Association between Proton Pump Inhibitor Use and Risk of Hepatocellular Carcinoma: A Korean Nationally Representative Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Key Variables
2.3. Statistical Analysis
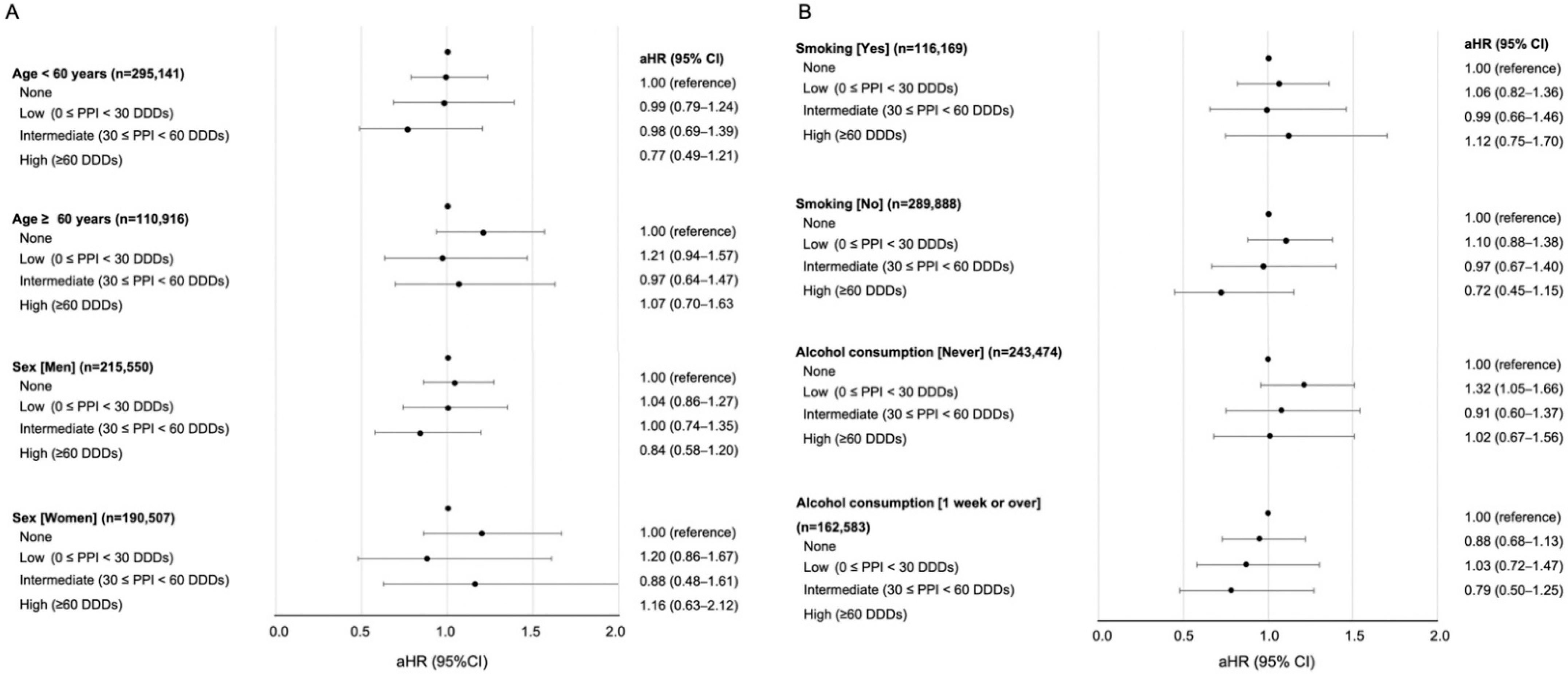
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.L.; Florio, A.A.; Znaor, A.; Ruggieri, D.; Laversanne, M.; Alvarez, C.S.; Ferlay, J.; Valery, P.C.; Bray, F.; McGlynn, K.A. International trends in hepatocellular carcinoma incidence, 1978–2012. Int. J. Cancer 2019, 147, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Ozakyol, A. Global Epidemiology of Hepatocellular Carcinoma (HCC Epidemiology). J. Gastrointest. Cancer 2017, 48, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Chon, Y.E.; Jeong, S.W.; Jun, D.W. Hepatocellular carcinoma statistics in South Korea. Clin. Mol. Hepatol. 2021, 27, 512–514. [Google Scholar] [CrossRef]
- Gomaa, A.; Khan, S.A.; Toledano, M.B.; Waked, I.; Taylor-Robinson, S.D. Hepatocellular carcinoma: Epidemiology, risk factors and pathogenesis. World J. Gastroenterol. 2008, 14, 4300–4308. [Google Scholar] [CrossRef]
- Polesel, J.; Zucchetto, A.; Montella, M.; Maso, L.D.; Crispo, A.; La Vecchia, C.; Serraino, D.; Franceschi, S.; Talamini, R. The impact of obesity and diabetes mellitus on the risk of hepatocellular carcinoma. Ann. Oncol. 2008, 20, 353–357. [Google Scholar] [CrossRef]
- Mittal, S.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma: Consider the population. J. Clin. Gastroenterol. 2013, 47, S2–S6. [Google Scholar] [CrossRef] [Green Version]
- Parkin, D.M. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer 2006, 118, 3030–3044. [Google Scholar] [CrossRef] [Green Version]
- Perz, J.F.; Armstrong, G.L.; Farrington, L.A.; Hutin, Y.J.; Bell, B.P. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J. Hepatol. 2006, 45, 529–538. [Google Scholar] [CrossRef]
- Donato, F.; Tagger, A.; Gelatti, U.; Parrinello, G.; Boffetta, P.; Albertini, A.; Decarli, A.; Trevisi, P.; Ribero, M.L.; Martelli, C.; et al. Alcohol and Hepatocellular Carcinoma: The Effect of Lifetime Intake and Hepatitis Virus Infections in Men and Women. Am. J. Epidemiol. 2002, 155, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.D.; Kim, W.R.; Coelho, R.; Mettler, T.A.; Benson, J.T.; Sanderson, S.O.; Therneau, T.M.; Kim, B.; Roberts, L. Cirrhosis Is Present in Most Patients With Hepatitis B and Hepatocellular Carcinoma. Clin. Gastroenterol. Hepatol. 2011, 9, 64–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, I.C.; Chang, J.; Park, S.M. Association between proton pump inhibitor use and the risk of pancreatic cancer: A Korean nationwide cohort study. PLoS ONE 2018, 13, e0203918. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.-H.J.; Chan, T.-S.; Tsai, K.; Wu, S.-Y. Association between proton pump inhibitors and the risk of hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2018, 48, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, A.J.; Grady, D. Adverse Effects Associated With Proton Pump Inhibitors. JAMA Intern. Med. 2016, 176, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-E.; Huang, Y.-S.; Perng, C.-L.; Hou, M.-C. Use of proton pump inhibitors and the risk of hepatocellular carcinoma. J. Chin. Med. Assoc. 2019, 82, 756–761. [Google Scholar] [CrossRef]
- Song, H.J.; Jiang, X.; Henry, L.; Nguyen, M.H.; Park, H. Proton pump inhibitors and risk of liver cancer and mortality in patients with chronic liver disease: A systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2020, 76, 851–866. [Google Scholar] [CrossRef]
- Seong, S.C.; Kim, Y.-Y.; Khang, Y.-H.; Heon Park, J.; Kang, H.-J.; Lee, H.; Do, C.-H.; Song, J.-S.; Hyon Bang, J.; Ha, S.; et al. Data Resource Profile: The National Health Information Database of the National Health Insurance Service in South Korea. Int. J. Epidemiol. 2017, 46, 799–800. [Google Scholar] [CrossRef] [Green Version]
- Seong, S.C.; Kim, Y.-Y.; Park, S.K.; Khang, Y.-H.; Kim, H.C.; Park, J.H.; Kang, H.-J.; Do, C.-H.; Song, J.-S.; Lee, E.-J.; et al. Cohort profile: The National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open 2017, 7, e016640. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Collaborating Centre for Drug Statistics Methodology, Guide-Lines for ATC Classification and DDD Assignment; WHO Collaborating Centre for Drug Statistics Methodology: Oslo, Norway, 2000. [Google Scholar]
- Cheung, K.S.; Chan, E.W.; Wong, A.Y.S.; Chen, L.; Wong, I.C.K.; Leung, W.K. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: A population-based study. Gut 2017, 67, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Abrahami, D.; McDonald, E.G.; Schnitzer, E.M.; Barkun, A.N.; Suissa, S.; Azoulay, L. Proton pump inhibitors and risk of gastric cancer: Population-based cohort study. Gut 2021, 71, 16–24. [Google Scholar] [CrossRef]
- Hwang, I.C.; Chang, J.; Park, S.M. Emerging hazard effects of proton pump inhibitor on the risk of colorectal cancer in low-risk populations: A Korean nationwide prospective cohort study. PLoS ONE 2017, 12, e0189114. [Google Scholar] [CrossRef] [PubMed]
- Alkhushaym, N.; Almutairi, A.R.; AlThagafi, A.; Fallatah, S.B.; Oh, M.; Martin, J.R.; Babiker, H.M.; McBride, A.; Abraham, I. Exposure to proton pump inhibitors and risk of pancreatic cancer: A meta-analysis. Expert Opin. Drug Saf. 2020, 19, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Brusselaers, N.; Sadr-Azodi, O.; Engstrand, L. Long-term proton pump inhibitor usage and the association with pancreatic cancer in Sweden. J. Gastroenterol. 2019, 55, 453–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, K.T.; McMenamin, C.; Hicks, B.; Murchie, P.; Thrift, A.P.; Coleman, H.G.; Iversen, L.; Johnston, B.T.; Lee, A.J.; Cardwell, C.R. Proton pump inhibitor and histamine-2 receptor antagonist use and risk of liver cancer in two population-based studies. Aliment. Pharmacol. Ther. 2018, 48, 55–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, S.J.; Franco, S.; Young, G.; O’Keefe, S. Altered bowel function and duodenal bacterial overgrowth in patients treated with omeprazole. Aliment. Pharmacol. Ther. 1996, 10, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Thorens, J.; Froehlich, F.; Schwizer, W.; Saraga, E.; Bille, J.; Gyr, K.; Duroux, P.; Nicolet, M.; Pignatelli, B.; Blum, A.L.; et al. Bacterial overgrowth during treatment with omeprazole compared with cimetidine: A prospective randomised double blind study. Gut 1996, 39, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Jansen, P.L. Endogenous bile acids as carcinogens. J. Hepatol. 2007, 47, 434–435. [Google Scholar] [CrossRef]
- Caplin, M.; Khan, K.; Savage, K.; Rode, J.; Varro, A.; Michaeli, D.; Grimes, S.; Brett, B.; Pounder, R.; Dhillon, A. Expression and processing of gastrin in hepatocellular carcinoma, fibrolamellar carcinoma and cholangiocarcinoma. J. Hepatol. 1999, 30, 519–526. [Google Scholar] [CrossRef]
- Fossmark, R.; Sagatun, L.; Nordrum, I.S.; Sandvik, A.K.; Waldum, H.L. Hypergastrinemia is associated with adenocarcinomas in the gastric corpus and shorter patient survival. APMIS 2015, 123, 509–514. [Google Scholar] [CrossRef]
- Thjodleifsson, B. Treatment of acid-related diseases in the elderly with emphasis on the use of proton pump inhibitors. Drugs Aging 2002, 19, 911–927. [Google Scholar] [CrossRef]

| Characteristic | Participant (n = 406,057) |
|---|---|
| Age, years | 51 (45–60) |
| Sex, n (%) | |
| Men | 215,550 (53.1) |
| Women | 190,507 (46.9) |
| Household income, n (%) | |
| First quartile | 64,001 (15.8) |
| Second quartile | 86,612 (21.3) |
| Third quartile | 117,440 (28.9) |
| Fourth quartile (highest) | 138,004 (34.0) |
| Charlson comorbidity index, n (%) | |
| 0 | 179,113 (44.1) |
| 1 | 123,439 (30.4) |
| ≥2 | 103,505 (25.5) |
| Systolic blood pressure, mmHg | 126 (114–138) |
| Diastolic blood pressure, mmHg | 80 (70–85) |
| Body mass index, kg/m2 | 23.9 (22.0–25.8) |
| Fasting serum glucose, mg/dL | 93 (84–104) |
| Cigarette smoking, n (%) | |
| Never | 289,888 (71.4) |
| Past | 34,783 (8.6) |
| Current | 81,386 (20.0) |
| Alcohol consumption frequency, n (%) | |
| 0 | 243,474 (60.0) |
| 2–3/month | 57,115 (14.1) |
| 1–2/week | 64,122 (15.8) |
| 2–3/week | 25,775 (6.3) |
| ≥5/week | 15,571 (3.8) |
| MVPA, time/week, n (%) | |
| 0 | 213,675 (52.6) |
| 1–2 | 103,074 (25.4) |
| 3–4 | 46,404 (11.4) |
| ≥5 | 42,904 (10.6) |
| None | Low (0 ≤ PPI < 30 DDDs) | Intermediate (30 ≤ PPI < 60 DDDs) | High (≥60 DDDs) | p for Trend | |
|---|---|---|---|---|---|
| Participant, n | 341,734 | 38,340 | 14,776 | 11,207 | |
| Overall HCC | |||||
| Event (%) | 1215 (0.4) | 153 (0.4) | 56 (0.4) | 42 (0.4) | |
| Person-year | 2,334,893 | 261,565 | 100,381 | 75,668 | |
| Crude rate/10,000 PY | 5.2 | 5.8 | 5.6 | 5.6 | |
| HR (95% CI) | 1.00 (reference) | 1.12 (0.95–1.33) | 1.07 (0.82–1.40) | 1.07 (0.78–1.45) | 0.546 |
| aHR (95% CI) a | 1.00 (reference) | 1.12 (0.94–1.32) | 1.00 (0.77–1.31) | 0.92 (0.67–1.25) | 0.559 |
| aHR (95% CI) b | 1.00 (reference) | 1.07 (0.91–1.27) | 0.96 (0.73–1.26) | 0.86 (0.63–1.17) | 0.613 |
| Cirrhosis-associated HCC | |||||
| Event (%) | 236 (0.1) | 27 (0.1) | 8 (0.1) | 11 (0.1) | |
| Person-year | 2,330,710 | 260,987 | 100,225 | 75,352 | |
| Crude rate/10,000 PY | 1.0 | 1.0 | 0.8 | 1.5 | |
| HR (95% CI) | 1.00 (reference) | 1.02 (0.69–1.52) | 0.79 (0.39–1.60) | 1.44 (0.79–2.79) | 0.594 |
| aHR (95% CI) a | 1.00 (reference) | 1.02 (0.68–1.51) | 0.74 (0.36–1.49) | 1.25 (0.68–2.28) | 0.731 |
| aHR (95% CI) b | 1.00 (reference) | 1.01 (0.68–1.51) | 0.73 (0.36–1.48) | 1.20 (0.65–2.22) | 0.762 |
| Non-cirrhosis-associated HCC | |||||
| Event (%) | 979 (0.3) | 126 (0.3) | 48 (0.3) | 31 (0.3) | |
| Person-year | 2,335,303 | 261,611 | 100,401 | 75,690 | |
| Crude rate/10,000 PY | 4.2 | 4.8 | 4.8 | 4.1 | |
| HR (95% CI) | 1.00 (reference) | 1.15 (0.95–1.38) | 1.14 (0.85–1.52) | 0.98 (0.68–1.40) | 0.420 |
| aHR (95% CI) a | 1.00 (reference) | 1.14 (0.95–1.37) | 1.06 (0.80–1.42) | 0.84 (0.59–1.20) | 0.364 |
| aHR (95% CI) b | 1.00 (reference) | 1.09 (0.90–1.31) | 1.01 (0.76–1.53) | 0.78 (0.54–1.12) | 0.424 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Jeong, S.; Park, S.J.; Chang, J.; Choi, S.; Cho, Y.; Ahn, J.C.; Lee, G.; Son, J.S.; Park, S.M. Association between Proton Pump Inhibitor Use and Risk of Hepatocellular Carcinoma: A Korean Nationally Representative Cohort Study. J. Clin. Med. 2022, 11, 2865. https://doi.org/10.3390/jcm11102865
Kim S, Jeong S, Park SJ, Chang J, Choi S, Cho Y, Ahn JC, Lee G, Son JS, Park SM. Association between Proton Pump Inhibitor Use and Risk of Hepatocellular Carcinoma: A Korean Nationally Representative Cohort Study. Journal of Clinical Medicine. 2022; 11(10):2865. https://doi.org/10.3390/jcm11102865
Chicago/Turabian StyleKim, Soungmun, Seogsong Jeong, Sun Jae Park, Jooyoung Chang, Seulggie Choi, Yoosun Cho, Joseph C. Ahn, Gyeongsil Lee, Joung Sik Son, and Sang Min Park. 2022. "Association between Proton Pump Inhibitor Use and Risk of Hepatocellular Carcinoma: A Korean Nationally Representative Cohort Study" Journal of Clinical Medicine 11, no. 10: 2865. https://doi.org/10.3390/jcm11102865
APA StyleKim, S., Jeong, S., Park, S. J., Chang, J., Choi, S., Cho, Y., Ahn, J. C., Lee, G., Son, J. S., & Park, S. M. (2022). Association between Proton Pump Inhibitor Use and Risk of Hepatocellular Carcinoma: A Korean Nationally Representative Cohort Study. Journal of Clinical Medicine, 11(10), 2865. https://doi.org/10.3390/jcm11102865






