Video Head Impulse Test (vHIT): Value of Gain and Refixation Saccades in Unilateral Vestibular Neuritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Control Group
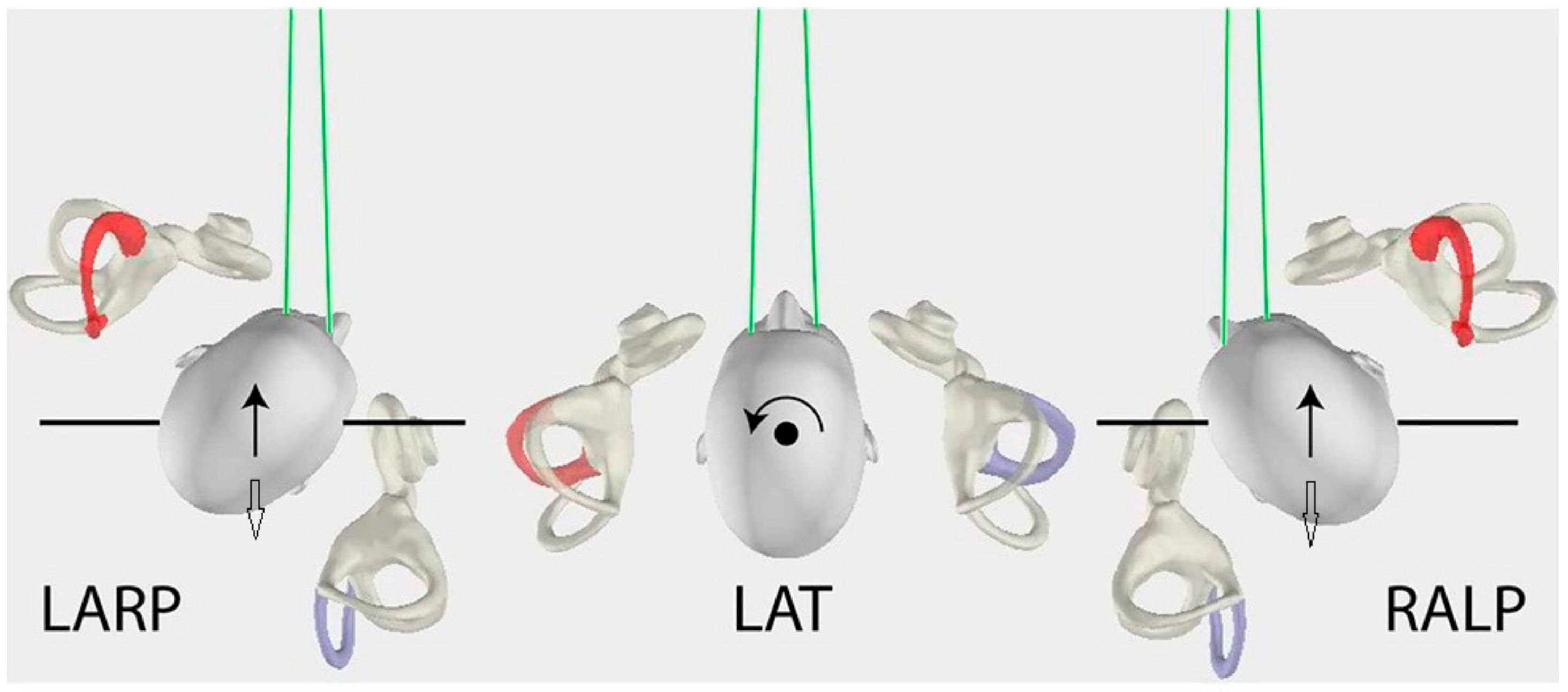
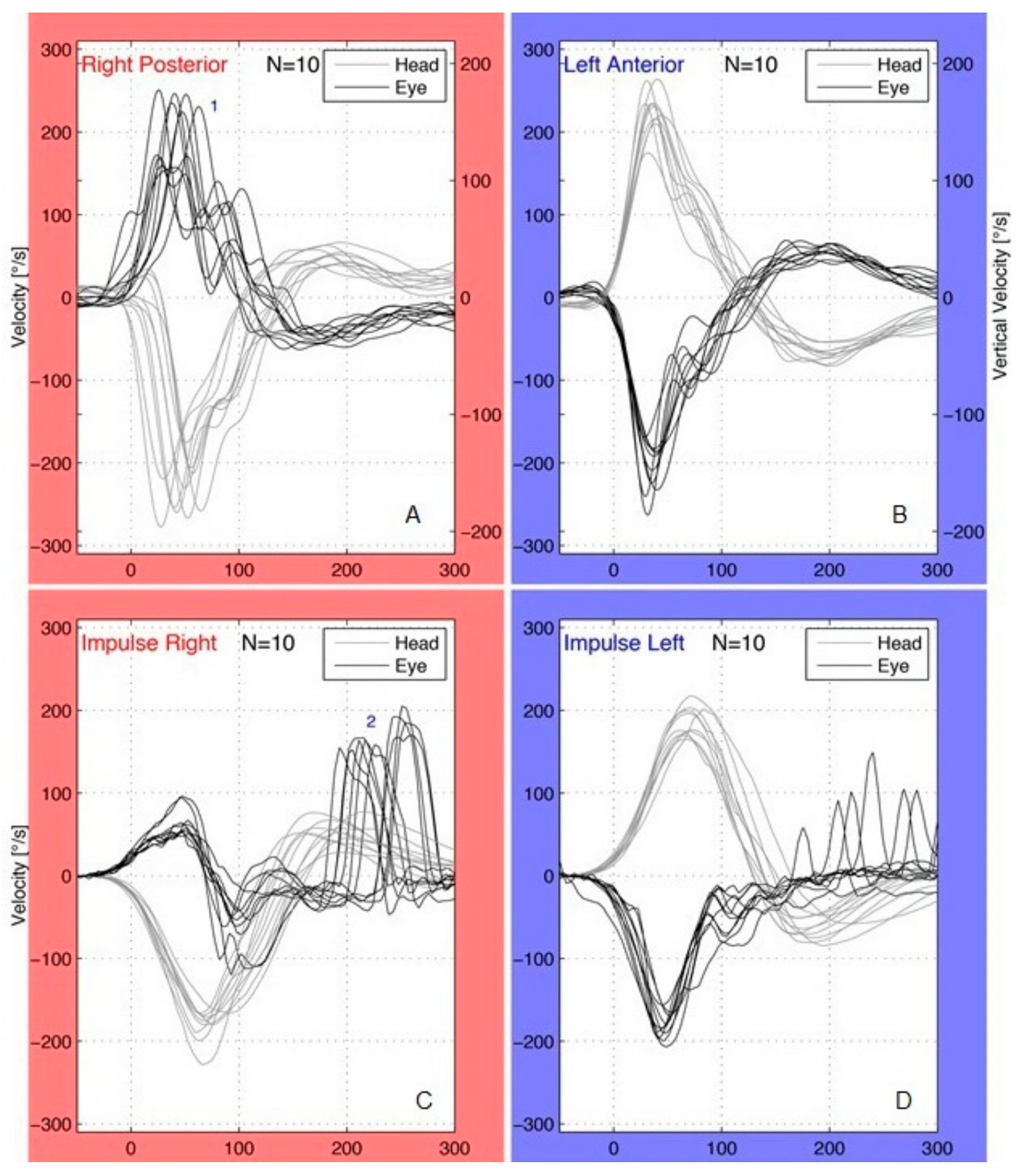
2.2. vHIT
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Strupp, M.; Brandt, T. Vestibular neuritis. Semin. Neurol. 2009, 5, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S. When the room is spinning: Experience of vestibular neuritis by a neurotologist. Front. Neurol. 2020, 11, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Himmelein, S.; Lindemann, A.; Sinicina, I.; Horn, A.K.E.; Brandt, T.; Strupp, M.; Hüfner, K. Differential involvement during latent herpes simplex virus 1 infection of the superior and inferior divisions of the vestibular ganglia: Implications for vestibular neuritis. J. Virol. 2017, 91, e00331-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riska, K.M.; Bellucci, J.; Garrison, D.; Hall, C. Relationship between corrective saccades and measures of physical function in unilateral and bilateral vestibular loss. Ear Hear. 2020, 41, 1568–1574. [Google Scholar] [CrossRef]
- Fu, W.; He, F.; Wei, D.; Bai, Y.; Shi, Y.; Wang, X.; Han, J. Recovery pattern of high-frequency acceleration vestibulo-ocular reflex in unilateral vestibular neuritis: A preliminary study. Front. Neurol. 2019, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Manzari, L.; Graziano, D.; Tramontano, M. The different stages of vestibular neuritis from the point of view of the video head impulse test. Audiol. Res. 2020, 10, 31–38. [Google Scholar] [CrossRef]
- Halmagyi, G.M.; Curthoys, I.S.; Cremer, P.D.; Henderson, C.J.; Staples, M. Head impulses after unilateral vestibular deafferentation validate Ewald’s second law. J. Vestib. Res. 1990–1991, 1, 187–197. [Google Scholar] [CrossRef]
- MacDougall, H.G.; Curthoys, I.S. Plasticity during vestibular compensation: The role of saccades. Front. Neurol. 2012, 3, 21. [Google Scholar] [CrossRef] [Green Version]
- MacDougall, H.G.; McGarvie, L.A.; Halmagyi, G.M.; Curthoys, I.S.; Weber, K.P. Application of the video head impulse test to detect vertical semicircular canal dysfunction. Otol. Neurotol. 2013, 34, 974–979. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R.L.; McGarvie, L.A.; Reid, N.; Young, A.S.; Halmagyi, G.M.; Welgampola, M.S. Vestibular neuritis affects both superior and inferior vestibular nerves. Neurology 2016, 87, 1704–1712. [Google Scholar] [CrossRef]
- Patel, M.; Arshad, Q.; Roberts, R.E.; Ahmad, H.; Bronstein, A.M. Chronic symptoms after vestibular neuritis and the high-velocity vestibulo-ocular reflex. Otol. Neurotol. 2016, 37, 179–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartolomeo, M.; Biboulet, R.; Pierre, G.; Mondain, M.; Uziel, A.; Venail, F. Value of the video head impulse test in assessing vestibular deficits following vestibular neuritis. Eur. Arch. Otorhinolaryngol. 2014, 271, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Magliulo, G.; Gagliardi, S.; Ciniglio Appiani, M.; Iannella, G.; Re, M. Vestibular neurolabyrinthitis: A follow-up study with cervical and ocular vestibular evoked myogenic potentials and the video head impulse test. Ann. Otol. Rhinol. Laryngol. 2014, 123, 162–173. [Google Scholar] [CrossRef]
- Sestak, A.; Maslovara, S.; Zubcic, Z.; Vceva, A. Influence of vestibular rehabilitation on the recovery of all vestibular receptor organs in patients with unilateral vestibular hypofunction. NeuroRehabilitation 2020, 47, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, G.P.; Newman, C.W. The development of the Dizziness Handicap Inventory. Arch. Otolaryngol. Head Neck Surg. 1990, 116, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.S.; Egilmez, O.K.; Kara, A.; Guven, M.; Demir, D.; Genc Elden, S. Comparison of the results of caloric and video head impulse tests in patients with Meniere’s disease and vestibular migraine. Eur. Arch. Otorhinolaryngol. 2021, 278, 1829–1834. [Google Scholar] [CrossRef]
- Gianoli, G.; Goebel, J.; Mowry, S.; Poomipannit, P. Anatomic differences in the lateral vestibular nerve channels and their implications in vestibular neuritis. Otol. Neurotol. 2005, 26, 489–494. [Google Scholar] [CrossRef] [Green Version]
- Lacour, M.; Helmchen, C.; Vidal, P.P. Vestibular compensation: The neuro-otologist’s best friend. J. Neurol. 2016, 263 (Suppl. S1), S54–S64. [Google Scholar] [CrossRef] [Green Version]
- Palla, A.; Straumann, D. Recovery of the high-acceleration vestibulo-ocular reflex after vestibular neuritis. J. Assoc. Res. Otolaryngol. 2004, 5, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Allum, J.H.J.; Honegger, F. Improvement of asymmetric vestibulo-ocular reflex responses following onset of vestibular neuritis is similar across canal planes. Front. Neurol. 2020, 11, 565125. [Google Scholar] [CrossRef]
- Manzari, L.; Princi, A.A.; De Angelis, S.; Tramontano, M. Clinical value of the video head impulse test in patients with vestibular neuritis: A systematic review. Eur. Arch. Otorhinolaryngol. 2021, 278, 4155–4167. [Google Scholar] [CrossRef] [PubMed]
- McGarvie, L.A.; MacDougall, H.G.; Curthoys, I.S.; Halmagyi, G.M. Spontaneous recovery of the vestibulo-ocular reflex after vestibular neuritis; Long-term monitoring with the video head impulse test in a single patient. Front. Neurol. 2020, 11, 732. [Google Scholar] [CrossRef] [PubMed]
- Büki, B.; Hanschek, M.; Jünger, H. Vestibular neuritis: Involvement and long-term recovery of individual semicircular canals. Auris Nasus Larynx 2017, 44, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.C.; Zee, D.S. Saccade and vestibular ocular motor adaptation. Restor. Neurol. Neurosci. 2010, 28, 9–18. [Google Scholar] [CrossRef]
- Cremer, P.D.; Halmagyi, G.M.; Aw, S.T.; Curthoys, I.S.; McGarvie, L.A.; Todd, M.J.; Black, R.A.; Hannigan, I.P. Semicircular canal plane head impulses detect absent function of individual semicircular canals. Brain 1998, 121, 699–716. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.J.; Cha, E.H.; Park, J.W.; Kang, B.C.; Yoo, M.H.; Kang, W.S.; Ahn, J.H.; Chung, J.W.; Park, H.J. Diagnostic value of gains and corrective saccades in video head impulse test in vestibular neuritis. Otolaryngol. Head Neck Surg. 2018, 159, 347–353. [Google Scholar] [CrossRef]
- Navari, E.; Cerchiai, N.; Casani, A.P. Assessment of vestibulo-ocular reflex gain and catch-up saccades during vestibular rehabilitation. Otol. Neurotol. 2018, 39, e1111–e1117. [Google Scholar] [CrossRef]
- Cerchiai, N.; Navari, E.; Sellari-Franceschini, S.; Re, C.; Casani, A.P. Predicting the outcome after acute unilateral vestibulopathy: Analysis of vestibulo-ocular reflex gain and catch-up saccades. Otolaryngol. Head Neck. Surg. 2018, 158, 527–533. [Google Scholar] [CrossRef]
- Wettstein, V.G.; Weber, K.P.; Bockisch, C.J.; Hegemann, S.C. Compensatory saccades in head impulse testing influence the dynamic visual acuity of patients with unilateral peripheral vestibulopathy1. J. Vestib. Res. 2016, 26, 395–402. [Google Scholar] [CrossRef]
- Perez-Fernandez, N.; Eza-Nuñez, P. Normal gain of VOR with refixation saccades in patients with unilateral vestibulopathy. J. Int. Adv. Otol. 2015, 11, 133–137. [Google Scholar] [CrossRef]
- Korsager, L.E.; Faber, C.E.; Schmidt, J.H.; Wanscher, J.H. Refixation saccades with normal gain values: A diagnostic problem in the video head impulse test: A case report. Front. Neurol. 2017, 8, 81. [Google Scholar] [CrossRef] [Green Version]
- Mutlu, B.; Serbetcioglu, B. Discussion of the dizziness handicap inventory. J. Vestib. Res. 2013, 23, 271–277. [Google Scholar] [CrossRef]
- Mandalà, M.; Nuti, D. Long-term follow-up of vestibular neuritis. Ann. N. Y. Acad. Sci. 2009, 1164, 427–429. [Google Scholar] [CrossRef]
| Gain | Mean ± SD | Significance | 95% C.I. for Mean | |
|---|---|---|---|---|
| Affected HC | Acute stage | 0.54 ± 0.36 | Acute—control *** Acute—follow-up ** | 0.41–0.67 |
| Follow-up | 0.77 ± 0.31 | 0.66–0.88 | ||
| Control | 0.86 ± 0.19 | 0.80–0.93 | ||
| Unaffected HC | Acute stage | 0.84 ± 0.36 | Acute—control ** | 0.72–0.97 |
| Follow-up | 0.98 ± 0.32 | 0.86–1.09 | ||
| Control | 1.10 ± 0.28 | 1.00–1.20 | ||
| Affected AC | Acute stage | 0.64 ± 0.16 | Acute—control * Acute—follow-up *** | 0.62–0.74 |
| Follow-up | 0.83 ± 0.15 | 0.77–0.88 | ||
| Control | 0.78 ± 0.10 | 0.74–0.82 | ||
| Unaffected AC | Acute stage | 0.79 ± 0.16 | Acute—control * | 0.73–0.85 |
| Follow-up | 0.84 ± 0.18 | 0.77–0.90 | ||
| Control | 0.90 ± 0.10 | 0.86–0.94 | ||
| Affected PC | Acute stage | 0.78 ± 0.17 | Acute—control * | 0.72–0.84 |
| Follow-up | 0.83 ± 0.16 | 0.77–0.89 | ||
| Control | 0.87 ± 0.12 | 0.83–0.92 | ||
| Unaffected PC | Acute stage | 0.77 ± 0.15 | Acute—control * | 0.72–0.83 |
| Follow-up | 0.85 ± 0.14 | 0.80–0.90 | ||
| Control | 0.86 ± 0.10 | 0.82–0.90 |
| Gain Asymmetry | Mean ± SD | Significance | 95% C.I. for Mean | |
|---|---|---|---|---|
| Affected HC | Acute stage | 17.86 ± 28.50 | 8.07–27.65 | |
| Follow-up | 9.83 ± 21.10 | 2.58–17.08 | ||
| Control | 12.81 ± 10.78 | 8.92–16.7 | ||
| Affected AC | Acute stage | 7.80 ± 11.16 | Acute—Follow-up * | 3.97–11.63 |
| Follow-up | 1.60 ± 8.45 | −1.30–4.50 | ||
| Control | 5.97 ± 6.31 | 3.69–8.24 | ||
| Affected PC | Acute stage | 0.46 ± 11.42 | Follow-up—control * | −3.47–4.38 |
| Follow-up | 0.11 ± 11.13 | −3.71–3.94 | ||
| Control | 5.94 ± 5.32 | 4.02–7.86 |
| Covert | Overt | Total (Covert and/or Overt Saccades) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Affected Side | Acute Stage | Follow-Up | Acute Stage | Follow-Up | Acute Stage | Follow-Up | |||||||||
| N | % | N | % | p-Value | N | % | N | % | p-Value | N | % | N | % | p-Value | |
| HC | 33 | 94% | 24 | 69% | * | 30 | 86% | 20 | 57% | * | 34 | 97% | 28 | 90% | |
| AC | 20 | 57% | 6 | 17% | ** | 17 | 49% | 4 | 11% | ** | 24 | 69% | 8 | 23% | *** |
| PC | 11 | 31% | 5 | 14% | 9 | 26% | 5 | 14% | 13 | 37% | 6 | 17% | |||
| Isolated Canal Involvement | Multi-Canal Involvement | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HC | AC | PC | HC + AC | HC + PC | AC + PC | HC + AC + PC | Normal | Total | ||
| Gain | 2 | 2 | 1 | 12 | 2 | 2 | 12 | 2 | 35 | |
| Saccades | 10 | - | - | 12 | 1 | 1 | 11 | - | 35 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psillas, G.; Petrou, I.; Printza, A.; Sfakianaki, I.; Binos, P.; Anastasiadou, S.; Constantinidis, J. Video Head Impulse Test (vHIT): Value of Gain and Refixation Saccades in Unilateral Vestibular Neuritis. J. Clin. Med. 2022, 11, 3467. https://doi.org/10.3390/jcm11123467
Psillas G, Petrou I, Printza A, Sfakianaki I, Binos P, Anastasiadou S, Constantinidis J. Video Head Impulse Test (vHIT): Value of Gain and Refixation Saccades in Unilateral Vestibular Neuritis. Journal of Clinical Medicine. 2022; 11(12):3467. https://doi.org/10.3390/jcm11123467
Chicago/Turabian StylePsillas, George, Ioanna Petrou, Athanasia Printza, Ioanna Sfakianaki, Paris Binos, Sofia Anastasiadou, and Jiannis Constantinidis. 2022. "Video Head Impulse Test (vHIT): Value of Gain and Refixation Saccades in Unilateral Vestibular Neuritis" Journal of Clinical Medicine 11, no. 12: 3467. https://doi.org/10.3390/jcm11123467








