Long-Term Outcomes of Robotic-Assisted, Video-Assisted and Open Surgery in Non-Small Cell Lung Cancer: A Matched Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preoperative Staging
2.2. Surgical Approach
2.3. Post-Operative Management and Follow-Up
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nwogu, C.E.; Groman, A.; Fahey, D.; Yendamuri, S.; Dexter, E.; Demmy, T.L.; Miller, A.; Reid, M. Number of Lymph Nodes and Metastatic Lymph Node Ratio Are Associated with Survival in Lung Cancer. Ann. Thorac. Surg. 2012, 93, 1614–1620. [Google Scholar] [CrossRef]
- Casiraghi, M.; Galetta, D.; Borri, A.; Tessitore, A.; Romano, R.; Diotti, C.; Brambilla, D.; Maisonneuve, P.; Spaggiari, L. Ten Years’ Experience in Robotic-Assisted Thoracic Surgery for Early Stage Lung Cancer. Thorac. Cardiovasc. Surg. 2019, 67, 564–572. [Google Scholar] [CrossRef]
- Howington, J.A.; Blum, M.G.; Chang, A.C.; Balekian, A.A.; Murthy, S.C. Treatment of Stage I and II Non-small Cell Lung Cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e278S–e313S. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Chair, V.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Lurie, R.H.; Bruno, D.S.; Chang, J.Y.; et al. Continue NCCN Guidelines Panel Disclosures NCCN Guidelines Version 3.2020 Non-Small Cell Lung Cancer. 2020. Available online: https://www2.tri-kobe.org/nccn/guideline/lung/english/non_small.pdf (accessed on 8 June 2022).
- Lutz, J.A.; Seguin-Givelet, A.; Grigoroiu, M.; Brian, E.; Girard, P.; Gossot, D. Oncological results of full thoracoscopic major pulmonary resections for clinical Stage I non-small-cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2018, 55, 263–270. [Google Scholar] [CrossRef]
- Oparka, J.; Yan, T.D.; Ryan, E.; Dunning, J. Does video-assisted thoracic surgery provide a safe alternative to conventional techniques in patients with limited pulmonary function who are otherwise suitable for lung resection? Interact. Cardiovasc. Thorac. Surg. 2013, 17, 159–162. [Google Scholar] [CrossRef]
- Whitson, B.A.; D’Cunha, J.; Andrade, R.S.; Kelly, R.F.; Groth, S.S.; Wu, B.; Miller, J.S.; Kratzke, R.A.; Maddaus, M.A. Thoracoscopic Versus Thoracotomy Approaches to Lobectomy: Differential Impairment of Cellular Immunity. Ann. Thorac. Surg. 2008, 86, 1735–1744. [Google Scholar] [CrossRef]
- Mahieu, J.; Rinieri, P.; Bubenheim, M.; Calenda, E.; Melki, J.; Peillon, C.; Baste, J.-M. Robot-Assisted Thoracoscopic Surgery versus Video-Assisted Thoracoscopic Surgery for Lung Lobectomy: Can a Robotic Approach Improve Short-Term Outcomes and Operative Safety? Thorac. Cardiovasc. Surg. 2016, 64, 354–362. [Google Scholar] [CrossRef]
- Novellis, P.; Bottoni, E.; Voulaz, E.; Cariboni, U.; Testori, A.; Bertolaccini, L.; Giordano, L.; Dieci, E.; Granato, L.; Vanni, E.; et al. Robotic surgery, video-assisted thoracic surgery, and open surgery for early stage lung cancer: Comparison of costs and outcomes at a single institute. J. Thorac. Dis. 2018, 10, 790–798. [Google Scholar] [CrossRef]
- Oh, D.S.; Reddy, R.M.; Gorrepati, M.L.; Mehendale, S.; Reed, M.F. Robotic-Assisted, Video-Assisted Thoracoscopic and Open Lobectomy: Propensity-Matched Analysis of Recent Premier Data. Ann. Thorac. Surg. 2017, 104, 1733–1740. [Google Scholar] [CrossRef]
- Paul, S.; Jalbert, J.; Isaacs, A.; Altorki, N.K.; Isom, O.W.; Sedrakyan, A. Comparative Effectiveness of Robotic-Assisted vs Thoracoscopic Lobectomy. Chest 2014, 146, 1505–1512. [Google Scholar] [CrossRef]
- Louie, B.E.; Wilson, J.L.; Kim, S.; Cerfolio, R.J.; Park, B.J.; Farivar, A.S.; Vallieres, E.; Aye, R.W.; Burfeind, W.R.; Block, M.I. Comparison of Video-Assisted Thoracoscopic Surgery and Robotic Approaches for Clinical Stage I and Stage II Non-Small Cell Lung Cancer Using the Society of Thoracic Surgeons Database. Ann. Thorac. Surg. 2016, 102, 917–924. [Google Scholar] [CrossRef]
- Guo, F.; Ma, D.; Li, S.; Adamek, M. Compare the prognosis of Da Vinci robot-assisted thoracic surgery (RATS) with video-assisted thoracic surgery (VATS) for non-small cell lung cancer. Medicine 2019, 98, e17089. [Google Scholar] [CrossRef]
- Emmert, A.; Straube, C.; Buentzel, J.; Roever, C. Robotic versus thoracoscopic lung resection. Medicine 2017, 96, e7633. [Google Scholar] [CrossRef]
- Liang, H.; Liang, W.; Zhao, L.; Chen, D.; Zhang, J.; Zhang, Y.; Tang, S.; He, J. Robotic Versus Video-assisted Lobectomy/Segmentectomy for Lung Cancer. Ann. Surg. 2018, 268, 254–259. [Google Scholar] [CrossRef]
- Casiraghi, M.; Sedda, G.; Diotti, C.; Mariolo, A.V.; Galetta, D.; Tessitore, A.; Maisonneuve, P.; Spaggiari, L. Postoperative outcomes of robotic-assisted lobectomy in obese patients with non-small-cell lung cancer. Interact. Cardiovasc. Thorac. Surg. 2019, 30, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, M.; Spaggiari, L. Robotic lobectomy has the greatest benefit in patients with marginal pulmonary function. J. Thorac. Dis. 2019, 11, S322–S324. [Google Scholar] [CrossRef] [PubMed]
- Toosi, K.; Velez-Cubian, F.O.; Glover, J.; Ng, E.P.; Moodie, C.C.; Garrett, J.R.; Fontaine, J.P.; Toloza, E.M. Upstaging and survival after robotic-assisted thoracoscopic lobectomy for non-small cell lung cancer. Surgery 2016, 160, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Galetta, D.; Casiraghi, M.; Pardolesi, A.; Borri, A.; Spaggiari, L. New stapling devices in robotic surgery. J. Vis. Surg. 2017, 3, 45. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Upham, T.C.; Onaitis, M.W. Video-assisted thoracoscopic surgery versus robot-assisted thoracoscopic surgery versus thoracotomy for early-stage lung cancer. J. Thorac. Cardiovasc. Surg. 2018, 156, 365–368. [Google Scholar] [CrossRef]
- Montagne, F.; Bottet, B.; Sarsam, M.; Mbadinga, F.; Chaari, Z.; Rinieri, P.; Melki, J.; Peillon, C.; Baste, J.-M. Robotic versus open and video-assisted thoracoscopic surgery approaches for lobectomy. Mini-Invasive Surg. 2020, 2020, 17. [Google Scholar] [CrossRef]
- Lee, B.E.; Shapiro, M.; Rutledge, J.R.; Korst, R.J. Nodal Upstaging in Robotic and Video Assisted Thoracic Surgery Lobectomy for Clinical N0 Lung Cancer. Ann. Thorac. Surg. 2015, 100, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.L.; Louie, B.E.; Cerfolio, R.J.; Park, B.J.; Vallières, E.; Aye, R.W.; Abdel-Razek, A.; Bryant, A.; Farivar, A.S. The Prevalence of Nodal Upstaging During Robotic Lung Resection in Early Stage Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2014, 97, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Licht, P.B.; Jørgensen, O.D.; Ladegaard, L.; Jakobsen, E. A National Study of Nodal Upstaging After Thoracoscopic Versus Open Lobectomy for Clinical Stage I Lung Cancer. Ann. Thorac. Surg. 2013, 96, 943–950. [Google Scholar] [CrossRef]
- Boffa, D.J.; Kosinski, A.S.; Paul, S.; Mitchell, J.D.; Onaitis, M. Lymph Node Evaluation by Open or Video-Assisted Approaches in 11,500 Anatomic Lung Cancer Resections. Ann. Thorac. Surg. 2012, 94, 347–353. [Google Scholar] [CrossRef]
- Kneuertz, P.J.; Cheufou, D.H.; D’Souza, D.M.; Mardanzai, K.; Abdel-Rasoul, M.; Theegarten, D.; Moffatt-Bruce, S.D.; Aigner, C.; Merritt, R.E. Propensity-Score Adjusted Comparison of Pathologic Nodal Upstaging by Robotic, Video-Assisted Thoraco-scopic, and Open Lobectomy for Non–Small Cell Lung Cancer. J. Thorac. Cardiovasc. Surg. 2019, 158, 1457–1466.e2. [Google Scholar] [CrossRef]
- Yang, H.-X.; Woo, K.M.; Sima, C.S.; Bains, M.S.; Adusumilli, P.S.; Huang, J.; Finley, D.J.; Rizk, N.P.; Rusch, V.; Jones, D.R.; et al. Long-Term Survival Based on the Surgical Approach to Lobectomy for Clinical Stage I Non-Small Cell Lung Cancer: Comparison of Robotic, Video Assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann. Surg. 2017, 265, 431–437. [Google Scholar] [CrossRef]
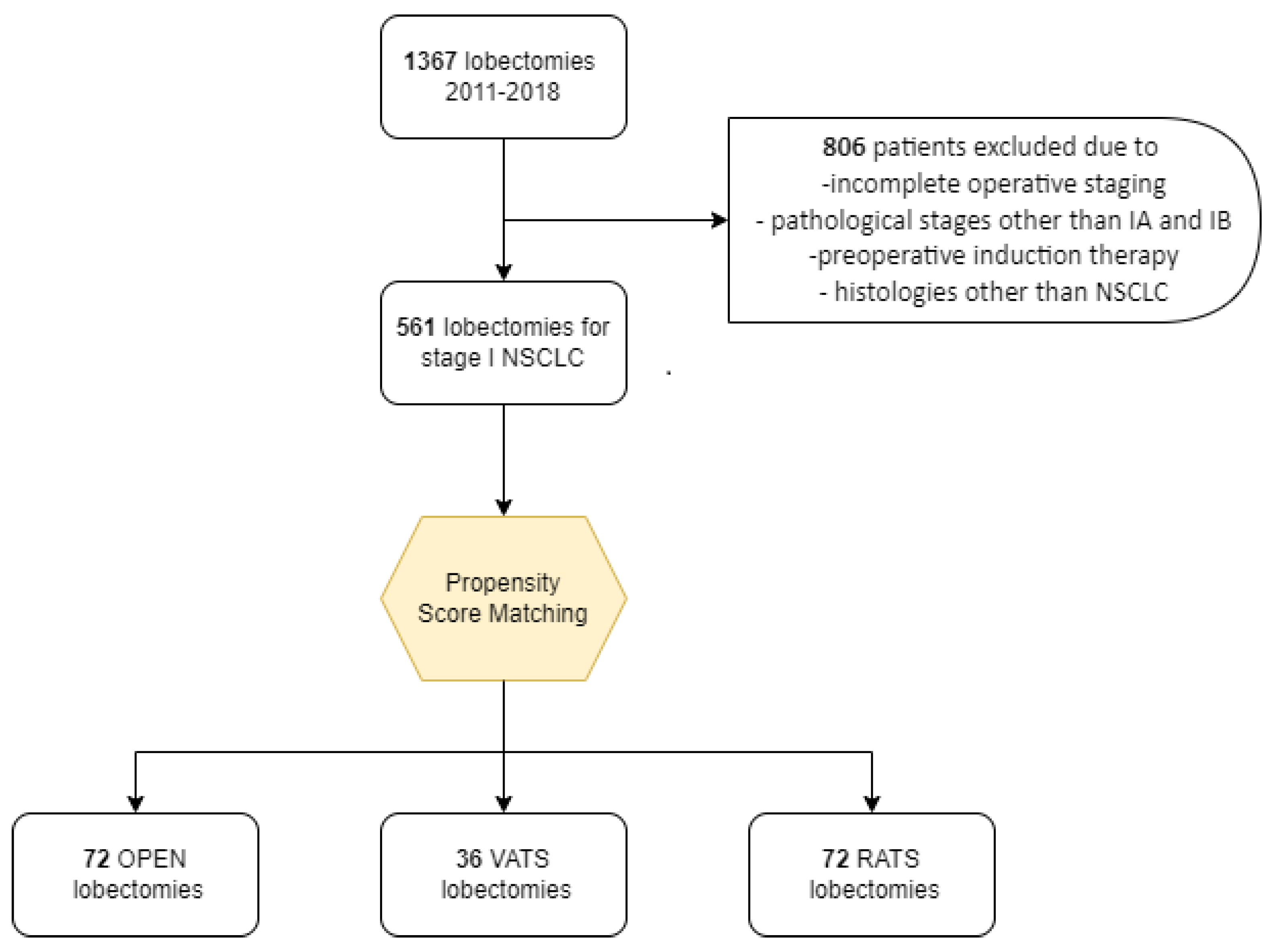
| Open | VATS | RATS | Open vs. VATS † | Open vs. RATS † | VATS vs. RATS † | ||
|---|---|---|---|---|---|---|---|
| 72 * (100.0) | 36 * (100.0) | 72 * (100.0) | |||||
| Age, years, median [range] | 68 (7–79) | 67 (52–80) | 68 (51–77) | MATCHING VARIABLE | |||
| Sex | Male | 32 (44.4) | 16 (44.4) | 32 (44.4) | MATCHING VARIABLE | ||
| Female | 40 (55.6) | 20 (55.6) | 40 (55.6) | ||||
| Body mass | Underweight | 4 (5.6) | 2 (5.6) | 1 (1.4) | |||
| index | Normal weight | 38 (52.8) | 15 (41.7) | 29 (40.3) | |||
| Overweight | 19 (26.4) | 13 (36.1) | 26 (36.1) | ||||
| Obese | 11 (15.3) | 6 (16.7) | 16 (22.2) | 0.66 | 0.19 | 0.62 | |
| Smoking status | Never | 20 (27.8) | 7 (19.4) | 17 (23.6) | |||
| Former | 27 (37.5) | 16 (44.4) | 16 (22.2) | ||||
| Current | 25 (34.7) | 13 (36.1) | 39 (54.2) | 0.58 | 0.06 | 0.05 | |
| Diabetes | No | 68 (94.4) | 30 (83.3) | 61 (84.7) | |||
| Yes | 4 (5.6) | 6 (16.7) | 11 (15.3) | 0.05 | 0.05 | 0.86 | |
| Cardiac comorbidity | No | 26 (36.1) | 16 (44.4) | 32 (44.4) | |||
| Yes | 46 (63.9) | 20 (55.6) | 40 (55.6) | 0.37 | 0.30 | 1.00 | |
| Pulmonary comorbidity | No | 64 (88.9) | 28 (77.8) | 68 (94.4) | |||
| Yes | 8 (11.1) | 8 (22.2) | (5.6) | 0.12 | 0.23 | 0.01 | |
| ASA score | 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | MATCHING VARIABLE | ||
| 2 | 66 (91.7) | 33 (91.7) | 66 (91.7) | ||||
| 3 | 6 (8.3) | 3 (8.3) | 6 (8.3) | ||||
| Clinical | Stage Ia1 | 6 (8.3) | 3 (8.3) | 6 (8.3) | MATCHING VARIABLE | ||
| Stage | Stage Ia2 | 34 (47.2) | 17 (47.2) | 34 (47.2) | |||
| Stage Ia3 | 22 (30.6) | 11 (30.6) | 22 (30.6) | ||||
| Stage Ib | 10 (13.9) | 5 (13.9) | 10 (13.9) | ||||
| Laterality | Left | 33 (45.8) | 16 (44.4) | 31 (43.1) | |||
| Right | 39 (54.2) | 20 (55.6) | 41 (56.9) | 0.89 | 0.74 | 0.88 | |
| Site | Upper lobe | 52 (72.2) | 19 (52.8) | 46 (63.9) | |||
| Medial lobe | 2 (2.8) | 1 (2.8) | 5 (6.9) | ||||
| Lower lobe | 18 (25.0) | 16 (44.4) | 21 (29.2) | 0.17 | 0.38 | 0.22 | |
| Histology | Adenocarcinoma | 60 (83.3) | 30 (83.3) | 58 (80.6) | |||
| Squamous | 9 (12.5) | 4 (11.1) | 7 (9.7) | ||||
| Adeno-squamous | 2 (2.8) | 1 (2.8) | 1 (1.4) | ||||
| Other | 1 (1.4) | 1 (2.8) | 6 (8.3) | 0.96 | 0.24 | 0.71 | |
| Diameter, median [range] | 21 [8–55] | 21 [10–60] | 21 [8–55] | ||||
| <10 mm | 4 (5.6) | 0 (0.0) | 1 (1.4) | ||||
| 10–19 mm | 25 (34.7) | 17 (47.2) | 30 (41.7) | ||||
| 20–29 mm | 24 (33.3) | 8 (22.2) | 25 (34.7) | ||||
| 30–49 mm | 17 (23.6) | 10 (27.8) | 15 (20.8) | ||||
| ≥50 mm | 2 (2.8) | 1 (2.8) | 1 (1.4) | 0.38 | 0.62 | 0.62 | |
| pT | 1 | 51 (70.8) | 26 (72.2) | 49 (68.1) | |||
| 2 | 18 (25.0) | 8 (22.2) | 22 (30.6) | ||||
| 3 | 3 (4.2) | 2 (5.6) | 1 (1.4) | ||||
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.90 | 0.52 | 0.35 | |
| pN | 0 | 50 (69.4) | 29 (80.6) | 66 (91.7) | |||
| 1 | 15 (20.8) | 5 (13.9) | 2 (2.8) | ||||
| 2 | 7 (9.7) | 2 (5.6) | 4 (5.6) | 0.41 | 0.002 | 0.10 | |
| Pathological stage | I | 46 (63.9) | 26 (72.2) | 65 (90.3) | |||
| (TNM 8th edition) | II | 19 (26.4) | 8 (22.2) | 3 (4.2) | |||
| III | 7 (9.7) | 2 (5.6) | 4 (5.6) | 0.56 | 0.0003 | 0.01 | |
| Open n = 72 | VATS n = 36 | RATS n = 72 | Open vs. VATS † | Open vs. RATS † | VATS vs. RATS † | ||
|---|---|---|---|---|---|---|---|
| Postoperative complications | None | 52 (72.2) | 31 (86.1) | 59 (81.9) | |||
| Minor | 18 (25.0) | 5 (13.9) | 9 (12.5) | ||||
| Major | 2 (2.8) | 0 (0.0) | 4 (5.6) | 0.28 | 0.11 | 0.36 | |
| Clavien 1 | 2 (2.8) | 1 (2.8) | 1 (1.4) | ||||
| Clavien 2 | 17 (23.6) | 4 (11.1) | 9 (12.5) | ||||
| Clavien 3a | 1 (1.4) | 0 (0.0) | 1 (1.4) | ||||
| Clavien 3b | 0 (0.0) | 0 (0.0) | 2 (2.8) | 0.45 | 0.27 | 0.78 | |
| Clavien 3a–3b | Complications | 1 (1.4) | 0 (0.0) | 3 (4.2) | 0.48 | 0.32 | 0.22 |
| only | Cardiac | 1 (1.4) | 0 (0.0) | 1 (1.4) | 0.48 | 1.00 | 0.49 |
| Pulmonary | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0.48 | 0.32 | - | |
| Surgical | 0 (0.0) | 0 (0.0) | 2 (2.8) | - | 0.16 | 0.32 | |
| Lymph node | median [range] | 22 [7–49] | 15 [4–25] | 17 [6–37] | 0.0001 | 0.004 | 0.04 |
| Removed | Positive | 0 [0–19] | 0 [0–13] | 0 [0–12] | 0.95 | 0.39 | 0.40 |
| Ratio Positive/Removed (%) | 3% ± 10% | 5% ± 16% | 2% ± 10% | 0.57 | 0.52 | 0.33 | |
| N1 Removed | median [range] | 10 [1–38] | 9 [1–20] | 10 [1–32] | 0.12 | 0.33 | 0.50 |
| Positive | 0 [0–3] | 0 [0–11] | 0 [0–9] | 0.31 | 0.34 | 0.17 | |
| Ratio Positive/Removed (%) | 5% ± 12% | 5% ± 18% | 3% ± 12% | 0.95 | 0.22 | 0.37 | |
| N2 Removed | median [range] | 10 [0–28] | 4 [1–14] | 6 [0–21] | <0.0001 | 0.002 | 0.04 |
| Positive | 0 [0–16] | 0 [0–2] | 0 [0–8] | 0.44 | 0.55 | 0.58 | |
| Ratio Positive/Removed (%) | 2% ± 11% | 3% ± 12% | 2% ± 8% | 0.90 | 0.59 | 0.65 | |
| Open | VATS | RATS | Open vs. VATS † | Open vs. RATS † | VATS vs. RATS † | |
|---|---|---|---|---|---|---|
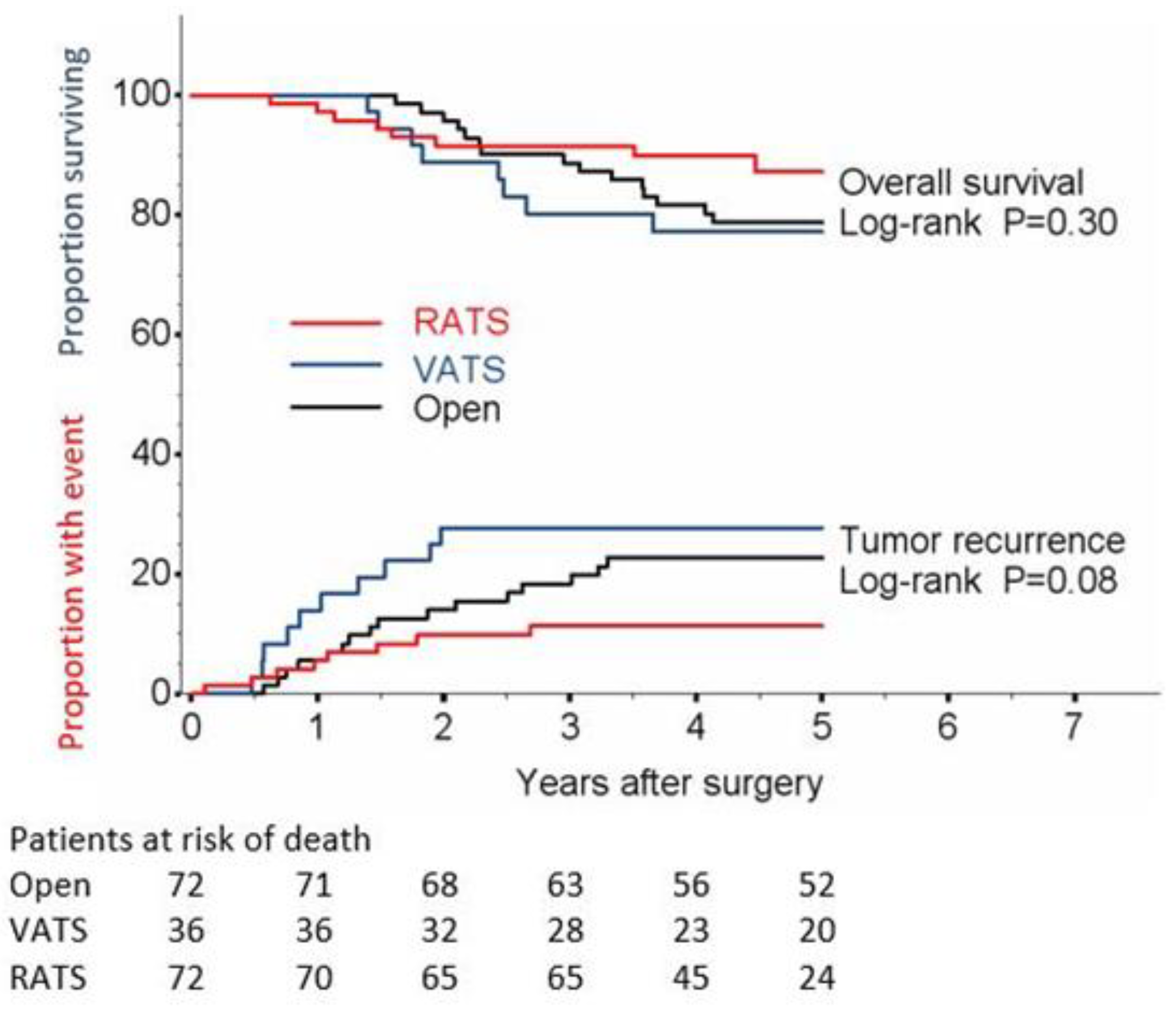
| Median follow-up | 5.0 years | 5.0 years | 4.4 years | |||
| Disease recurrence | Log-rank | Log-rank | Log-rank | |||
| Number of events | 16 (22.2) | 10 (27.8) | 8 (11.1) | 0.45 | 0.09 | 0.03 |
| Local (±regional ± distant) | 2 (2.8) | 4 (11.1) | 3 (4.2) | 0.07 | 0.66 | 0.15 |
| Regional (±distant) | 6 (8.3) | 2 (5.6) | 3 (4.2) | 0.71 | 0.30 | 0.69 |
| Distant only | 8 (11.1) | 4 (11.1) | 2 (2.8) | 0.89 | 0.05 | 0.07 |
| 1-year relapse, % (95% CI) | 5.6 (2.1−14.2) | 13.9 (6.0−30.2) | 5.6 (2.1−14.2) | |||
| 2-year relapse, % (95% CI) | 14.1 (7.9−24.6) | 27.8 (16.0−45.5) | 9.9 (4.9−19.7) | |||
| 3-year relapse, % (95% CI) | 18.4 (11.1−29.6) | 27.8 (16.0−45.5) | 11.4 (5.9−21.5) | |||
| 4-year relapse, % (95% CI) | 22.9 (14.7−34.7) | 27.8 (16.0−45.5) | 11.4 (5.9−21.5) | |||
| 5-year relapse, % (95% CI) | 22.9 (14.7−34.7) | 27.8 (16.0−45.5) | 11.4 (5.9−21.5) | |||
| HR (95% CI) | 1.00 (ref) | 1.35 (0.61−2.98) | 0.49 (0.21−1.15) | |||
| p-value | 0.45 | 0.10 | ||||
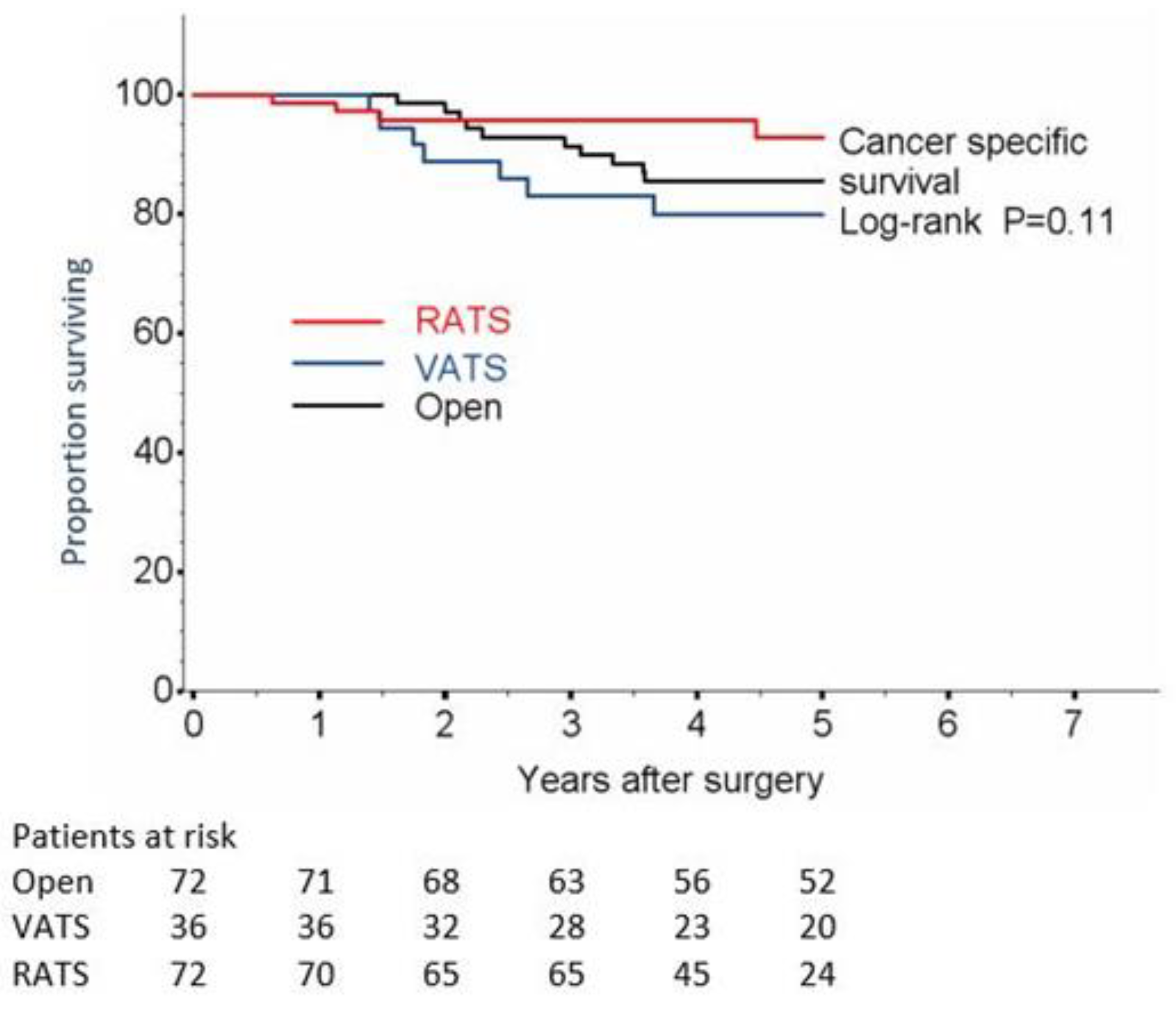
| Cancer-specific survival (CSS) | Log-rank | Log-rank | Log-rank | |||
| Number of deaths | 10 (13.9) | 7 (19.4) | 4 (5.6) | 0.40 | 0.14 | 0.10 |
| 1-year CSS, % (95% CI) | 100 | 100 | ||||
| 2-year CSS, % (95% CI) | 97.2 (89.1−99.3) | 88.9 (73.1−95.7) | 98.6 (90.5−99.8) | |||
| 3-year CSS, % (95% CI) | 91.4 (81.9−96.0) | 83.2 (66.3−92.1) | 95.7 (87.3−98.6) | |||
| 4-year CSS, % (95% CI) | 85.5 (74.6−91.9) | 80.0 (62.4−89.9) | 95.7 (87.3−98.6) | |||
| 5-year CSS, % (95% CI) | 85.5 (74.6−91.9) | 80.0 (62.4−89.9) | 95.7 (87.3−98.6) | |||
| HR (95% CI) | 1.00 (ref) | 1.50 (0.57−3.94) | 0.42 (0.13−1.35) | |||
| p-value | 0.41 | 0.15 | ||||
| Overall survival (OS) | Log-rank | Log-rank | Log-rank | |||
| Number of deaths | 15 (20.8) | 10 (27.8) | 8 (11.1) | 0.72 | 0.21 | 0.17 |
| 1-year OS, % (95% CI) | 100 | 100 | 97.2 (89.3−99.3) | |||
| 2-year OS, % (95% CI) | 95.8 (87.5−98.6) | 88.9 (73.1−95.7) | 91.6 (82.3−96.1) | |||
| 3-year OS, % (95% CI) | 88.7 (78.7−94.2) | 80.4 (63.3−90.2) | 91.6 (82.3−96.1) | |||
| 4-year OS, % (95% CI) | 81.6 (70.4−88.9) | 77.3 (59.7−88.0) | 90.0 (80.1−95.1) | |||
| 5-year OS, % (95% CI) | 78.6 (67.0−86.5) | 77.3 (59.7−88.0) | 87.4 (75.8−93.7) | |||
| HR (95% CI) | 1.00 (ref) | 1.16 (0.49−2.74) | 0.57 (0.24−1.35) | |||
| p-value | 0.74 | 0.20 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casiraghi, M.; Mariolo, A.V.; Mohamed, S.; Sedda, G.; Maisonneuve, P.; Mazzella, A.; Lo Iacono, G.; Petrella, F.; Spaggiari, L. Long-Term Outcomes of Robotic-Assisted, Video-Assisted and Open Surgery in Non-Small Cell Lung Cancer: A Matched Analysis. J. Clin. Med. 2022, 11, 3363. https://doi.org/10.3390/jcm11123363
Casiraghi M, Mariolo AV, Mohamed S, Sedda G, Maisonneuve P, Mazzella A, Lo Iacono G, Petrella F, Spaggiari L. Long-Term Outcomes of Robotic-Assisted, Video-Assisted and Open Surgery in Non-Small Cell Lung Cancer: A Matched Analysis. Journal of Clinical Medicine. 2022; 11(12):3363. https://doi.org/10.3390/jcm11123363
Chicago/Turabian StyleCasiraghi, Monica, Alessio Vincenzo Mariolo, Shehab Mohamed, Giulia Sedda, Patrick Maisonneuve, Antonio Mazzella, Giorgio Lo Iacono, Francesco Petrella, and Lorenzo Spaggiari. 2022. "Long-Term Outcomes of Robotic-Assisted, Video-Assisted and Open Surgery in Non-Small Cell Lung Cancer: A Matched Analysis" Journal of Clinical Medicine 11, no. 12: 3363. https://doi.org/10.3390/jcm11123363







