Long-Term Sex-Specific Effects of Cadmium Exposure on Osteoporosis and Bone Density: A 10-Year Community-Based Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Methods
2.2. Assessment of Cadmium Exposure
2.3. Assessment of Bone Mineral Density and Outcome
2.4. Confounders
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics
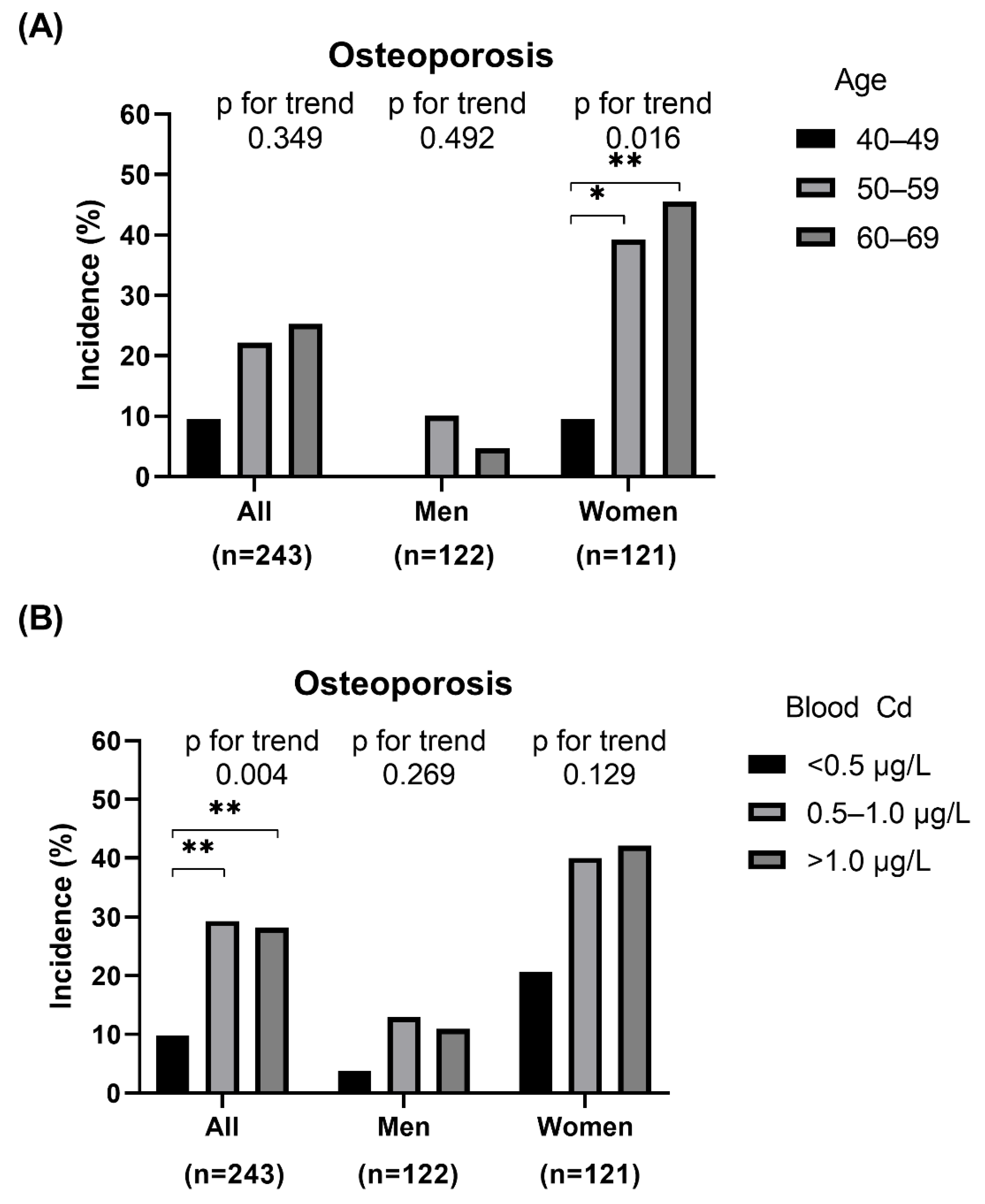
3.2. Associations between Blood Cadmium Level and Osteoporosis and ΔBMD
3.3. Adjusted Regression Models between Blood Cadmium and Osteoporosis and ΔBMD
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elonheimo, H.; Lange, R.; Tolonen, H.; Kolossa-Gehring, M. Environmental Substances Associated with Osteoporosis-A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 738. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.M.; Moon, J.S.; Yoon, J.S.; Won, K.C.; Lee, H.W. Sex-specific effects of blood cadmium on thyroid hormones and thyroid function status: Korean nationwide cross-sectional study. J. Trace Elem. Med. Biol. 2019, 53, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.M.; Moon, J.S.; Yoon, J.S.; Won, K.C.; Lee, H.W. The sex-specific effects of blood lead, mercury, and cadmium levels on hepatic steatosis and fibrosis: Korean nationwide cross-sectional study. J. Trace Elem. Med. Biol. 2020, 62, 126601. [Google Scholar] [CrossRef] [PubMed]
- Tinkov, A.A.; Filippini, T.; Ajsuvakova, O.P.; Aaseth, J.; Gluhcheva, Y.G.; Ivanova, J.M.; Bjorklund, G.; Skalnaya, M.G.; Gatiatulina, E.R.; Popova, E.V.; et al. The role of cadmium in obesity and diabetes. Sci. Total Environ. 2017, 601–602, 741–755. [Google Scholar] [CrossRef]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Diab, D.L.; Eldeiry, L.S.; Farooki, A.; Harris, S.T.; Hurley, D.L.; Kelly, J.; Lewiecki, E.M.; et al. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis- 2020 Update Executive Summary. Endocr. Pract. 2020, 26, 564–570. [Google Scholar] [CrossRef]
- Kijima, T. “Itai-itai” disease (osteoporosis and osteomalacia due to industrial cadmium poisoning). Kangogaku Zasshi 1969, 33, 56–60. [Google Scholar]
- James, K.A.; Meliker, J.R. Environmental cadmium exposure and osteoporosis: A review. Int. J. Public Health 2013, 58, 737–745. [Google Scholar] [CrossRef]
- Ougier, E.; Fiore, K.; Rousselle, C.; Assuncao, R.; Martins, C.; Buekers, J. Burden of osteoporosis and costs associated with human biomonitored cadmium exposure in three European countries: France, Spain and Belgium. Int. J. Hyg. Environ. Health 2021, 234, 113747. [Google Scholar] [CrossRef]
- Kim, Y.; Han, B.G.; Ko, G.E.S.G. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol 2017, 46, e20. [Google Scholar] [CrossRef]
- Prozialeck, W.C.; Edwards, J.R. Early biomarkers of cadmium exposure and nephrotoxicity. BioMetals 2010, 23, 793–809. [Google Scholar] [CrossRef]
- Angerer, J.; Ewers, U.; Wilhelm, M. Human biomonitoring: State of the art. Int. J. Hyg. Environ. Health 2007, 210, 201–228. [Google Scholar] [CrossRef] [PubMed]
- Krieg, M.A.; Barkmann, R.; Gonnelli, S.; Stewart, A.; Bauer, D.C.; del Rio Barquero, L.; Kaufman, J.J.; Lorenc, R.; Miller, P.D.; Olszynski, W.P.; et al. Quantitative ultrasound in the management of osteoporosis: The 2007 ISCD Official Positions. J. Clin. Densitom. 2008, 11, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Hans, D.; Krieg, M.A. The clinical use of quantitative ultrasound (QUS) in the detection and management of osteoporosis. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2008, 55, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Hans, D.; Genton, L.; Allaoua, S.; Pichard, C.; Slosman, D.O. Hip fracture discrimination study: QUS of the radius and the calcaneum. J. Clin. Densitom. 2003, 6, 163–172. [Google Scholar] [CrossRef]
- Rhee, Y.; Lee, J.; Jung, J.Y.; Lee, J.E.; Park, S.Y.; Kim, Y.M.; Lee, S.; Choi, H.S.; Kim, S.H.; Lim, S.K. Modifications of T-scores by quantitative ultrasonography for the diagnosis of osteoporosis in koreans. J. Korean Med. Sci. 2009, 24, 232–236. [Google Scholar] [CrossRef]
- Rivas-Ruiz, R.; Clark, P.; Talavera, J.O.; Huitron, G.; Tamayo, J.A.; Salmeron, J. Bone speed of sound throughout lifetime assessed with quantitative ultrasound in a Mexican population. J. Clin. Densitom. 2015, 18, 68–75. [Google Scholar] [CrossRef]
- Jalili, C.; Kazemi, M.; Taheri, E.; Mohammadi, H.; Boozari, B.; Hadi, A.; Moradi, S. Exposure to heavy metals and the risk of osteopenia or osteoporosis: A systematic review and meta-analysis. Osteoporos. Int. 2020, 31, 1671–1682. [Google Scholar] [CrossRef]
- DeFlorio-Barker, S.A.; Turyk, M.E. Associations between bone mineral density and urinary phthalate metabolites among post-menopausal women: A cross-sectional study of NHANES data 2005–2010. Int. J. Environ. Health Res. 2016, 26, 326–345. [Google Scholar] [CrossRef]
- Youness, E.R.; Mohammed, N.A.; Morsy, F.A. Cadmium impact and osteoporosis: Mechanism of action. Toxicol. Mech. Methods 2012, 22, 560–567. [Google Scholar] [CrossRef]
- Abnosi, M.H.; Golami, S. Cadmium chloride treatment of rats significantly impairs membrane integrity of mesenchymal stem cells via electrolyte imbalance and lipid peroxidation, a possible explanation of Cd related osteoporosis. Iran. J. Basic Med. Sci. 2017, 20, 280–287. [Google Scholar] [CrossRef]
- Luo, H.; Gu, R.; Ouyang, H.; Wang, L.; Shi, S.; Ji, Y.; Bao, B.; Liao, G.; Xu, B. Cadmium exposure induces osteoporosis through cellular senescence, associated with activation of NF-kappaB pathway and mitochondrial dysfunction. Environ. Pollut. 2021, 290, 118043. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ran, D.; Cao, Y.; Zhao, H.; Song, R.; Zou, H.; Gu, J.; Yuan, Y.; Bian, J.; Zhu, J.; et al. The effect of P2X7 on cadmium-induced osteoporosis in mice. J. Hazard. Mater. 2021, 405, 124251. [Google Scholar] [CrossRef] [PubMed]
- Jarup, L.; Alfven, T.; Persson, B.; Toss, G.; Elinder, C.G. Cadmium may be a risk factor for osteoporosis. Occup. Environ. Med. 1998, 55, 435–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La-Up, A.; Mahasakpan, P.; Saengow, U. The current status of osteoporosis after 15 years of reduced cadmium exposure among residents living in cadmium-contaminated areas in northwestern Thailand. Environ. Sci. Pollut. Res. Int. 2021, 28, 20121–20127. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, T.; Geusens, P.; Nulens, T.S.; Nemery, B. Occupational cadmium exposure and calcium excretion, bone density, and osteoporosis in men. J. Bone Miner. Res. 2010, 25, 1441–1445. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Nordberg, G.; Ye, T.; Bo, M.; Wang, H.; Zhu, G.; Kong, Q.; Bernard, A. Osteoporosis and renal dysfunction in a general population exposed to cadmium in China. Environ. Res. 2004, 96, 353–359. [Google Scholar] [CrossRef]
- Kim, Y.D.; Yim, D.H.; Eom, S.Y.; Moon, S.I.; Park, C.H.; Kim, G.B.; Yu, S.D.; Choi, B.S.; Park, J.D.; Kim, H. Differences in the susceptibility to cadmium-induced renal tubular damage and osteoporosis according to sex. Environ. Toxicol. Pharm. 2014, 38, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wallin, M.; Barregard, L.; Sallsten, G.; Lundh, T.; Ohlsson, C.; Mellstrom, D.; Andersson, E.M. Smoking-Induced Risk of Osteoporosis Is Partly Mediated by Cadmium From Tobacco Smoke: The MrOS Sweden Study. J. Bone Miner. Res. 2020, 35, 1424–1429. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, C.M.; Kovach, J.S.; Meliker, J.R. Urinary cadmium and osteoporosis in U.S. Women >or = 50 years of age: NHANES 1988–1994 and 1999–2004. Environ. Health Perspect. 2008, 116, 1338–1343. [Google Scholar] [CrossRef] [Green Version]
- Engstrom, A.; Michaelsson, K.; Vahter, M.; Julin, B.; Wolk, A.; Akesson, A. Associations between dietary cadmium exposure and bone mineral density and risk of osteoporosis and fractures among women. Bone 2012, 50, 1372–1378. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Zhu, G.; Nordberg, G.F.; Jin, T.; Ding, X. The association between cumulative cadmium intake and osteoporosis and risk of fracture in a Chinese population. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Magnus, J.H.; Hentz, J.G. Urinary cadmium, osteopenia, and osteoporosis in the US population. Osteoporos. Int. 2010, 21, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Alfven, T.; Elinder, C.G.; Carlsson, M.D.; Grubb, A.; Hellstrom, L.; Persson, B.; Pettersson, C.; Spang, G.; Schutz, A.; Jarup, L. Low-level cadmium exposure and osteoporosis. J. Bone Miner. Res. 2000, 15, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gan, C.; Zhu, G.; Jin, T. Benchmark dose for estimation of cadmium reference level for osteoporosis in a Chinese female population. Food Chem. Toxicol. 2013, 55, 592–595. [Google Scholar] [CrossRef] [PubMed]
- HBM4EU Substances—Cadmium. Available online: https://www.hbm4eu.eu/hbm4eu-substances/cadmium-and-chromium/ (accessed on 8 February 2022).
- Lim, H.S.; Lee, H.H.; Kim, T.H.; Lee, B.R. Relationship between Heavy Metal Exposure and Bone Mineral Density in Korean Adult. J. Bone Metab. 2016, 23, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.-S.; Shin, S.; Lee, Y.J.; Ha, I.-H. Association between blood cadmium levels and the risk of osteopenia and osteoporosis in Korean post-menopausal women. Arch. Osteoporos. 2021, 16, 1–10. [Google Scholar] [CrossRef]
- Banjabi, A.A.; Kannan, K.; Kumosani, T.A.; Yousef, J.M.; Abulnaja, K.O.; Moselhy, S.S. Association of blood heavy metal levels with osteocalcin abnormality and incidence of osteoporosis in Saudi subjects. Braz. J. Biol. 2021, 83, e248828. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, H.; Cui, W.; Wang, Z.; Zhu, G.; Chen, X.; Jin, T. Nomogram to Predict Cadmium-Induced Osteoporosis and Fracture in a Chinese Female Population. Biol. Trace Elem. Res. 2021, 199, 4028–4035. [Google Scholar] [CrossRef]

| BCd | p-Value | |||
|---|---|---|---|---|
| <0.5 µg/L | 0.50–1.0 µg/L | >1.0 µg/L | ||
| N (%) | 82 | 58 | 103 | |
| Sex, n (%) | ||||
| Male | 53 (64.6) | 23 (39.7) | 46 (44.7) | 0.005 |
| Female | 29 (35.4) | 35 (60.3) | 57 (55.3) | |
| Age, years, n (%) | ||||
| 40–49 | 5 (6.1) | 5 (8.6) | 11 (10.7) | 0.175 |
| 50–59 | 43 (52.4) | 28 (48.3) | 64 (62.1) | |
| 60–69 | 34 (41.5) | 25 (43.1) | 28 (27.2) | |
| Age, mean ± SD | 57.4 ± 6.1 | 57.2 ± 6.1 | 55.9 ± 5.8 | 0.203 |
| Smoking, n (%) | ||||
| Never | 46 (56.1) | 39 (69.6) | 61 (60.4) | 0.016 |
| Former | 20 (24.4) | 6 (10.7) | 9 (8.9) | |
| Ever | 16 (19.5) | 11 (19.6) | 31 (30.7) | |
| Drinking, n (%) | ||||
| Never | 32 (39.5) | 30 (52.6) | 47 (46.1) | 0.27 |
| Former | 12 (14.8) | 3 (5.3) | 8 (7.8) | |
| Current | 37 (45.7) | 24 (42.1) | 47 (46.1) | |
| Moderate-intensity physical activity, n (%) | ||||
| 0–30 min/day | 42 (54.5) | 29 (52.7) | 65 (69.1) | 0.018 |
| 30–60 min/day | 20 (26.0) | 7 (12.7) | 12 (12.8) | |
| >60 min/day | 15 (19.5) | 19 (34.5) | 17 (18.1) | |
| Treatment of rheumatoid arthritis, n (%) | ||||
| No | 57 (95.0) | 34 (94.4) | 50 (89.3) | 0.499 |
| Yes | 3 (5.0) | 2 (5.6) | 6 (10.7) | |
| Prior use of systemic glucocorticoids, n (%) | ||||
| No | 0 (0) | 0 (0) | 0 (0) | NA |
| Yes | 0 (0) | 0 (0) | 0 (0) | |
| BMI, kg/m2 | 24.4 ± 3.4 | 24.6 ± 3.4 | 24.6 ± 3.3 | 0.963 |
| Cre, mg/dL | 0.9 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.201 |
| Baseline BMD, T-score | 0.3 ± 1.7 | 0.3 ± 1.5 | 0.3 ± 1.5 | 0.950 |
| Model 1 HR (95% CI) | Model 2 HR (95% CI) | Model 3 HR (95% CI) | |
|---|---|---|---|
| Total | |||
| Blood Cd: ref. < 0.5 µg/L | |||
| 0.5–1.0 µg/L | 2.46 (1.06, 5.72) * | 1.69 (0.72, 3.95) | 2.33 (0.87, 6.2) |
| >1.0 µg/L | 2.34 (1.07, 5.15) * | 1.9 (0.87, 4.19) | 2.67 (1.03, 6.91) * |
| Men | |||
| Blood Cd: ref. < 0.5 µg/L | |||
| 0.5–1.0 µg/L | 2.27 (0.37, 13.88) | 2.27 (0.37, 13.77) | 0.84 (0.07, 9.71) |
| >1.0 µg/L | 2.08 (0.4, 10.93) | 2.14 (0.41, 11.17) | 1.53 (0.21, 11.15) |
| Women | |||
| Blood Cd: ref. < 0.5 µg/L | |||
| 0.5–1.0 µg/L | 1.86 (0.71, 4.84) | 1.59 (0.61, 4.15) | 3.8 (1.12, 12.84) * |
| >1.0 µg/L | 1.82 (0.74, 4.45) | 1.95 (0.79, 4.79) | 4.24 (1.25, 14.42) * |
| Model 1 Coefficient (95% CI) | Model 2 Coefficient (95% CI) | Model 3 Coefficient (95% CI) | |
|---|---|---|---|
| Total | |||
| Blood Cd: ref. <0.5 µg/L | |||
| 0.5–1.0 µg/L | −0.14 (−0.28, 0) * | −0.12 (−0.26, 0.02) | −0.08 (−0.22, 0.06) |
| >1.0 µg/L | −0.18 (−0.3, −0.06) ** | −0.17 (−0.3, −0.05) ** | −0.15 (−0.28, −0.03) * |
| Men | |||
| Blood Cd: ref. <0.5 µg/L | |||
| 0.5–1.0 µg/L | 0.02 (−0.16, 0.2) | 0.02 (−0.16, 0.2) | 0.08 (−0.1, 0.25) |
| >1.0 µg/L | −0.12 (−0.26, 0.03) | −0.12 (−0.26, 0.03) | −0.08 (−0.22, 0.07) |
| Women | |||
| Blood Cd: ref. <0.5 µg/L | |||
| 0.5–1.0 µg/L | −0.28 (−0.5, −0.06) * | −0.27 (−0.49, −0.05) * | −0.13 (−0.36, 0.1) |
| >1.0 μg/L | −0.26 (−0.46, −0.06) * | −0.26 (−0.46, −0.06) * | −0.2 (−0.41, 0.01) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, S.M. Long-Term Sex-Specific Effects of Cadmium Exposure on Osteoporosis and Bone Density: A 10-Year Community-Based Cohort Study. J. Clin. Med. 2022, 11, 2899. https://doi.org/10.3390/jcm11102899
Chung SM. Long-Term Sex-Specific Effects of Cadmium Exposure on Osteoporosis and Bone Density: A 10-Year Community-Based Cohort Study. Journal of Clinical Medicine. 2022; 11(10):2899. https://doi.org/10.3390/jcm11102899
Chicago/Turabian StyleChung, Seung Min. 2022. "Long-Term Sex-Specific Effects of Cadmium Exposure on Osteoporosis and Bone Density: A 10-Year Community-Based Cohort Study" Journal of Clinical Medicine 11, no. 10: 2899. https://doi.org/10.3390/jcm11102899
APA StyleChung, S. M. (2022). Long-Term Sex-Specific Effects of Cadmium Exposure on Osteoporosis and Bone Density: A 10-Year Community-Based Cohort Study. Journal of Clinical Medicine, 11(10), 2899. https://doi.org/10.3390/jcm11102899






