Association between Non-Steroidal Anti-Inflammatory Drugs Use and the Risk of Type 2 Diabetes Mellitus: A Nationwide Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
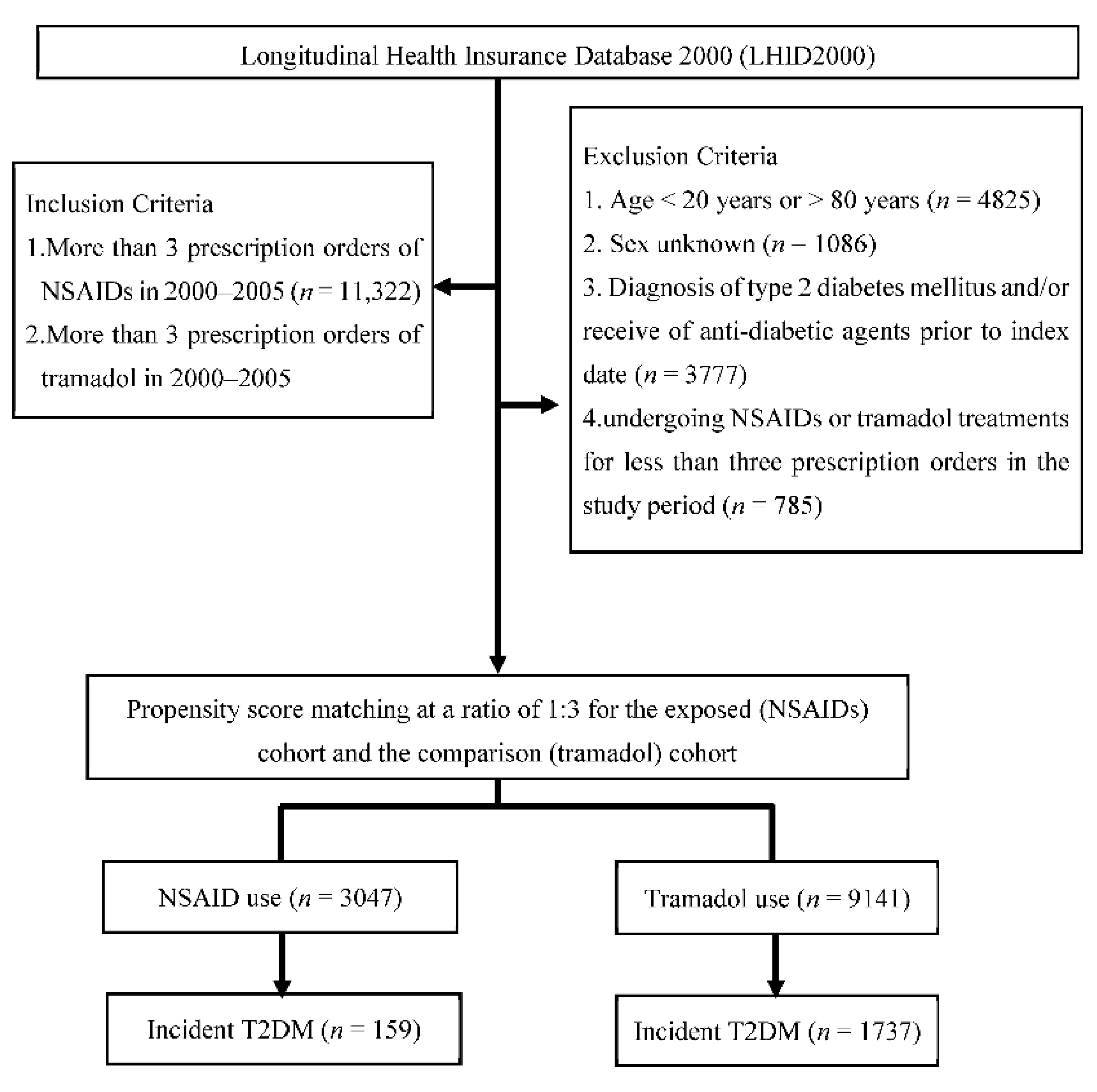
2.2. Study Design and Study Population
2.3. Clinical Outcomes
2.4. Covariate Assessment and Adjustment
2.5. Statistical Analysis
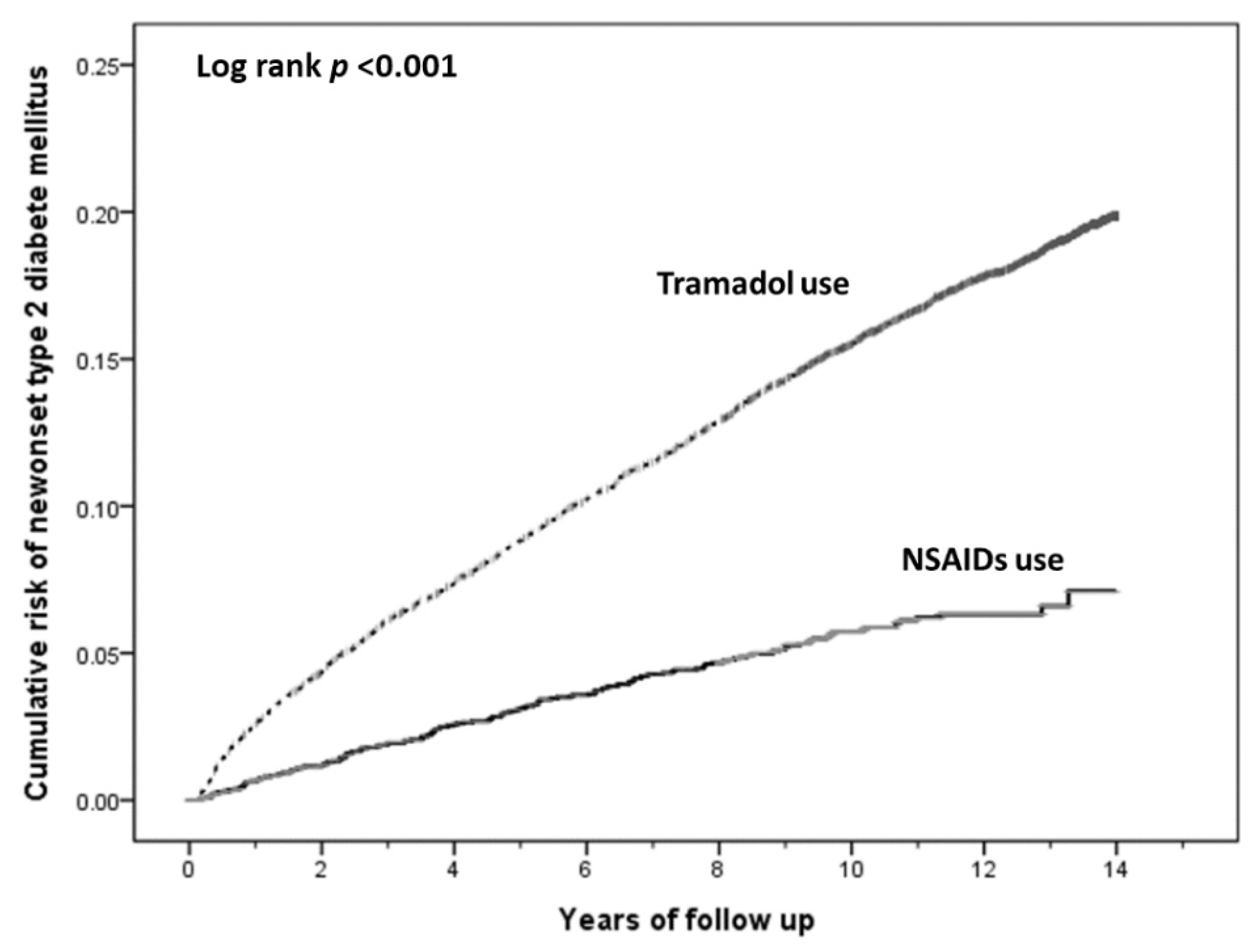
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dahlhamer, J.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; Kerns, R.; Von Korff, M.; Porter, L.; Helmick, C. Prevalence of chronic pain and high-Impact chronic pain among adults—United States, 2016. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Gureje, O.; Von Korff, M.; Simon, G.E.; Gater, R. Persistent pain and well-being: A World Health Organization Study in primary care. JAMA 1998, 280, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Hider-Mlynarz, K.; Cavalié, P.; Maison, P. Trends in analgesic consumption in France over the last 10 years and comparison of patterns across Europe. Br. J. Clin. Pharmacol. 2018, 84, 1324–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMurray, R.W.; Hardy, K.J. Cox-2 inhibitors: Today and tomorrow. Am. J. Med. Sci. 2002, 323, 181–189. [Google Scholar]
- Thomas, T.; Nadackal, G.; Thomas, K. Aspirin and diabetes: Inhibition of amylin aggregation by nonsteroidal anti-inflammatory drugs. Exp. Clin. Endocrinol. Diabetes 2003, 111, 8–11. [Google Scholar]
- Kumar, N.S.; Muktesh, G.; Samra, T.; Sharma, P.; Samanta, J.; Sinha, S.K.; Dhaka, N.; Yadav, T.D.; Gupta, V.; Kochhar, R. Comparison of efficacy of diclofenac and tramadol in relieving pain in patients of acute pancreatitis: A randomized parallel group double blind active controlled pilot study. Eur. J. Pain 2020, 24, 639–648. [Google Scholar] [CrossRef]
- Fournier, J.P.; Azoulay, L.; Yin, H.; Montastruc, J.L.; Suissa, S. Tramadol use and the risk of hospitalization for hypoglycemia in patients with noncancer pain. JAMA Intern. Med. 2015, 175, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Golightly, L.K.; Simendinger, B.A.; Barber, G.R.; Stolpman, N.M.; Kick, S.D.; McDermott, M.T. Hypoglycemic effect of tramadol analgesia in hospitalized patients: A case-control study. J. Diabetes Metab. Disord. 2017, 16, 30. [Google Scholar] [CrossRef] [Green Version]
- Juba, K.M.; van Manen, R.P.; Fellows, S.E. A review of the Food and Drug Administration Adverse Event Reporting System for tramadol-related hypoglycemia. Ann. Pharmacother. 2020, 54, 247–253. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.Y.; Su, C.C.; Shao, S.C.; Sung, S.F.; Lin, S.J.; Kao Yang, Y.H.; Lai, E.C. Taiwan’s National Health Insurance Research Database: Past and future. Clin. Epidemiol. 2019, 11, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.Y.; Chen, Y.J.; Ho, H.J.; Hsu, Y.C.; Kuo, K.N.; Wu, M.S.; Lin, J.T. Association between nucleoside analogues and risk of hepatitis B virus–elated hepatocellular carcinoma recurrence following liver resection. JAMA 2012, 308, 1906–1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.H.; Chou, I.J.; Yeh, Y.H.; Chiou, M.J.; Wen, M.S.; Kuo, C.T.; See, L.C.; Kuo, C.F. Association between use of non–vitamin K oral anticoagulants with and without concurrent medications and risk of major bleeding in nonvalvular atrial fibrillation. JAMA 2017, 318, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Gau, C.S.; Chang, I.S.; Wu, F.L.L.; Yu, H.T.; Huang, Y.W.; Chi, C.L.; Chien, S.Y.; Lin, K.M.; Liu, M.Y.; Wang, H.P. Usage of the claim database of national health insurance programme for analysis of cisapride-erythromycin co-medication in Taiwan. Pharmacoepidemiol. Drug Saf. 2007, 16, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.L.; Kao, Y.H.; Lin, S.J.; Lee, C.H.; Lai, M.L. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol. Drug Saf. 2011, 20, 236–242. [Google Scholar] [CrossRef]
- Johnson, E.S.; Bartman, B.A.; Briesacher, B.A.; Fleming, N.S.; Gerhard, T.; Kornegay, C.J.; Nourjah, P.; Sauer, B.; Schumock, G.T.; Sedrakyan, A.; et al. The incident user design in comparative effectiveness research. Pharmacoepidemiol. Drug Saf. 2013, 22, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Seeger, J.D.; Walker, A.M.; Williams, P.L.; Saperia, G.M.; Sacks, F.M. A propensity score-matched cohort study of the effects of statins, mainly fluvastatin, on the occurrence of acute myocardial infarction. Am. J. Cardiol. 2003, 92, 1447–1451. [Google Scholar] [CrossRef]
- Patorno, E.; Grotta, A.; Bellocco, R.; Schneeweiss, S. Propensity score methodology for confounding control in health care utilization databases. Epidemiol. Biostat. Public Health. 2013, 10, e8940. [Google Scholar]
- Gill, R.; Schumacher, M. A simple test of the proportional hazards assumption. Biometrika 1987, 74, 289–300. [Google Scholar] [CrossRef]
- Wertheimer, A.I. The defined daily dose system (DDD) for drug utilization review. Hosp. Pharm. 1986, 21, 233–234. [Google Scholar]
- Zhou, Y.; Boudreau, D.M.; Freedman, A.N. Trends in the use of aspirin and nonsteroidal anti-inflammatory drugs in the general U.S. population. Pharmacoepidemiol. Drug Saf. 2013, 23, 43–50. [Google Scholar] [CrossRef]
- Hawkey, C.J. COX-1 and COX-2 inhibitors. Best Pract. Res. Clin. Gastroenterol. 2001, 15, 801–820. [Google Scholar] [CrossRef]
- Simon, L.S. Role and regulation of cyclooxygenase-2 during inflammation. Am. J. Med. 1999, 106, 37S–42S. [Google Scholar] [CrossRef]
- Conaghan, P.G. A turbulent decade for NSAIDs: Update on current concepts of classification, epidemiology, comparative efficacy, and toxicity. Rheumatol. Int. 2011, 32, 1491–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabatabaie, T.; Waldon, A.M.; Jacob, J.M.; Floyd, R.A.; Kotake, Y. COX-2 inhibition prevents insulin-dependent diabetes in low-dose streptozotocin-treated mice. Biochem. Biophys. Res. Commun. 2000, 273, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, N.; Ye, B.; Ju, W.; Orser, B.; Fox, J.E.M.; Wheeler, M.B.; Wang, Q.; Lu, W.Y. Non-steroidal anti-inflammatory drugs increase insulin release from beta cells by inhibiting ATP-sensitive potassium channels. Br. J. Pharmacol. 2007, 151, 483–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hieronymus, L.; Griffin, S. Role of amylin in type 1 and type 2 diabetes. Diabetes Educ. 2015, 41, 47S–56S. [Google Scholar] [CrossRef]
- Mugunthan, N.; Davoren, P. Danger of hypoglycemia due to acute tramadol poisoning. Endocr. Pract. 2012, 18, e151–e152. [Google Scholar] [CrossRef]
- Li, S.Y.; Chen, H.H.; Lin, C.L.; Yeh, S.Y.; Kao, C.H. Association of tramadol and hypoglycemia in diabetic Asians. J. Clin. Med. 2018, 7, 380. [Google Scholar] [CrossRef] [Green Version]
- Hyman, J. The limitations of using insurance data for research. J. Am. Dent. Assoc. 2015, 146, 283–285. [Google Scholar] [CrossRef]
| Variable | Study Cohorts | p Value | |
|---|---|---|---|
| Tramadol (n = 9141) | NSAIDs (n = 3047) | ||
| Age group (No., %) | |||
| 18 ≤ age < 30 | 1378 (11.3) | 993 (10.9) | |
| 30 ≤ age < 40 | 1847 (15.2) | 1394 (15.2) | |
| 40 ≤ age < 50 | 2628 (21.6) | 2011 (22.0) | |
| 50 ≤ age < 60 | 2202 (18.1) | 1715 (18.8) | |
| 60 ≤ age < 70 | 2074 (17.0) | 1596 (17.5) | |
| 70 ≤ age < 80 | 2059 (16.9) | 1432 (15.7) | |
| Gender (No., %) | |||
| Female | 3959 (43.3) | 1257 (41.3) | |
| Male | 5182 (56.7) | 1790 (58.7) | |
| Comorbidities (No., %) | |||
| Chronic liver disease | 1340 (14.7) | 441 (14.5) | 0.801 |
| Malignant neoplasms | 2205 (24.1) | 702 (23.0) | 0.224 |
| Hyperlipidemia | 646 (7.1) | 215 (7.1) | 0.984 |
| Hypertension | 3048 (33.3) | 981 (32.2) | 0.243 |
| Coronary artery disease | 1148 (12.6) | 408 (13.4) | 0.234 |
| Concomitant medications (No., %) | |||
| Beta-blockade | 2568 (28.1) | 851 (27.9) | 0.861 |
| Statins | 848 (9.3) | 276 (9.1) | 0.718 |
| Corticosteroids | 4061 (44.4) | 1422 (46.7) | 0.031 |
| Variable | No. of Subjects | No. of T2DM Cases | Crude HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|
| Overall | ||||
| Tramadol | 9141 | 1737 | 1.00 | 1.00 |
| NSAIDs | 3047 | 159 | 0.34 (0.29–0.40) | 0.31 (0.26–0.36) |
| Exposure duration (days) | ||||
| 1–3215 | 10154 | 1873 | 1.00 | 1.00 |
| 3216–4013 | 1016 | 20 | 0.12 (0.07–0.18) | 0.11 (0.07–0.17) |
| ≧4014 | 3 | 0.06 (0.02–0.14) | 0.04 (0.01–0.12) | |
| p for trend | 1018 | <0.001 | <0.001 | |
| cDDD | ||||
| 0–15 | 10,164 | 1791 | 1.00 | 1.00 |
| 16–32 | 1017 | 55 | 0.56 (0.43–0.73) | 0.74 (0.51–1.08) |
| ≧32 | 1007 | 50 | 0.34 (0.26–0.46) | 0.50 (0.34–0.73) |
| p for trend | <0.001 | 0.002 |
| Variable | No. of Subjects | No. of T2DM Cases | Crude HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|
| Gender | ||||
| Male | ||||
| Tramadol | 5182 | 1087 | 1.00 | 1.00 |
| NSAIDs | 1790 | 101 | 0.33 (0.27–0.40) | 0.30 (0.25–0.37) |
| Female | ||||
| Tramadol | 3959 | 650 | 1.00 | 1.00 |
| NSAIDs | 1257 | 58 | 0.36 (0.27–0.47) | 0.35 (0.27–0.46) |
| Age (years) | ||||
| <40 | ||||
| Tramadol | 2387 | 194 | 1.00 | 1.00 |
| NSAIDs | 838 | 15 | 0.34 (0.20–0.57) | 0.33 (0.19–0.55) |
| 40–59 | ||||
| Tramadol | 3726 | 874 | 1.00 | 1.00 |
| NSAIDs | 1104 | 92 | 0.42 (0.34–0.52) | 0.38 (0.31–0.48) |
| ≧60 | ||||
| Tramadol | 3028 | 669 | 1.00 | 1.00 |
| NSAIDs | 1105 | 52 | 0.26 (0.20–0.35) | 0.26 (0.19–0.34) |
| Stratified Variable | Crude HR (95% CI) | p-Value | Adjusted HR (95% CI) | p-Value |
|---|---|---|---|---|
| Baseline comorbidities | ||||
| Chronic liver disease | ||||
| Without | 0.35 (0.29–0.42) | <0.001 | 0.33 (0.28–0.40) | <0.001 |
| With | 0.27 (0.17–0.45) | <0.001 | 0.25 (0.15–0.41) | <0.001 |
| Malignant neoplasms | ||||
| Without | 0.33 (0.28–0.39) | <0.001 | 0.33 (0.28–0.38) | <0.001 |
| With | 0.26 (0.11–0.59) | 0.001 | 0.25 (0.11–0.56) | 0.001 |
| Hyperlipidemia | ||||
| Without | 0.34 (0.28–0.40) | <0.001 | 0.32 (0.27–0.38) | <0.001 |
| With | 0.36 (0.21–0.63) | <0.001 | 0.37 (0.21–0.63) | <0.001 |
| Hypertension | ||||
| Without | 0.29 (0.24–0.37) | <0.001 | 0.28 (0.22–0.34) | <0.001 |
| With | 0.43 (0.33–0.55) | <0.001 | 0.42 (0.33–0.54) | <0.001 |
| Coronary artery disease | ||||
| Without | 0.34 (0.29–0.41) | <0.001 | 0.32 (0.27–0.38) | <0.001 |
| With | 0.33 (0.20–0.54) | <0.001 | 0.34 (0.20–0.55) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.-H.; Wu, W.-T.; Chen, Y.-C.; Lu, C.-H.; Su, S.-C.; Kuo, F.-C.; Chou, Y.-C.; Sun, C.-A. Association between Non-Steroidal Anti-Inflammatory Drugs Use and the Risk of Type 2 Diabetes Mellitus: A Nationwide Retrospective Cohort Study. J. Clin. Med. 2022, 11, 3186. https://doi.org/10.3390/jcm11113186
Lin M-H, Wu W-T, Chen Y-C, Lu C-H, Su S-C, Kuo F-C, Chou Y-C, Sun C-A. Association between Non-Steroidal Anti-Inflammatory Drugs Use and the Risk of Type 2 Diabetes Mellitus: A Nationwide Retrospective Cohort Study. Journal of Clinical Medicine. 2022; 11(11):3186. https://doi.org/10.3390/jcm11113186
Chicago/Turabian StyleLin, Ming-Hsun, Wen-Tung Wu, Yong-Chen Chen, Chieh-Hua Lu, Sheng-Chiang Su, Feng-Chih Kuo, Yu-Ching Chou, and Chien-An Sun. 2022. "Association between Non-Steroidal Anti-Inflammatory Drugs Use and the Risk of Type 2 Diabetes Mellitus: A Nationwide Retrospective Cohort Study" Journal of Clinical Medicine 11, no. 11: 3186. https://doi.org/10.3390/jcm11113186
APA StyleLin, M.-H., Wu, W.-T., Chen, Y.-C., Lu, C.-H., Su, S.-C., Kuo, F.-C., Chou, Y.-C., & Sun, C.-A. (2022). Association between Non-Steroidal Anti-Inflammatory Drugs Use and the Risk of Type 2 Diabetes Mellitus: A Nationwide Retrospective Cohort Study. Journal of Clinical Medicine, 11(11), 3186. https://doi.org/10.3390/jcm11113186






