Port Placement Strategies for Robotic Pulmonary Lobectomy: A Narrative Review
Abstract
:1. Introduction
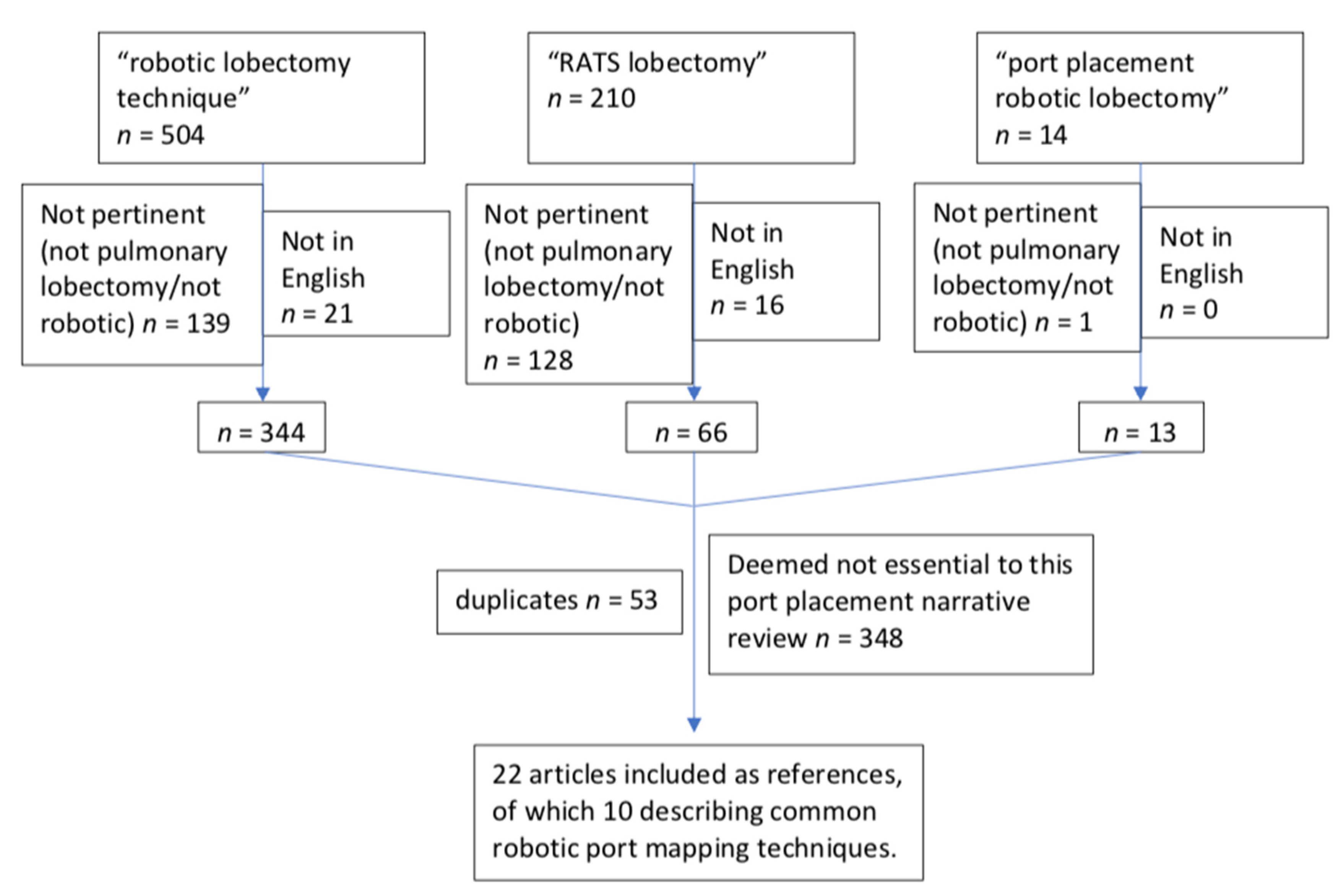
2. Materials and Methods
3. Results and Discussion
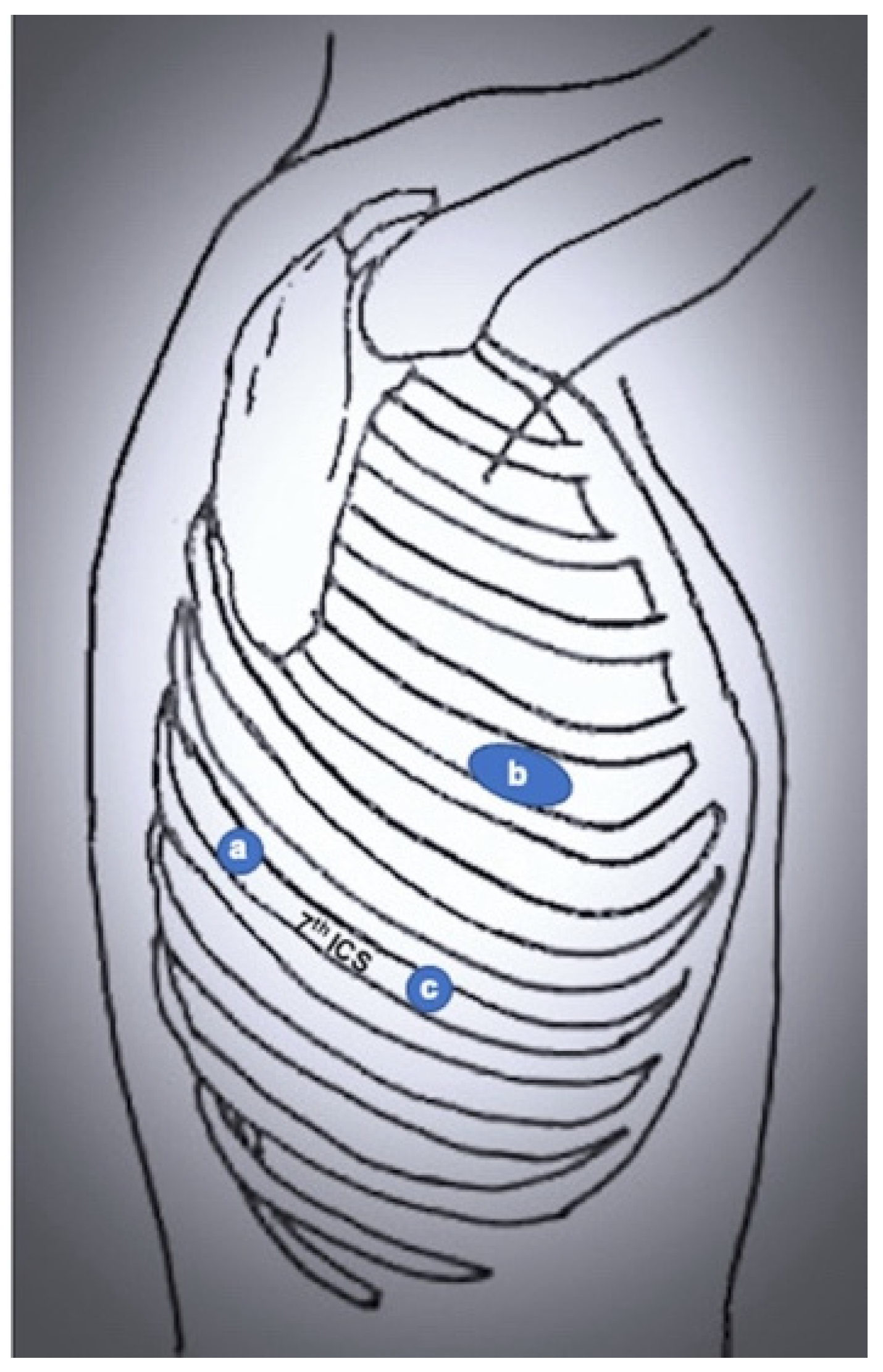
3.1. Robot-Assisted Thoracic Surgery (RATS)
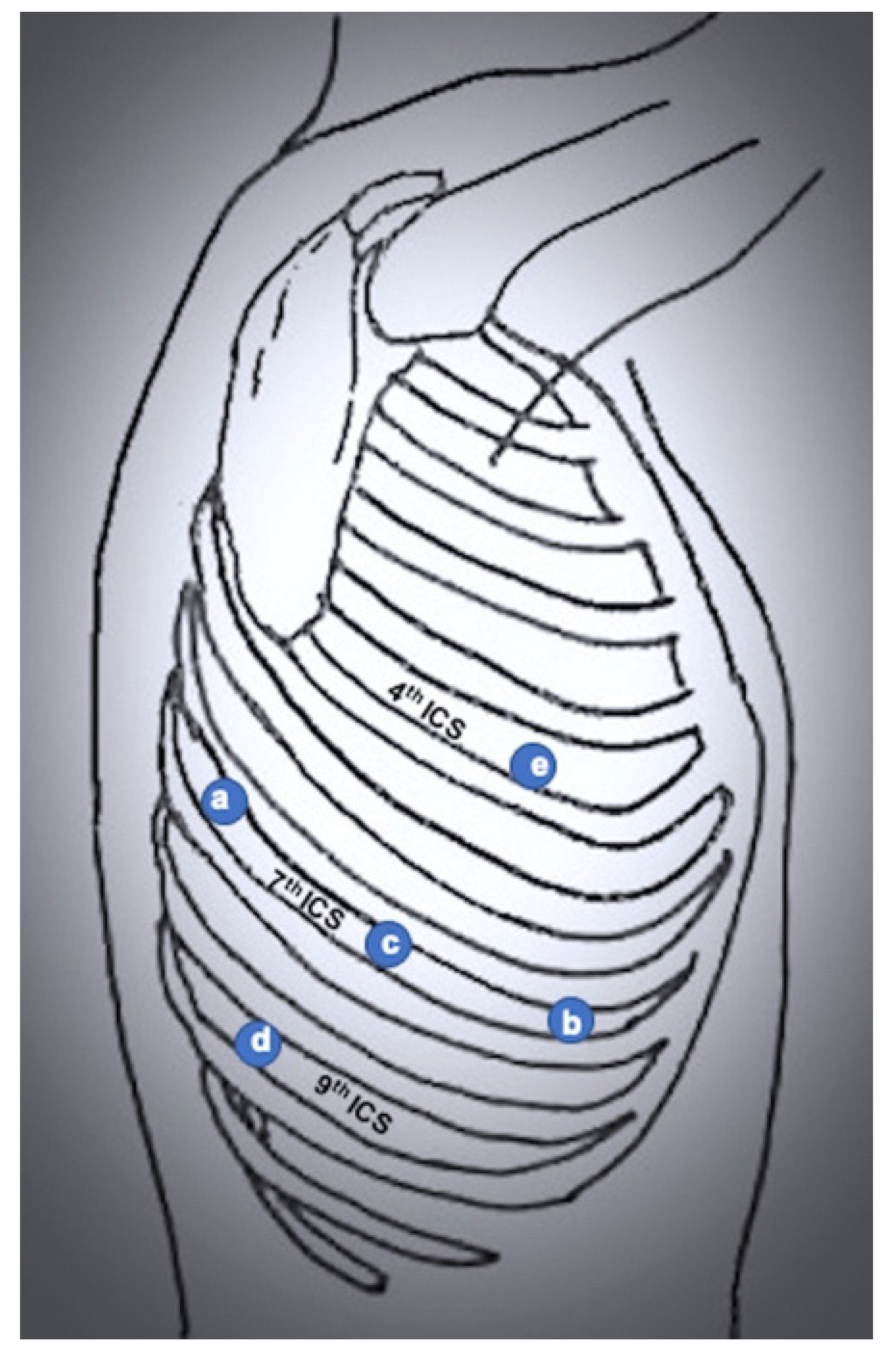
3.2. Completely Portal Robotic Lobectomy (CPRL)
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RATS | Robot-Assisted Thoracic Surgery |
| VATS | Video-Assisted Thoracic Surgery |
| CPRL | Completely Portal Robotic Lobectomy |
References
- Cerfolio, R.J. Total Port Approach for Robotic Lobectomy. Thorac. Surg. Clin. 2014, 24, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Soliman, B.G.; Nguyen, D.T.; Chan, E.Y.; Chihara, R.K.; Meisenbach, L.M.; Graviss, E.A.; Kim, M.P. Impact of da Vinci Xi robot in pulmonary resection. J. Thorac. Dis. 2020, 12, 3561–3572. [Google Scholar] [CrossRef]
- Melfi, F.M.; Menconi, G.F.; Mariani, A.M.; Angeletti, C.A. Early experience with robotic technology for thoracoscopic surgery. Eur. J. Cardio-Thorac. Surg. 2002, 21, 864–868. [Google Scholar] [CrossRef] [Green Version]
- Zirafa, C.C.; Romano, G.; Key, T.H.; Davini, F.; Melfi, F. The evolution of robotic thoracic surgery. Ann. Cardiothorac. Surg. 2019, 8, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Park, B.J.; Flores, R.M.; Rusch, V. Robotic assistance for video-assisted thoracic surgical lobectomy: Technique and initial results. J. Thorac. Cardiovasc. Surg. 2006, 131, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Veronesi, G.; Galetta, D.; Maisonneuve, P.; Melfi, F.; Schmid, R.A.; Borri, A.; Vannucci, F.; Spaggiari, L. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J. Thorac. Cardiovasc. Surg. 2010, 140, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; He, X.; Bai, Q.; Cui, B.; Gou, Y. Analysis of the short-term outcomes of biportal robot-assisted lobectomy. Int. J. Med. Robot. 2021, 17, e2326. [Google Scholar] [CrossRef]
- Yang, Y.; Song, L.; Huang, J.; Cheng, X.; Luo, Q. A uniportal right upper lobectomy by three-arm robotic-assisted thoracoscopic surgery using the da Vinci (Xi) Surgical System in the treatment of early-stage lung cancer. Transl. Lung Cancer Res. 2021, 10, 1571–1575. [Google Scholar] [CrossRef]
- Jayakumar, S.; Nardini, M.; Papoulidis, P.; Dunning, J. Robotic right middle lobectomy with a subxiphoid utility port. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 1049–1050. [Google Scholar] [CrossRef] [Green Version]
- Slight, R.D.; Koulaxouzidis, G.; Stamenkovic, S. Sequential robotic-assisted lung resection with a subxiphoid utility incision. Asian Cardiovasc. Thorac. Ann. 2018, 26, 404–406. [Google Scholar] [CrossRef] [Green Version]
- Cerfolio, R.; Louie, B.E.; Farivar, A.S.; Onaitis, M.; Park, B.J. Consensus statement on definitions and nomenclature for robotic thoracic surgery. J. Thorac. Cardiovasc. Surg. 2017, 154, 1065–1069. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.R. Anesthetic considerations for robotic surgery. Korean J. Anesthesiol. 2014, 66, 3–11. [Google Scholar] [CrossRef] [PubMed]
- El-Dawlatly, A.A.; Al-Dohayan, A.; Abdel-Meguid, M.E.; Turkistani, A.; Alotaiby, W.M.; Abdelaziz, E.M. Variations in dynamic lung compliance during endoscopic thoracic sympathectomy with CO2 insufflation. Clin. Auton. Res. 2003, 13, i94–i97. [Google Scholar] [CrossRef] [PubMed]
- Cerfolio, R.J.; Bryant, A.; Minnich, D.J. Starting a Robotic Program in General Thoracic Surgery: Why, How, and Lessons Learned. Ann. Thorac. Surg. 2011, 91, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Cerfolio, R.J.; Watson, C.; Minnich, D.J.; Calloway, S.; Wei, B. One Hundred Planned Robotic Segmentectomies: Early Results, Technical Details, and Preferred Port Placement. Ann. Thorac. Surg. 2016, 101, 1089–1096. [Google Scholar] [CrossRef] [Green Version]
- Wei, B.; Cerfolio, R. Robotic Lobectomy and Segmentectomy: Technical Details and Results. Surg. Clin. N. Am. 2017, 97, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Zirafa, C.C.; Davini, F.; Romano, G.; Melfi, F. Robotic Lobectomy: Left Lower Lobectomy by Surgery. Oper. Tech. Thorac. Cardiovasc. Surg. 2017, 22, 43–57. [Google Scholar] [CrossRef] [Green Version]
- Melfi, F.M.; Fanucchi, O.; Davini, F.; Mussi, A. VATS-based Approach for Robotic Lobectomy. Thorac. Surg. Clin. 2014, 24, 143–149. [Google Scholar] [CrossRef]
- Ninan, M.; Dylewski, M.R. Total port-access robot-assisted pulmonary lobectomy without utility thoracotomy. Eur. J. Cardio-Thorac. Surg. 2010, 38, 231–232. [Google Scholar] [CrossRef] [Green Version]
- Oh, D.S.; Tisol, W.B.; Cesnik, L.; Crosby, A.; Cerfolio, R. Port Strategies for Robot-Assisted Lobectomy by High-Volume Thoracic Surgeons: A Nationwide Survey. Innovations 2019, 14, 545–552. [Google Scholar] [CrossRef]
- Kim, M.P.; Chan, E.Y. “Five on a dice” port placement for robot-assisted thoracoscopic right upper lobectomy using robotic stapler. J. Thorac. Dis. 2017, 9, 5355–5362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, N.; Fikfak, V.; Chan, E.Y.; Kim, M.P. “Five on a Dice” Port Placement Allows for Successful Robot-Assisted Left Pneumonectomy. Thorac. Cardiovasc. Surg. Rep. 2017, 6, e42–e44. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Potential Advantages | Potential Disadvantages | |
|---|---|---|
| Robot-assisted thoracic surgery (RATS) |
|
|
| 3 or 4 arms ± assistant port [3,5,6] |
|
|
| Biportal [7] |
|
|
| Uniportal [8] |
|
|
| Completely Portal Robotic Lobectomy (CPRL) |
|
|
| 4 arms + assistant port [15,16] |
|
|
| 4 arms, no assistant port [18] |
|
|
| 3 arms + assistant port, 11th intercostal space [19] |
|
|
| “Five on a dice” [21] |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parini, S.; Massera, F.; Papalia, E.; Baietto, G.; Bora, G.; Rena, O. Port Placement Strategies for Robotic Pulmonary Lobectomy: A Narrative Review. J. Clin. Med. 2022, 11, 2612. https://doi.org/10.3390/jcm11092612
Parini S, Massera F, Papalia E, Baietto G, Bora G, Rena O. Port Placement Strategies for Robotic Pulmonary Lobectomy: A Narrative Review. Journal of Clinical Medicine. 2022; 11(9):2612. https://doi.org/10.3390/jcm11092612
Chicago/Turabian StyleParini, Sara, Fabio Massera, Esther Papalia, Guido Baietto, Giulia Bora, and Ottavio Rena. 2022. "Port Placement Strategies for Robotic Pulmonary Lobectomy: A Narrative Review" Journal of Clinical Medicine 11, no. 9: 2612. https://doi.org/10.3390/jcm11092612
APA StyleParini, S., Massera, F., Papalia, E., Baietto, G., Bora, G., & Rena, O. (2022). Port Placement Strategies for Robotic Pulmonary Lobectomy: A Narrative Review. Journal of Clinical Medicine, 11(9), 2612. https://doi.org/10.3390/jcm11092612








