Provides New Insights Into Tricuspid Valve Geometry and Dynamics. JACC Cardiovasc. Imaging. 2019, 12, 401–412. [Google Scholar] [CrossRef]
- van Rosendael, P.J.; Joyce, E.; Katsanos, S.; Debonnaire, P.; Kamperidis, V.; van der Kley, F.; Schalij, M.J.; Bax, J.J.; Ajmone Marsan, N.; Delgado, V. Tricuspid Valve Remodelling in Functional Tricuspid Regurgitation: Multidetector Row Computed Tomography Insights. Eur. Heart J. Cardiovasc. Imaging 2015, jev140, 96–105. [Google Scholar] [CrossRef][Green Version]
- Ussia, G.P.; Cammalleri, V.; Mehta, J.L.; Sarkar, K.; Muscoli, S.; de Vico, P.; Ruvolo, G.; Romeo, F. Transcatheter Mitral Valve Replacement with a Novel Self-Expandable Prosthesis: Single Institutional Experience Procedural Outcomes and Follow-Up. J. Cardiovasc. Med. 2017, 18, 415–424. [Google Scholar] [CrossRef]
- Romeo, F.; Cammalleri, V.; Ruvolo, G.; Quadri, A.; De Vico, P.; Muscoli, S.; Marchei, M.; Meloni, S.; Conti, F.; Ussia, G.P. Trans-Catheter Mitral Valve Implantation for Mitral Regurgitation: Clinical Case Description and Literature Review. J. Cardiovasc. Med. 2016, 17, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Pulerwitz, T.C.; Khalique, O.K.; Leb, J.; Hahn, R.T.; Nazif, T.M.; Leon, M.B.; George, I.; Vahl, T.P.; D’Souza, B.; Bapat, V.N.; et al. Optimizing Cardiac CT Protocols for Comprehensive Acquisition Prior to Percutaneous MV and TV Repair/Replacement. JACC Cardiovasc. Imaging. 2020, 13, 836–850. [Google Scholar] [CrossRef] [PubMed]
- Ranard, L.S.; Vahl, T.P.; Chung, C.J.; Sadri, S.; Khalique, O.K.; Hamid, N.; Nazif, T.; George, I.; Ng, V.; Patel, A.; et al. Impact of Inferior Vena Cava Entry Characteristics on Tricuspid Annular Access during Transcatheter Interventions. Catheter. Cardiovasc. Interv. 2022, ccd.30048, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Lopes, B.B.C.; Sorajja, P.; Hashimoto, G.; Fukui, M.; Bapat, V.N.; Du, Y.; Bae, R.; Schwartz, R.S.; Stanberry, L.I.; Enriquez-Sarano, M.; et al. Tricuspid Anatomic Regurgitant Orifice Area by Functional DSCT. JACC Cardiovasc. Imaging 2021, 14, 1669–1672. [Google Scholar] [CrossRef]
- Khalique, O.K.; Jelnin, V.; Hueske, A.; Lawlor, M.; Leon, M.B.; Kodali, S.K.; Akkoc, D.; Pettway, E.; Hahn, R.T.; Hamid, N.B.; et al. Right Heart Morphology of Candidate Patients for Transcatheter Tricuspid Valve Interventions. Cardiovasc. Eng. Technol. 2021. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Hahn, R.T.; Zamorano, J.L. The Need for a New Tricuspid Regurgitation Grading Scheme. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1342–1343. [Google Scholar] [CrossRef]
- Silver, M.D.; Lam, J.H.C.; Ranganathan, N.; Wigle, E.D. Morphology of the Human Tricuspid Valve. Circulation 1971, 43, 333–348. [Google Scholar] [CrossRef]
- Joudinaud, T.M.; Flecher, E.M.; Duran, C.M.G. Functional Terminology for the Tricuspid Valve. J. Heart Valve Dis. 2006, 15, 382–388. [Google Scholar]
- Muraru, D.; Addetia, K.; Guta, A.C.; Ochoa-Jimenez, R.C.; Genovese, D.; Veronesi, F.; Basso, C.; Iliceto, S.; Badano, L.P.; Lang, R.M. Right atrial volume is a major determinant of tricuspid annulus area in functional tricuspid regurgitation: A three-dimensional echocardiographic study. Eur. Heart J. Cardiovasc. Imaging. 2021, 22, 660–669. [Google Scholar] [CrossRef]
- Hahn, R.T.; Badano, L.P.; Bartko, P.E.; Muraru, D.; Maisano, F.; Zamorano, J.L.; Donal, E. Tricuspid Regurgitation: Recent Advances in Understanding Pathophysiology, Severity Grading and Outcome. Eur. Heart J. Cardiovasc. Imaging 2022, jeac009. [Google Scholar] [CrossRef]
- Florescu, D.R.; Muraru, D.; Volpato, V.; Gavazzoni, M.; Caravita, S.; Tomaselli, M.; Ciampi, P.; Florescu, C.; Bălșeanu, T.A.; Parati, G.; et al. Atrial Functional Tricuspid Regurgitation as a Distinct Pathophysiological and Clinical Entity: No Idiopathic Tricuspid Regurgitation Anymore. J. Clin. Med. 2022, 11, 382. [Google Scholar] [CrossRef] [PubMed]
- Tei, C.; Pilgrim, J.P.; Shah, P.M.; Ormiston, J.A.; Wong, M. The Tricuspid Valve Annulus: Study of Size and Motion in Normal Subjects and in Patients with Tricuspid Regurgitation. Circulation 1982, 66, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Hołda, M.K.; Zhingre Sanchez, J.D.; Bateman, M.G.; Iaizzo, P.A. Right Atrioventricular Valve Leaflet Morphology Redefined. JACC Cardiovasc. Interv. 2019, 12, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Victor, S.; Nayak, V.M. The Tricuspid Valve Is Bicuspid. J. Heart Valve Dis. 1994, 3, 27–36. [Google Scholar] [CrossRef]
- Hahn, R.T.; Weckbach, L.T.; Noack, T.; Hamid, N.; Kitamura, M.; Bae, R.; Lurz, P.; Kodali, S.K.; Sorajja, P.; Hausleiter, J.; et al. Proposal for a Standard Echocardiographic Tricuspid Valve Nomenclature. JACC Cardiovasc. Imaging 2021, 14, 1299–1305. [Google Scholar] [CrossRef]
- Victor, S.; Nayak, V.M. Skirts, Slits, Scallops and Semantics. J. Heart Valve Dis. 1995, 4, 576–579; discussion 580. [Google Scholar]
- Lamers, W.H.; Virágh, S.; Wessels, A.; Moorman, A.F.M.; Anderson, R.H. Formation of the Tricuspid Valve in the Human Heart. Circulation 1995, 91, 111–121. [Google Scholar] [CrossRef]
- Wafae, N.; Hayashi, H.; Gerola, L.R.; Vieira, M.C. Anatomical Study of the Human Tricuspid Valve. Surg. Radiol. Anat. 1990, 12, 37–41. [Google Scholar] [CrossRef]
- Sugiura, A.; Tanaka, T.; Kavsur, R.; Öztürk, C.; Vogelhuber, J.; Wilde, N.; Becher, M.U.; Zimmer, S.; Nickenig, G.; Weber, M. Leaflet Configuration and Residual Tricuspid Regurgitation After Transcatheter Edge-to-Edge Tricuspid Repair. JACC Cardiovasc Interv. 2021, 14, 2260–2270. [Google Scholar] [CrossRef]
- Kitamura, M.; Kresoja, K.P.; Besler, C.; Leontyev, S.; Kiefer, P.; Rommel, K.P.; Otto, W.; Forner, A.F.; Ender, J.; Holzhey, D.M.; et al. Impact of Tricuspid Valve Morphology on Clinical Outcomes After Transcatheter Edge-to-Edge Repair. JACC Cardiovasc. Interv. 2021, 14, 1616–1618. [Google Scholar] [CrossRef] [PubMed]
- Ton-Nu, T.-T.; Levine, R.A.; Handschumacher, M.D.; Dorer, D.J.; Yosefy, C.; Fan, D.; Hua, L.; Jiang, L.; Hung, J. Geometric Determinants of Functional Tricuspid Regurgitation: Insights From 3-Dimensional Echocardiography. Circulation 2006, 114, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Tei, C.; Hopkins, J.M.; Shah, P.M. Assessment of Right Ventricular Function Using Two-Dimensional Echocardiography. Am. Heart J. 1984, 107, 526–531. [Google Scholar] [CrossRef]
- Aloia, E.; Cameli, M.; D’Ascenzi, F.; Sciaccaluga, C.; Mondillo, S. TAPSE: An Old but Useful Tool in Different Diseases. Int. J. Cardiol. 2016, 225, 177–183. [Google Scholar] [CrossRef]
| Patient Characteristics | N = 22 |
|---|---|
| Mean age, years old ± SD | 78 ± 7.66 |
| Female, n (%) | 17 (77.3) |
| BMI, kg/m2 ± SD | 24.42 ± 3.89 |
| Previous CABG, n (%) | 2 (9) |
| Previous left side valve surgery, n (%) | 3 (14) |
| Atrial fibrillation, n (%) | 20 (91) |
| Heart rate, bpm ± SD | 78.36 ± 14.45 |
| Permanent pacemaker, n (%) | 6 (27) |
| ICD/CRT, n (%) | 4 (18) |
| LVEF, % ± SD | 46.27 ± 10.69 |
| TR grade, n (%) | |
| 3+ | 17 (77) |
| 4+ | 2 (9) |
| 5+ | 3 (14) |
| Variable | Diastole | Systole | Difference (95% CI) | p Value |
|---|---|---|---|---|
| Annulus area (cm2 ± SD) | 17.78 ± 3.39 | 16.41 ± 3.47 | 1.37 (0.74 to 1.99) | <0.001 |
| Anulus perimeter (mm ± SD) | 152.32 ± 16.32 | 147.63 ± 15.45 | 4.69 (1.32 to 8.06) | 0.009 |
| SL diameter (mm ± SD) | 48.27 ± 6.40 | 45.92 ± 6.26 | 2.35 (1.30 to 3.40) | <0.001 |
| AP diameter (mm ± SD) | 47.03 ± 3.63 | 44.98 ± 3.76 | 2.05 (1.16 to 2.94) | <0.001 |
| Eccentricity (index ± SD) | 0.99 ± 0.10 | 0.99 ± 0.12 | −0.01 (−0.03 to 0.02) | 0.572 |
| AS-PS (mm ± SD) | 39.64 ± 5.89 | 37.58 ± 5.40 | 2.06 (1.03 to 3.10) | <0.001 |
| PS-AP (mm ± SD) | 42.48 ± 5.31 | 41.86 ± 5.93 | 0.61 (−0.54 to 1.77) | 0.280 |
| AP-AS (mm ± SD) | 39.72 ± 4.88 | 38.75 ± 4.86 | 0.97 (0.07 to 1.87) | 0.037 |
| Ce-AS (mm ± SD) | 22.53 ± 2.88 | 21.19 ± 2.71 | 1.34 (0.85 to 1.83) | <0.001 |
| Ce-PS (mm ± SD) | 23.91 ± 3.98 | 23.06 ± 3.46 | 0.85 (0.07 to 1.64) | 0.034 |
| Ce-AP (mm ± SD) | 23.84 ± 2.57 | 23.76 ± 3.15 | 0.07 (−0.67 to 0.82) | 0.841 |
| α (° ± SD) | 116.32 ± 10.05 | 115.82 ± 9.25 | 0.50 (−1.92 to 2.92) | 0.672 |
| β (° ± SD) | 125.50 ± 7.60 | 126.00 ± 6.60 | −0.50 (−3.00 to 2.00) | 0.682 |
| γ (° ± SD) | 117.68 ± 9.78 | 118.18 ± 8.46 | −0.5 (−2.96 to 1.96) | 0.677 |
| RA 4 chambers (mm ± SD) | 65.57 ± 11.57 | 70.79 ± 9.34 | −5.22 (−8.29 to 2.15) | 0.002 |
| RA 2 chambers (mm ± SD) | 63.34 ± 12.32 | 67.85 ± 10.01 | −4.52 (−8.54 to −0.50) | 0.030 |
| RV 4 chambers (mm ± SD) | 70.07 ± 7.85 | 58.80 ± 8.00 | 11.27 (7.90 to 14.64) | <0.001 |
| RV 2 chambers (mm ± SD) | 67.95 ± 7.49 | 57.57 ± 8.26 | 10.38 (6.79 to 13.96) | <0.001 |
| Variables | Diastole | Systole |
|---|---|---|
| Annulus perimeter | r = 0.92 p = <0.001 | r = 0.98 p = <0.001 |
| SL diameter | r = 0.93 p = <0.001 | r = 0.92 p = <0.001 |
| AP diameter | r = 0.87 p = <0.001 | r = 0.86 p = <0.001 |
| Eccentricity index | r = −0.61 p = 0.001 | r = −0.52 p = 0.001 |
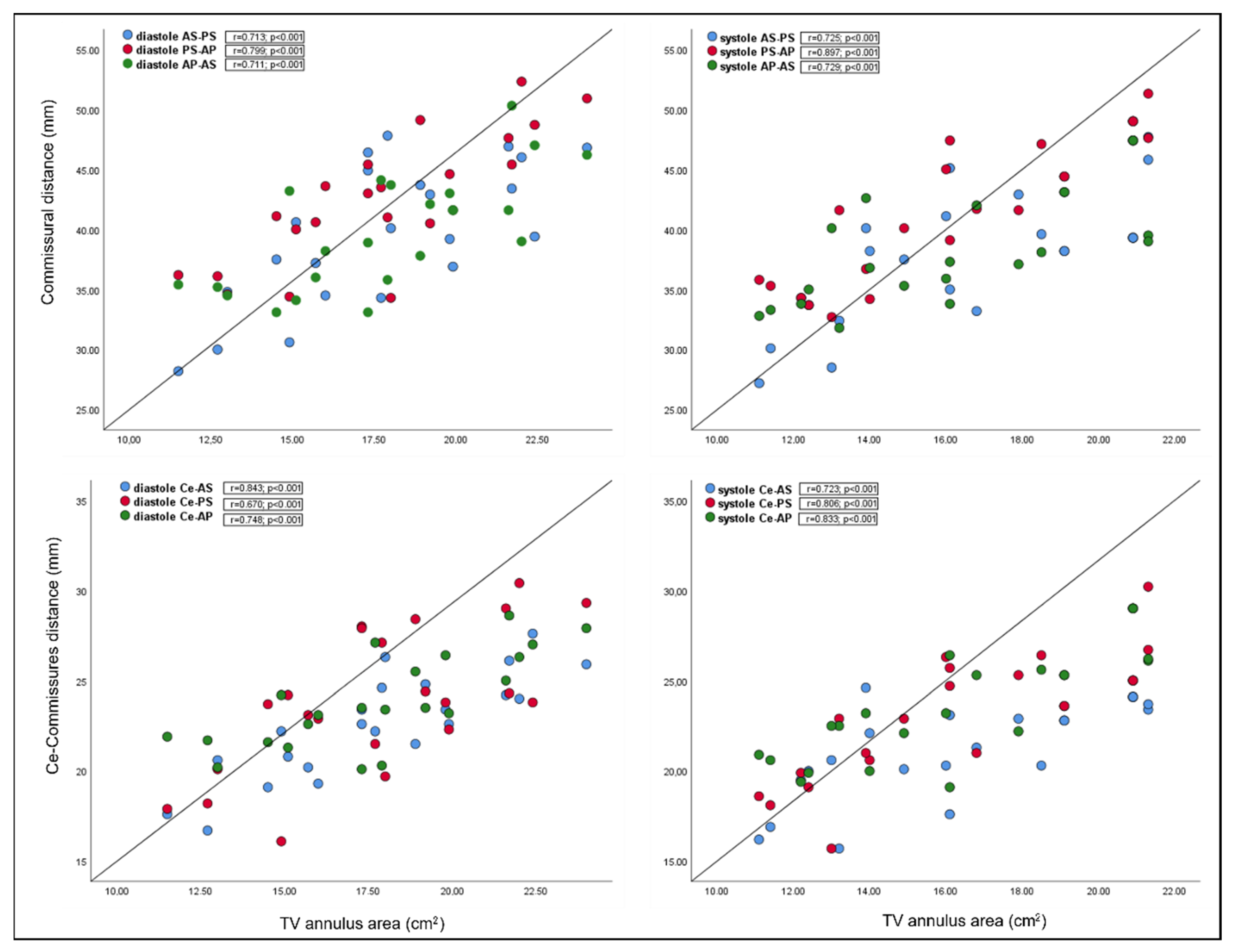
| AS-PS | r = 0.72 p = <0.001 | r = 0.72 p = <0.001 |
| PS-AP | r = 0.80 p = <0.001 | r = 0.90 p = <0.001 |
| AP-AS | r = 0.71 p = <0.001 | r = 0.73 p = <0.001 |
| Ce-AS | r = 0.84 p = <0.001 | r = 0.72 p = <0.001 |
| Ce-PS | r = 0.67 p = <0.001 | r = 0.81 p = <0.001 |
| Ce-AP | r = 0.75 p = <0.001 | r = 0.83 p = <0.001 |
| α | r = 0.07 p = 0.384 | r = −0.01 p = 0.495 |
| β | r = 0.17 p = 0.230 | r = 0.34 p = 0.062 |
| γ | r = −0.19 p = 0.196 | r = −0.26 p = 0.121 |
| RA 4Ch | r = 0.53 p = 0.005 | r = 0.56 p = 0.003 |
| RA 2Ch | r = 0.51 p = 0.008 | r = 0.24 p = 0.138 |
| RV 4Ch | r = 0.50 p = 0.008 | r = 0.34 p = 0.059 |
| RV 2Ch | r = 0.53 p = 0.005 | r = 0.48 p = 0.011 |
| Tenting 4Ch | - | r = 0.01 p = 0.492 |
| Tenting 2Ch | - | r = 0.35 p = 0.057 |
| Anterior tethering degree | - | r = 0.24 p = 0.301 |
| Septal tethering degree | - | r = 0.98 p = 0.682 |
| Posterior tethering degree | - | r = 0.39 p = 0.084 |
| EROA Area | - | r = 0.26 p = 0.117 |
| EROA Perimeter | - | r = 0.26 p = 0.12 |
| EROA Max | - | r = 0.36 p = 0.051 |
| EROA Min | - | r = 0.28 p = 0.106 |
| EROA SA-gap | - | r = 0.39 p = 0.037 |
| EROA SP-gap | - | r = 0.22 p = 0.167 |
| Diastole | Systole | |||||
|---|---|---|---|---|---|---|
| Variables | Coefficient | 95% CI | p Value | Coefficient | 95% CI | p Value |
| AS-PS | 0.228 | 0.133–0.322 | <0.001 | 0.205 | 0.111–0.300 | <0.001 |
| PS-AP | 0.227 | 0.117–0.338 | <0.001 | 0.297 | 0.197–0.397 | <0.001 |
| AP-AS | 0.364 | 0.273–0.456 | <0.001 | 0.252 | 0.150–0.354 | <0.001 |
| Ce-AS | 0.541 | 0.332–0.749 | <0.001 | 0.411 | 0.234–0.588 | <0.001 |
| Ce-PS | 0.310 | 0.182–0.439 | <0.001 | 0.408 | 0.262–0.555 | <0.001 |
| Ce-AP | 0.521 | 0.305–0.737 | <0.001 | 0.508 | 0.347–0.670 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cammalleri, V.; Carpenito, M.; De Stefano, D.; Ussia, G.P.; Bono, M.C.; Mega, S.; Nusca, A.; Cocco, N.; Nobile, E.; De Filippis, A.; et al. Novel Computed Tomography Variables for Assessing Tricuspid Valve Morphology: Results from the TRIMA (Tricuspid Regurgitation IMAging) Study. J. Clin. Med. 2022, 11, 2825. https://doi.org/10.3390/jcm11102825
Cammalleri V, Carpenito M, De Stefano D, Ussia GP, Bono MC, Mega S, Nusca A, Cocco N, Nobile E, De Filippis A, et al. Novel Computed Tomography Variables for Assessing Tricuspid Valve Morphology: Results from the TRIMA (Tricuspid Regurgitation IMAging) Study. Journal of Clinical Medicine. 2022; 11(10):2825. https://doi.org/10.3390/jcm11102825
Chicago/Turabian StyleCammalleri, Valeria, Myriam Carpenito, Domenico De Stefano, Gian Paolo Ussia, Maria Caterina Bono, Simona Mega, Annunziata Nusca, Nino Cocco, Edoardo Nobile, Aurelio De Filippis, and et al. 2022. "Novel Computed Tomography Variables for Assessing Tricuspid Valve Morphology: Results from the TRIMA (Tricuspid Regurgitation IMAging) Study" Journal of Clinical Medicine 11, no. 10: 2825. https://doi.org/10.3390/jcm11102825
APA StyleCammalleri, V., Carpenito, M., De Stefano, D., Ussia, G. P., Bono, M. C., Mega, S., Nusca, A., Cocco, N., Nobile, E., De Filippis, A., Vitez, L., Quattrocchi, C. C., & Grigioni, F. (2022). Novel Computed Tomography Variables for Assessing Tricuspid Valve Morphology: Results from the TRIMA (Tricuspid Regurgitation IMAging) Study. Journal of Clinical Medicine, 11(10), 2825. https://doi.org/10.3390/jcm11102825









