Sepsis Management in Southeast Asia: A Review and Clinical Experience
Abstract
:1. Introduction
2. Methodology
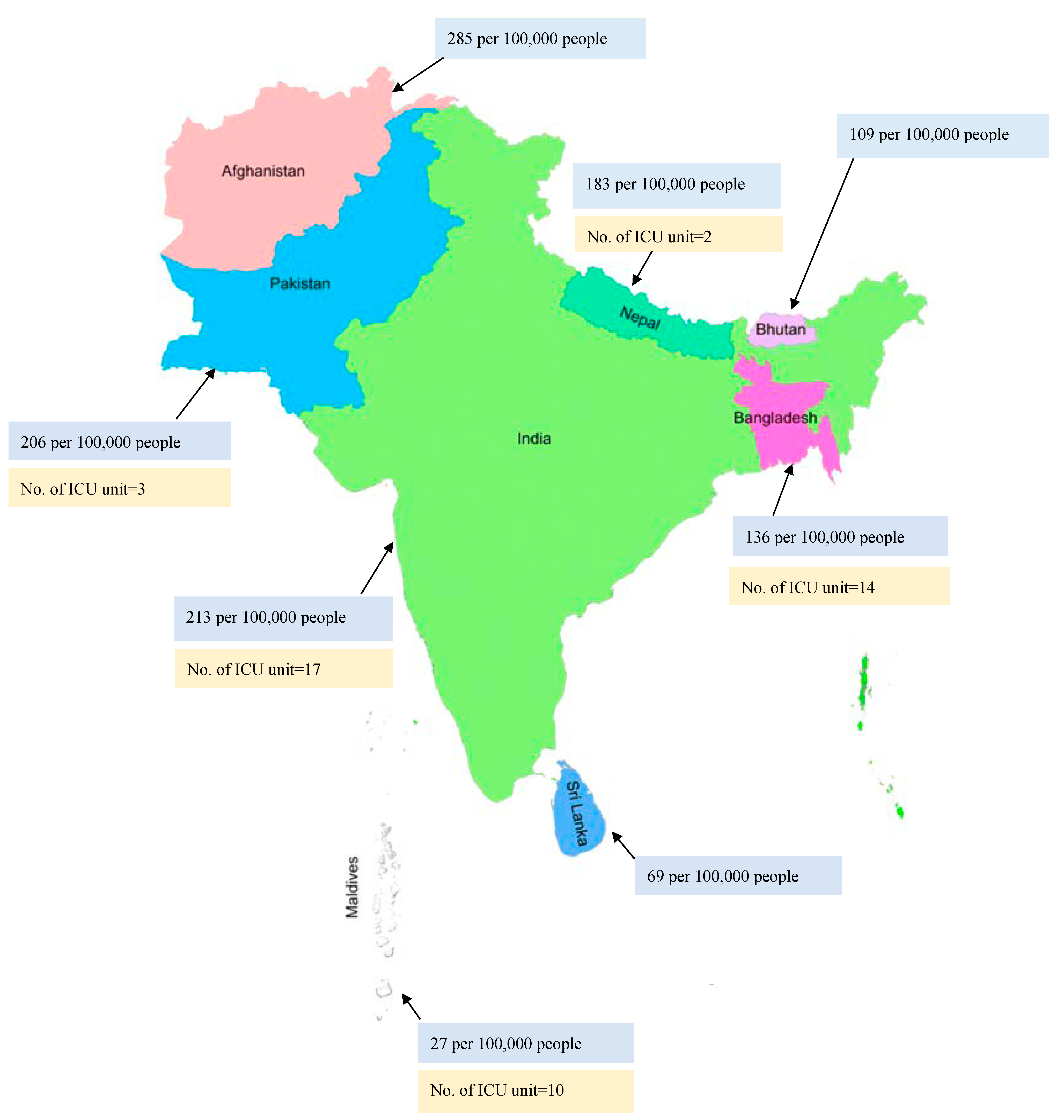
3. Epidemiological Data Associated with Sepsis Patients in Southeast Asia
4. Clinical Investigation of Sepsis Patients in Southeast Asia
5. Time Factor Associated with Sepsis Patients in Southeast Asia
6. Chloramphenicol Advantage over Colistin Resistance in Sepsis Patients and Antibiotic Abuse in Southeast Asia
7. Resource Constraints about High Population and Limitations of Funds, Personnel, Infrastructure, etc. Sepsis Patients in Southeast Asia
8. Sepsis and COVID-19 in Southeast Asia
9. Recommendations by the Authors
9.1. Data Collection and Sharing
9.2. Personalized Approach to Sepsis Management
9.3. Conventional Approach to Sepsis Management
9.4. Innovative Therapeutic Alternatives
9.5. CytoSorb® Therapy: Optimal Dosage and Early Initiation Improves Clinical Outcome
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rello, J.; Valenzuela-Sánchez, F.; Ruiz-Rodriguez, M.; Moyano, S. Sepsis: A review of advances in management. Adv. Ther. 2017, 34, 2393–2411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, M.; Cai, S.; Su, J. The pathogenesis of sepsis and potential therapeutic targets. Int. J. Mol. Sci. 2019, 20, 5376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salomão, R.; Ferreira, B.L.; Salomão, M.C.; Santos, S.S.; Azevedo, L.C.P.; Brunialti, M.K.C. Sepsis: Evolving concepts and challenges. Braz. J. Med. Biol. Res. 2019, 52, e8595. [Google Scholar] [CrossRef] [PubMed]
- Monard, C.; Rimmele, T.; Ronco, C. Extracorporeal blood purification therapies for sepsis. Blood Purif. 2019, 47, 2–15. [Google Scholar] [CrossRef]
- Research, Southeast Asia Infectious Disease Clinical. Causes and outcomes of sepsis in southeast Asia: A multinational multicentre cross-sectional study. Lancet Glob. Health 2017, 5, e157–e167. [Google Scholar] [CrossRef] [Green Version]
- Ansari, S.; Nepal, H.P.; Gautam, R.; Shrestha, S.; Neopane, P.; Chapagain, M.L. Neonatal septicemia in Nepal: Early-onset versus late-onset. Int. J. Pediatr. 2015, 2015, 379806. [Google Scholar] [CrossRef] [Green Version]
- Rehman, Z.U.; Shah, M.H.; Afridi, M.N.S.; Sardar, H.; Shiraz, A. Bacterial Sepsis Pathogens and Resistance Patterns in a South Asian Tertiary Care Hospital. Cureus 2021, 13, e15082. [Google Scholar] [CrossRef]
- Polat, G.; Ugan, R.A.; Cadirci, E.; Halici, Z. Sepsis and septic shock: Current treatment strategies and new approaches. Eurasian J. Med. 2017, 49, 53. [Google Scholar] [CrossRef]
- Bonjar, M.R.S.; Bonjar, L.S. A prospective treatment for sepsis. Drug Des. Dev. Ther. 2015, 9, 2537. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Applegate, J.A.; Mitra, D.K.; Callaghan-Koru, J.A.; Mousumi, M.; Khan, A.M.; Joarder, T.; Harrison, M.; Ahmed, S.; Begum, N.; et al. Implementation research to support Bangladesh Ministry of Health and Family Welfare to implement its national guidelines for management of infections in young infants in two rural districts. J. Health Popul. Nutr. 2019, 38, 41. [Google Scholar] [CrossRef] [Green Version]
- Haniffa, R.; Mukaka, M.; Munasinghe, S.B.; De Silva, A.P.; Jayasinghe, K.S.A.; Beane, A.; de Keizer, N.; Dondorp, A.M. Simplified prognostic model for critically ill patients in resource limited settings in South Asia. Crit. Care 2017, 21, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meshram, R.M.; Gajimwar, V.S.; Bhongade, S.D. Predictors of mortality in outborns with neonatal sepsis: A prospective observational study. Niger. Postgrad. Med. J. 2019, 26, 216. [Google Scholar] [PubMed]
- Godara, S.M.; Kute, V.B.; Trivedi, H.L.; Vanikar, A.V.; Shah, P.R.; Gumber, M.R.; Patel, H.V.; Gumber, V.M. Clinical profile and outcome of acute kidney injury related to pregnancy in developing countries: A single-center study from India. Saudi J. Kidney Dis. Transplant. 2014, 25, 906. [Google Scholar]
- Lie, K.C.; Lau, C.Y.; Chau, N.V.V.; West, T.E.; Limmathurotsakul, D. Utility of SOFA score, management and outcomes of sepsis in Southeast Asia: A multinational multicenter prospective observational study. J. Intensive Care 2018, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Pradipta, I.S.; Sodik, D.C.; Lestari, K.; Parwati, I.; Halimah, E.; Diantini, A.; Abdulah, R. Antibiotic resistance in sepsis patients: Evaluation and recommendation of antibiotic use. N. Am. J. Med. Sci. 2013, 5, 344. [Google Scholar] [CrossRef]
- Nepal, D.; Agrawal, S.; Shrestha, S.; Rayamajhi, A. Bacteriological profile and antibiotic susceptibility pattern of Neonatal Septicemia in Kanti Children Hospital, Nepal. J. Gandaki Med. Coll.-Nepal 2020, 13, 97–103. [Google Scholar] [CrossRef]
- Ghimire, R.; Shakya, Y.M.; Shrestha, T.M.; Neupane, R.P. The utility of red cell distribution width to predict mortality of septic patients in a tertiary hospital of Nepal. BMC Emerg. Med. 2020, 20, 43. [Google Scholar] [CrossRef]
- Dassanayake, V.G. Sepsis and septic shock: Can we win the battle against this hidden crisis? Sri Lanka J. Surg. 2016, 34, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Matthias, T.; Ranasinghe, T.; Mallawaarachchi, C.; Wijekoon, S.; Indrakumar, J. A study on adherence to surviving sepsis campaign bundle at a tertiary care hospital in Sri Lanka. Int. J. Infect. Dis. 2020, 101, 217. [Google Scholar] [CrossRef]
- Teparrukkul, P.; Hantrakun, V.; Imwong, M.; Teerawattanasook, N.; Wongsuvan, G.; Day, N.P.; Dondorp, A.M.; West, T.E.; Limmathurotsakul, D. Utility of qSOFA and modified SOFA in severe malaria presenting as sepsis. PLoS ONE 2019, 14, e0223457. [Google Scholar] [CrossRef]
- Do, S.N.; Luong, C.Q.; Pham, D.T.; Nguyen, M.H.; Nguyen, N.T.; Huynh, D.Q.; Hoang, Q.T.A.; Dao, C.X.; Le, T.M.; Bui, H.N.; et al. Factors relating to mortality in septic patients in Vietnamese intensive care units from a subgroup analysis of MOSAICS II study. Sci. Rep. 2021, 11, 18924. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Rieder, M.; Zahn, T.; Benk, C.; Lother, A.; Bode, C.; Staudacher, D.; Duerschmied, D.; Supady, A. Cytokine adsorption in a patient with severe coronavirus disease 2019 related acute respiratory distress syndrome requiring extracorporeal membrane oxygenation therapy: A case report. Artif. Organs 2021, 45, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.L.; Miller, N.S.; Lee, J.; Remick, D.G. Diagnosing sepsis–The role of laboratory medicine. Clin. Chim. Acta 2016, 460, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Divatia, J.V.; Mehta, Y.; Govil, D.; Zirpe, K.; Amin, P.R.; Ramakrishnan, N.; Kapadia, F.N.; Sircar, M.; Sahu, S.; Bhattacharya, P.K.; et al. Intensive Care in India in 2018–2019: The Second Indian Intensive Care Case Mix and Practice Patterns Study. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2021, 25, 1093. [Google Scholar]
- Kshirsagar, A.; Kale, S.; More, S.; Anturkar, R.; Vispute, S. An Application of Predictive Analytics for Early Detection of Sepsis: An Overview. Int. J. Eng. Res. Technol. 2021, 10, 602–609. [Google Scholar]
- Sarker, S.K.; Azam, M.S.; Mondal, M.K.; Goswami, U.K.; Mohsin, M. Demographic Profiles and Sources of Infection among Septic Patients admitted at ICU of a Public Hospital in Dhaka City. Bangladesh J. Infect. Dis. 2020, 7, 40–43. [Google Scholar] [CrossRef]
- Oeschger, T.; McCloskey, D.; Kopparthy, V.; Singh, A.; Erickson, D. Point of care technologies for sepsis diagnosis and treatment. Lab Chip 2019, 19, 728–737. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock [Sepsis-3]. JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Baig, M.A.; Sheikh, S.; Hussain, E.; Bakhtawar, S.; Khan, M.S.; Mujtaba, S.; Waheed, S. Comparison of qSOFA and SOFA score for predicting mortality in severe sepsis and septic shock patients in the emergency department of a low middle income country. Turk. J. Emerg. Med. 2018, 18, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.P.; Lauffenburger, J.C. Effectiveness of corticosteroids in patients with sepsis or septic shock using the new third international consensus definitions [Sepsis-3]: A retrospective observational study. PLoS ONE 2020, 15, e0243149. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.R.; Ferreira, E.M.; Schippers, P.; de Paula, I.C.; Saes, L.S.V.; de Oliveira, F.I.; Tuma, P.; Nogueira Filho, W.; Piza, F.; Guare, S.; et al. Implementation of sepsis bundles in public hospitals in Brazil: A prospective study with heterogeneous results. Critical Care 2017, 21, 268. [Google Scholar] [CrossRef] [Green Version]
- Noritomi, D.T.; Ranzani, O.T.; Monteiro, M.B.; Ferreira, E.M.; Santos, S.R.; Leibel, F.; Machado, F.R. Implementation of a multifaceted sepsis education program in an emerging country setting: Clinical outcomes and cost-effectiveness in a long-term follow-up study. Intensive Care Med. 2014, 40, 182–191. [Google Scholar] [CrossRef] [PubMed]
- National Health Service UK. Sepsis Guidance Implementation Advice for Adults. Available online: https://www.england.nhs.uk/wp-content/uploads/2017/09/sepsis-guidance-implementation-advice-for-adults.pdf (accessed on 2 June 2022).
- Ekman, B.; Paudel, P.; Basnet, O.; Ashish, K.C.; Wrammert, J. Adherence to World Health Organisation guidelines for treatment of early onset neonatal sepsis in low-income settings; a cohort study in Nepal. BMC Infect. Dis. 2020, 20, 666. [Google Scholar] [CrossRef]
- Busani, S.; Serafini, G.; Mantovani, E.; Venturelli, C.; Giannella, M.; Viale, P.; Mussini, C.; Cossarizza, A.; Girardis, M. Mortality in patients with septic shock by multidrug resistant bacteria: Risk factors and impact of sepsis treatments. J. Intensive Care Med. 2019, 34, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Zilahi, G.; Artigas, A.; Martin-Loeches, I. What’s new in multidrug-resistant pathogens in the ICU? Ann. Intensive Care 2016, 6, 96. [Google Scholar] [CrossRef] [Green Version]
- Tosi, M.; Roat, E.; De Biasi, S.; Munari, E.; Venturelli, S.; Coloretti, I.; Biagioni, E.; Cossarizza, A.; Girardis, M. Multidrug resistant bacteria in critically ill patients: A step further antibiotic therapy. J. Emerg. Crit. Care Med. 2018, 2, 103. [Google Scholar] [CrossRef]
- Shorr, A.F.; Micek, S.T.; Welch, E.C.; Doherty, J.A.; Reichley, R.M.; Kollef, M.H. Inappropriate antibiotic therapy in Gram-negative sepsis increases hospital length of stay. Crit. Care Med. 2011, 39, 46–51. [Google Scholar] [CrossRef]
- Exner, M.; Bhattacharya, S.; Christiansen, B.; Gebel, J.; Goroncy-Bermes, P.; Hartemann, P.; Heeg, P.; Ilschner, C.; Kramer, A.; Larson, E.; et al. Antibiotic resistance: What is so special about multidrug-resistant Gram-negative bacteria? GMS Hyg. Infect. Control 2017, 12, Doc05. [Google Scholar]
- Qureshi, Z.A.; Hittle, L.E.; O’Hara, J.A.; Rivera, J.I.; Syed, A.; Shields, R.K.; Pasculle, A.W.; Ernst, R.K.; Doi, Y. Colistin-resistant Acinetobacter baumannii: Beyond carbapenem resistance. Clin. Infect. Dis. 2015, 60, 1295–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papathanakos, G.; Andrianopoulos, I.; Papathanasiou, A.; Priavali, E.; Koulenti, D.; Koulouras, V. Colistin-resistant Acinetobacter baumannii bacteremia: A serious threat for critically ill patients. Microorganisms 2020, 8, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambreen, G.; Salat, M.S.; Hussain, K.; Raza, S.S.; Ali, U.; Azam, I.; Iqbal, J.; Fatmi, Z. Efficacy of colistin in multidrug-resistant neonatal sepsis: Experience from a tertiary care center in Karachi, Pakistan. Arch. Dis. Child. 2020, 105, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Hamel, M.; Rolain, J.M.; Baron, S.A. The History of Colistin Resistance Mechanisms in Bacteria: Progress and Challenges. Microorganisms 2021, 9, 442. [Google Scholar] [CrossRef]
- Eiamphungporn, W.; Yainoy, S.; Jumderm, C.; Tan-Arsuwongkul, R.; Tiengrim, S.; Thamlikitkul, V. Prevalence of the colistin resistance gene mcr-1 in colistin-resistant Escherichia coli and Klebsiella pneumoniae isolated from humans in Thailand. J. Glob. Antimicrob. Resist. 2018, 15, 32–35. [Google Scholar] [CrossRef]
- Ramesh, N.; Prasanth, M.; Ramkumar, S.; Suresh, M.; Tamhankar, A.J.; Gothandam, K.M.; Karthikeyan, S.; Bozdogan, B. Colistin susceptibility of gram-negative clinical isolates from Tamil Nadu, India. Asian Biomed. 2016, 10, 35–39. [Google Scholar]
- Arjun, R.; Gopalakrishnan, R.; Nambi, P.S.; Kumar, D.S.; Madhumitha, R.; Ramasubramanian, V. A study of 24 patients with colistin-resistant Gram-negative isolates in a tertiary care hospital in South India. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2017, 21, 317. [Google Scholar] [CrossRef]
- Bialvaei, A.Z.; Samadi Kafil, H. Colistin, mechanisms and prevalence of resistance. Curr. Med. Res. Opin. 2015, 31, 707–721. [Google Scholar] [CrossRef]
- Rahal, J.J.; Simberkoff, M.S. Bacteriocidal and bacteriostatic action of chloramphenicol against meningeal pathogens. Antimicrob. Agents Chemother. 1979, 16, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Livermore, D.M.; Warner, M.; Mushtaq, S.; Doumith, M.; Zhang, J.; Woodford, N. What remains against carbapenem-resistant Enterobacteriaceae? Evaluation of chloramphenicol, ciprofloxacin, colistin, fosfomycin, minocycline, nitrofurantoin, temocillin and tigecycline. Int. J. Antimicrob. Agents 2011, 37, 415–419. [Google Scholar] [CrossRef] [Green Version]
- Batty, E.M.; Cusack, T.P.; Thaipadungpanit, J.; Watthanaworawit, W.; Carrara, V.; Sihalath, S.; Hopkins, J.; Soeng, S.; Ling, C.; Turner, P.; et al. The spread of chloramphenicol-resistant Neisseria meningitidis in Southeast Asia. Int. J. Infect. Dis. 2020, 95, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Fuursted, K.; Schumacher, H. Significance of low-level resistance to ciprofloxacin in Klebsiella pneumoniae and the effect of increased dosage of ciprofloxacin in vivo using the rat granuloma pouch model. J. Antimicrob. Chemother. 2002, 50, 421–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sueke, H.; Kaye, S.; Neal, T.; Murphy, C.; Hall, A.; Whittaker, D.; Tuft, S.; Parry, C. Minimum inhibitory concentrations of standard and novel antimicrobials for isolates from bacterial keratitis. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2519–2524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Citron, D.M.; Tyrrell, K.L.; Merriam, C.V.; Goldstein, E.J. In vitro activities of CB-183,315, vancomycin, and metronidazole against 556 strains of Clostridium difficile, 445 other intestinal anaerobes, and 56 Enterobacteriaceae species. Antimicrob. Agents Chemother. 2012, 56, 1613–1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grillon, A.; Schramm, F.; Kleinberg, M.; Jehl, F. Comparative activity of ciprofloxacin, levofloxacin and moxifloxacin against Klebsiella pneumoniae, Pseudomonas aeruginosa and Stenotrophomonas maltophilia assessed by minimum inhibitory concentrations and time-kill studies. PLoS ONE 2016, 11, e0156690. [Google Scholar] [CrossRef]
- Kuti, J.L.; Wang, Q.; Chen, H.; Li, H.; Wang, H.; Nicolau, D.P. Defining the potency of amikacin against Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii derived from Chinese hospitals using CLSI and inhalation-based breakpoints. Infect. Drug Resist. 2018, 11, 783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michael, C.A.; Dominey-Howes, D.; Labbate, M. The antimicrobial resistance crisis: Causes, consequences, and management. Front. Public Health 2014, 2, 145. [Google Scholar] [CrossRef]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Schechner, V.; Temkin, E.; Harbarth, S.; Carmeli, Y.; Schwaber, M.J. Epidemiological interpretation of studies examining the effect of antibiotic usage on resistance. Clin. Microbiol. Rev. 2013, 26, 289–307. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Jin, Y.; Duan, Y.; He, M.; Lin, Z.; Lin, J. Multi-drug resistant Escherichia coli causing early-onset neonatal sepsis—A single center experience from China. Infect. Drug Resist. 2019, 12, 3695. [Google Scholar] [CrossRef] [Green Version]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shyam, R.; Patel, M.L.; Kumar, D. Etiology and outcome of patients with sepsis: A tertiary centre study. J. Med. Sci. Clin. Res. 2020, 8, 279–286. [Google Scholar]
- Chaurasia, S.; Sivanandan, S.; Agarwal, R.; Ellis, S.; Sharland, M.; Sankar, M.J. Neonatal sepsis in South Asia: Huge burden and spiralling antimicrobial resistance. BMJ 2019, 364, k5314. [Google Scholar] [CrossRef] [Green Version]
- Moran, E.; Munang, M.; Chan, C.; Chaudhri, S.; Himayakanthan, M.; Laird, S.; Moltu, A.; Naworynsky, N.; Pollard, C.; Saeed, T.; et al. Sepsis quality standards are laudable but have low specificity. BMJ 2017, 357, j1974. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.P.; Safford, M.M.; Shapiro, N.I.; Baddley, J.W.; Wang, H.E. Application of the Third International Consensus Definitions for Sepsis [Sepsis-3] Classification: A retrospective population-based cohort study. Lancet Infect. Dis. 2017, 17, 661–670. [Google Scholar] [CrossRef]
- Oliver, D. David Oliver: Sepsis—What’s behind the “hype”? BMJ 2019, 367, l6327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuehn, B.M. IDSA: Better, faster diagnostics for infectious diseases needed to curb overtreatment, antibiotic resistance. JAMA 2013, 310, 2385–2386. [Google Scholar] [CrossRef]
- Antibiotics. Available online: https://www.hopkinsmedicine.org/health/wellness-and-prevention/antibiotics (accessed on 2 June 2022).
- Sulis, G.; Adam, P.; Nafade, V.; Gore, G.; Daniels, B.; Daftary, A.; Das, J.; Gandra, S.; Pai, M. Antibiotic prescription practices in primary care in low-and middle-income countries: A systematic review and meta-analysis. PLoS Med. 2020, 17, e1003139. [Google Scholar] [CrossRef]
- Barker, A.K.; Brown, K.; Ahsan, M.; Sengupta, S.; Safdar, N. Social determinants of antibiotic misuse: A qualitative study of community members in Haryana, India. BMC Public Health 2017, 17, 333. [Google Scholar] [CrossRef] [Green Version]
- Kotwani, A.; Joshi, J.; Lamkang, A.S. Over-the-Counter Sale of Antibiotics in India: A Qualitative Study of Providers’ Perspectives across Two States. Antibiotics 2021, 10, 1123. [Google Scholar] [CrossRef]
- Vercelli, C.; Gambino, G.; Amadori, M.; Re, G. Implications of veterinary medicine in the comprehension and stewardsship of antimicrobial phenomenon: From the origins till nowadays. Vet. Anim. Sci. 2022, 16, 100249. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Weize, Y.; Jie, W. Guiding effect of serum procalcitonin [PCT] on the antibiotic application to patients with sepsis. Iran. J. Public Health 2017, 46, 1535. [Google Scholar]
- Schroeder, S.; Hochreiter, M.; Koehler, T.; Schweiger, A.M.; Bein, B.; Keck, F.S.; Von Spiegel, T. Procalcitonin [PCT]-guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: Results of a prospective randomized study. Langenbeck’s Arch. Surg. 2009, 394, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yuan, X.; Yu, L.; Wang, B.; Gao, F.; Ma, J. Procalcitonin-guided antibiotic therapy in acute exacerbation of chronic obstructive pulmonary disease: An updated meta-analysis. Medicine 2019, 98, e16775. [Google Scholar] [CrossRef]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef]
- Kwizera, A.; Baelani, I.; Mer, M.; Kissoon, N.; Schultz, M.J.; Patterson, A.J.; Musa, N.; Farmer, J.C.; Dünser, M.W. The long sepsis journey in low- and middle-income countries begins with a first step... but on which road? Crit. Care 2018, 22, 64. [Google Scholar] [CrossRef] [Green Version]
- Schultz, M.J.; Dünser, M.W.; Dondorp, A.M.; Adhikari, N.K.; Iyer, S.; Kwizera, A.; Lubell, Y.; Papali, A.; Pisani, L.; Riviello, E.D.; et al. Current challenges in the management of sepsis in ICUs in resource-poor settings and suggestions for the future. In Sepsis Management in Resource-Limited Settings; Springer: Cham, Switzerland, 2019; pp. 1–24. [Google Scholar] [CrossRef] [Green Version]
- Phua, J.; Faruq, M.O.; Kulkarni, A.P.; Redjeki, I.S.; Detleuxay, K.; Mendsaikhan, N.; Sann, K.K.; Shrestha, B.R.; Hashmi, M.; Palo, J.E.M.; et al. Critical care bed capacity in Asian countries and regions. Crit. Care Med. 2020, 48, 654–662. [Google Scholar] [CrossRef]
- Booraphun, S.; Hantrakun, V.; Siriboon, S.; Boonsri, C.; Poomthong, P.; Singkaew, B.O.; Wasombat, O.; Chamnan, P.; Champunot, R.; Rudd, K.; et al. Effectiveness of a sepsis programme in a resource-limited setting: A retrospective analysis of data of a prospective observational study [Ubon-sepsis]. BMJ Open 2021, 11, e041022. [Google Scholar] [CrossRef]
- Shrestha, G.S.; Lamsal, R.; Tiwari, P.; Acharya, S.P. Anesthesiology and Critical Care Response to COVID-19 in Resource-limited Settings: Experiences from Nepal. Anesthesiol. Clin. 2021, 39, 285–292. [Google Scholar] [CrossRef]
- Popp, W.; Rasslan, O.; Unahalekhaka, A.; Brenner, P.; Fischnaller, E.; Fathy, M.; Goldman, C.; Gillespie, E. What is the use? An international look at reuse of single-use medical devices. Int. J. Hyg. Environ. Health 2010, 213, 302–307. [Google Scholar] [CrossRef]
- Mer, M.; Schultz, M.J.; Adhikari, N.K. Core elements of general supportive care for patients with sepsis and septic shock in resource-limited settings. Intensive Care Med. 2017, 43, 1690–1694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duke, T.; Wandi, F.; Jonathan, M.; Matai, S.; Kaupa, M.; Saavu, M.; Subhi, R.; Peel, D. Improved oxygen systems for childhood pneumonia: A multihospital effectiveness study in Papua New Guinea. Lancet 2008, 372, 1328–1333. [Google Scholar] [CrossRef]
- Barker, A.K.; Brown, K.; Siraj, D.; Ahsan, M.; Sengupta, S.; Safdar, N. Barriers and facilitators to infection control at a hospital in northern India: A qualitative study. Antimicrob. Resist. Infect. Control 2017, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Permpikul, C.; Tongyoo, S.; Viarasilp, T.; Trainarongsakul, T.; Chakorn, T.; Udompanturak, S. Early Use of Norepinephrine in Septic Shock Resuscitation (CENSER) A Randomized Trial. Am. J. Respir. Crit. Care Med. 2019, 199, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Bima, P.; Orlotti, C.; Smart, O.G.; Morello, F.; Trunfio, M.; Brazzi, L.; Montrucchio, G. Norepinephrine may improve survival of septic shock patients in a low-resource setting: A proof-of-concept study on feasibility and efficacy outside the intensive care unit. Pathog. Glob. Health 2022, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.; Lau, E.H.; Wong, J.Y.; et al. Early transmission dynamics in Wuhan, China, of novel coronavirus—Infected pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Jain, V.K.; Iyengar, K.P.; Vaishya, R. Differences between First wave and Second wave of COVID-19 in India. Diabetes Metab. Syndr. 2021, 15, 1047. [Google Scholar] [CrossRef]
- Available online: https://covid19.who.int/region/searo/country/lk (accessed on 2 June 2022).
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Damiani, M.; Gandini, L.; Landi, F.; Borleri, G.; Fabretti, F.; Gritti, G.; Riva, I. Extracorporeal cytokine hemadsorption in severe COVID-19 respiratory failure. Respir. Med. 2021, 185, 106477. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.; Brown, M.; Sanchez, E.; Tattersall, R.; Manson, J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, J.; Zhang, D.; Xu, Z.; Ji, J.; Wen, C. Cytokine storm in COVID-19: The current evidence and treatment strategies. Front. Immunol. 2020, 11, 1708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhou, X.; Qiu, Y.; Song, Y.; Feng, F.; Feng, J.; Song, Q.; Jia, Q.; Wang, J. Clinical characteristics of 82 cases of death from COVID-19. PLoS ONE 2020, 15, e0235458. [Google Scholar] [CrossRef] [PubMed]
- As Covid-19 Devastates India, Deaths Go Undercounted. N. Y. Times 2021, 16, 2021. Available online: https://www.nytimes.com/2021/04/24/world/asia/india-coronavirus-deaths.html (accessed on 2 June 2022).
- Ghonimi, T.A.L.; Alkad, M.M.; Abuhelaiqa, E.A.; Othman, M.M.; Elgaali, M.A.; Ibrahim, R.A.M.; Joseph, S.M.; Al-Malki, H.A.; Hamad, A.I. Mortality and associated risk factors of COVID-19 infection in dialysis patients in Qatar: A nationwide cohort study. PLoS ONE 2021, 16, e0254246. [Google Scholar] [CrossRef]
- Yam, E.L.Y.; Hsu, L.Y.; Yap, E.P.H.; Yeo, T.W.; Lee, V.; Schlundt, J.; Lwin, M.O.; Limmathurotsakul, D.; Jit, M.; Dedon, P.; et al. Antimicrobial Resistance in the Asia Pacific region: A meeting report. Antimicrob. Resist. Infect. Control 2019, 8, 202. [Google Scholar] [CrossRef]
- Ray, S.; Goyal, S. Precision medicine: From concept to clinical practice—A promising challenge! J. Mar. Med. Soc. 2020, 22, 1. [Google Scholar] [CrossRef]
- da Silva, F.P.; Machado, M.C.C. Personalized medicine for sepsis. Am. J. Med. Sci. 2015, 350, 409–413. [Google Scholar] [CrossRef]
- Chong, H.Y.; Allotey, P.A.; Chaiyakunapruk, N. Current landscape of personalized medicine adoption and implementation in Southeast Asia. BMC Med. Genom. 2018, 11, 94. [Google Scholar] [CrossRef]
- Evangelatos, N.; Bauer, P.; Reumann, M.; Satyamoorthy, K.; Lehrach, H.; Brand, A. Metabolomics in sepsis and its impact on public health. Public Health Genom. 2017, 20, 274–285. [Google Scholar] [CrossRef]
- Podder, V.; Dhakal, B.; Shaik, G.U.S.; Sundar, K.; Sivapuram, M.S.; Chattu, V.K.; Biswas, R. Developing a case-based blended learning ecosystem to optimize precision medicine: Reducing overdiagnosis and overtreatment. Healthcare 2018, 6, 78. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.ahrq.gov/sites/default/files/wysiwyg/antibiotic-use/best-practices/sepsis-facilitator-guide.pdf (accessed on 2 June 2022).
- Dunser, M.W.; Festic, E.; Dondorp, A.; Kissoon, N.; Ganbat, T.; Kwizera, A.; Haniffa, R.; Baker, T.; Shultz, M.J. Recommendations for sepsis management in resource-limited settings. Intensive Care Med. 2012, 38, 557–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, J.; Vuylsteke, A. Extracorporeal membrane oxygenation: Indications, technique and contemporary outcomes. Heart 2019, 105, 1437–1443. [Google Scholar] [PubMed]
- Gotur, D.B. Sepsis Diagnosis and Management. J. Med. Sci. Health 2017, 3, 1–12. [Google Scholar] [CrossRef]
- Bonavia, A.; Groff, A.; Karamchandani, K.; Singbartl, K. Clinical utility of extracorporeal cytokine hemoadsorption therapy: A literature review. Blood Purif. 2018, 46, 337–349. [Google Scholar] [CrossRef]
- Cytosorbent Corporation, CytoSorb Fields of Application. Available online: https://cytosorb-therapy.com/en/the-therapy/fields-of-application/ (accessed on 2 June 2022).
- Available online: https://cytosorb-therapy.com/en/covid-19/ (accessed on 2 June 2022).
- CytoSorbents Corporation, CytoSorb: Broad Cytokine and Toxin Reduction to Control Deadly Inflammation. Available online: http://cytosorbents.com/products/cyto-sorb/ (accessed on 2 June 2022).
- Schultz, P.; Schwier, E.; Eickmeyer, C.; Henzletr, D.; Kohler, T. High dose CytoSorb haemadsorption is associated with improved survival in patients with septic shock: A retrospective cohort study. J. Crit. Care 2021, 64, 184–192. [Google Scholar] [CrossRef]
- Available online: https://cytosorb-therapy.com/en/the-therapy/ (accessed on 2 June 2022).
- Basu, R.; Pathak, S.; Goyal, J.; Chaudhry, R.; Goel, R.B.; Barwal, A. Use of a novel hemoadsorption device for cytokine removal as adjuvant therapy in a patient with septic shock with multi-organ dysfunction: A case study. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2014, 18, 822. [Google Scholar] [CrossRef]
- Paul, R.; Sathe, P.; Kumar, S.; Prasad, S.; Aleem, M.; Sakhalvalkar, P. Multicentered prospective investigator initiated study to evaluate the clinical outcomes with extracorporeal cytokine adsorption device [CytoSorb®] in patients with sepsis and septic shock. World J. Crit. Care Med. 2021, 10, 22. [Google Scholar] [CrossRef]
- Cytosorbents Corporation. CytoSorb Literature Database; Cytosorbents Corporation: Monmouth Junction, NJ, USA, 2018; Available online: https://literature.cytosorb-therapy.com/ (accessed on 2 June 2022).
- Rizvi, S.; Danic, M.; Silver, M.; LaBond, V. Cytosorb filter: An adjunct for survival in the COVID-19 patient in cytokine storm? A case report. Heart Lung 2021, 50, 44–50. [Google Scholar] [CrossRef]
- Alharthy, A.; Faqihi, F.; Memish, Z.A.; Balhamar, A.; Nasim, N.; Shahzad, A.; Tamim, H.; Alqahtani, S.A.; Brindley PGKarakitsos, D. Continuous renal replacement therapy with the addition of CytoSorb cartridge in critically ill patients with COVID-19 plus acute kidney injury: A case-series. Artif. Organs 2021, 45, E101–E112. [Google Scholar] [CrossRef]
- Mehta, Y.; Mehta, C.; Nanda, S.; Kochar, G.; George, J.V.; Singh, M.K. Use of CytoSorb therapy to treat critically ill coronavirus disease 2019 patients: A case series. J. Med. Case Rep. 2021, 15, 476. [Google Scholar] [CrossRef]
- Song, T.; Hayanga, J.; Durham, L.; Garrison, L.; McCarthy, P.; Barksdale, A.; Smith, D.; Bartlett, R.; Jaros, M.; Nelson, P.; et al. CytoSorb therapy in COVID-19 (CTC) patients requiring extracorporeal membrane oxygenation: A multicentric retrospective registry. Front. Med. 2021, 8, 773461. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Damera, S. Paediatric patient with dengue fever and associated multi-organ dysfunction syndrome (MODS) receiving hemoadsorption using Cytosorb® A case report on clinical experience. IJMDAT 2020, 3, e233. [Google Scholar]
- Khan, Z.A. A Clinical Experience Of Using Extracorporeal Cytokine Adsorption Device [Cytosorb®] In A Case Of Dengue Fever. J. Evid. Based Med. Healthc. 2016, 3, 4779–4781. [Google Scholar] [CrossRef] [PubMed]
- Krishan, K.; Dutta, R.; Chand, R.; Malhotra, R. Experience of using an extracorporeal cytokine hemoadsorber [CytoSorb®] in systemic inflammatory response syndrome after heart transplantation. Indian J. Transplant. 2020, 14, 166. [Google Scholar] [CrossRef]
- Mehta, Y.; Singh, A.; Singh, A.; Gupta, A.; Bhan, A. Modulating the Inflammatory Response with Hemadsorption [CytoSorb] in Patients Undergoing Major Aortic Surgery. J. Cardiothorac. Vasc. Anesth. 2021, 35, 673–675. [Google Scholar] [CrossRef]
- Padiyar, S.; Deokar, A.; Birajdar, S.; Walawalkar, A.; Doshi, H. Cytosorb for management of acute kidney injury due to rhabdomyolysis in a child. Indian Paediatr. 2019, 56, 974–976. [Google Scholar] [CrossRef]
- Sairam, R. Safety and Efficacy of Cytosorb Hemadsorption in Children with Multiorgan Dysfunction Syndrome. Available online: https://www.ijccm.org/doi/IJCCM/pdf/10.5005/ijccm-24-S2-S1 (accessed on 2 June 2022).
- Available online: https://literature.cytosorb-therapy.com/infoitem/use-of-cytosorb-in-a-patient-diagnosed-with-sepsis-and-mods-due-to-infection-with-salmonella-typhi?lang= (accessed on 2 June 2022).
- Available online: http://cytosorb-therapy.com/wp-content/uploads/2016/01/CaseStudy_Booklet_10-001-20_EN_241115_low.pdf (accessed on 2 June 2022).
- Available online: https://literature.cytosorb-therapy.com/infoitem/combined-application-of-cytosorb-and-sustained-low-efficiency-dialysis-sled-in-a-patient-with-septic-shock-and-multiple-organ-failure?lang=en (accessed on 2 June 2022).
- Mehta, Y.; Mehta, C.; Kumar, A.; George, J.V.; Gupta, A.; Nanda, S.; Kochhar, G.; Raizada, A. Experience with hemoadsorption [CytoSorb®] in the management of septic shock patients. World J. Crit. Care Med. 2020, 9, 1–12. [Google Scholar] [CrossRef]
- Singh, Y.P.; Chhabra, S.C.; Lashkari, K.; Taneja, A.; Garg, A.; Chandra, A.; Kochhar, G.; Jain, S. Hemoadsorption by extracorporeal cytokine adsorption therapy [CytoSorb®] in the management of septic shock: A retrospective observational study. Int. J. Artif. Organs 2020, 43, 372–378. [Google Scholar] [CrossRef]
- Brouwer, W.P.; Duran, S.; Kuijper, M.; Ince, C. Hemadsorption with CytoSorb shows a decreased observed versus expected 28-day all-cause mortality in ICU patients with septic shock: A propensity-score-weighted-retrospective study. Crit. Care 2019, 23, 317. [Google Scholar] [CrossRef] [Green Version]
- Rugg, C.; Klose, R.; Hornung, R.; Innerhofer, N.; Bachler, M.; Schmid, S.; Fries, D.; Strohle, M. Hemadsorption with CytoSorb in septic shock reduces catecholamine requirements and in-hospital mortality: A single centre retrospective genetic matched analysis. Biomedicines 2020, 8, 539. [Google Scholar] [CrossRef]
- Kogelmann, K.; Hubner, T.; Schwameis, F.; Druner, M.; Scheller, M.; Jarczak, D. First evaluation of new dynamic scoring system intended to support prescription of adjuvant CytoSorb hemadsorption therapy in patients with septic shock. J. Clin. Med. 2021, 11, 334. [Google Scholar]
- Nassiri, A.A.; Hakemi, M.S.; Shahrami, R.; Koomleh, A.A.; Sabaghian, T. Blood purification CytoSorb in critically-ill COVID-19 patients: A case series of 26 patients. Artif. Organs 2021, 45, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich-Sperl, F.; Kautzky, S.; Pickem, C.; Hormann, C. Adjuvant hemadsorption therapy in patients with severe COVID-19 and related organ failure requiring CRRT or ECMO therapy: A case series. Int. J. Artif. Organs 2021, 44, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Hawcher, F.; Laszlo, I.; Oveges, N.; Trasy, D.; Ondrik, Z.; Molnar, Z. Extracorporeal cytokine adsorption in septic shock: A proof of concept randomized, controlled pilot study. J. Crit. Care 2019, 49, 172–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schadler, D.; Pausch, C.; Heise, D.; Meier-Hellmann, A.; Brederlau, J.; Weiler, N.; Marx, G.; Putensen, C.; Spies, C.; Jorres, A.; et al. The effect of a novel extracorporeal cytokine hemadsorption device on IL-6 elimination in septic patients: A randomized controlled trial. PLoS ONE 2017, 12, e0187015. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.-L.; Singer, M.; Einav, S.; Moreno, R.; Wendon, J.; Teboul, J.-L.; Bakker, J.; Hernandez, G.; Annane, D.; de Man, A.M.; et al. Equilibrating SSC guidelines with individualized care. Crit. Care 2021, 25, 397. [Google Scholar] [CrossRef]


| Organisms | Klebsiella pneumonia | Escherichia coli | Staphylococcus aureus |
|---|---|---|---|
| Chloramphenicol | 4–256 | 0.015–10,000 | 0.200–256 |
| Ciprofloxacin | 0.25–0.5 | 15–300 | 0.09–64.00 |
| Vancomycin | 256–1024 | 64–1024 | 0.5–3.0 |
| Tigecycline | 50–200 | 0.03–4 | 0.016–0.47 |
| Meropenem | 4000–16,000 | 0.015–0.25 | 0.12–3.00 |
| Amikacin | 320–256 00 | 2–16 | 0.500–8.00 |
| S. No. | No. of Patients | Study Type | Comorbidities/Indication of CytoSorb® | Clinical Outcomes |
|---|---|---|---|---|
| 1. | 198 | Retrospective control study | Septic shock | Early start of CytoSorb® therapy significantly improved the survival of septic shock patients. A dynamic scoring system to assess the efficacy of CytoSorb® therapy was also developed. |
| 2. | 116 | Retrospective study | Septic shock | Improvement in 28-day survival, both on the basis of observed versus predicted mortality rates, was observed in CytoSorb® + CRRT group as compared CRRT alone group. |
| 3. | 100 | Observational and retrospective study | Sepsis and septic shock. | Survivors (n = 40) Non-survivors (n = 60) |
| 4. | 84 (Group 1: CytoSorb + CRRT, 42; Group 2: CRRT) | Retrospective genetic matched control study | Septic Shock | Catecholamines levels were reduced to half within 24 h after initiation of CytoSorb therapy. In hospital and 28-day mortality were reduced in CytoSorb® group |
| 5. | 45 | Observational multicenter study | Sepsis and septic shock. | Mortality rate: 48.8% after CytoSorb® therapy. 75% survival rate in patients given treatment in <24 h of ICU admission and 68% survival rates within 24–48 h of ICU admission |
| 6. | 36 | Observational and retrospective study | Sepsis and septic shock | Procalcitonin and total leucocyte count was reduced within 24 h of initiation of therapy. Sepsis related SOFA score was reduced. Survivors (17) Non-survivors (n = 19) |
| 7. | 26 | Retrospective case series | COVID-19 and acute respiratory distress syndrome | Significant reductions in norepinephrine, and inflammatory markers, with improvements in respiratory and other organ functions by use of CytoSorb®. |
| 8. | 25 (Group 1: CRRT, 15; Group 2: CRRT + CytoSorb®) | Retrospective analysis | Multiorgan dysfunction syndrome | Mortality rate: 53.3% in Group I and 60.0% in Group II |
| 9. | 16 (CytoSorb®: 8; control: 8) | Retrospective pilot study | Elective major aortic surgery for aortic aneurysm and/or aortic dissection. | |
| 10. | 13 | Retrospective case series | COVID-19, organ failure and acute respiratory distress syndrome | Significant reduction in inflammatory mediators (interleukin 6), hemodynamic stabilization with a concomitant decrease in requirements for vasoactive substances (norepinpethrine), and a pronounced improvement in lung function were observed with combined therapy of CRRT and CytoSorb® |
| 11. | 3 1 | Case Series Case Study | Hypertension Respiratory failure type-1/Other Hypertension Diabetes/Septic with shock Septic shock with multi-organ dysfunction (MODS) and a low perfusion state with a history of diabetes mellitus type II, hypertension, obstructive sleep apnea, hypothyroidism and morbid obesity. | Significant improvement in biochemical parameters and mean arterial pressure was observed with reduction in C-reactive proteins. All patients survived |
| 12. | 1 | Case Study | Septic shock with multi-organ dysfunction (MODS) and a low perfusion state with a history of diabetes mellitus type II, hypertension, obstructive sleep apnea, hypothyroidism and morbid obesity. | Reduction in lactate levels with improved clinical parameters were observed post-CytoSorb® therapy. Vasopressor requirement was reduced to nil. Patient survived |
| 13. | 1 | Case Study | Dengue haemorrhagic fever associated with SIRS, acute fulminant hepatic failure with encephalopathy and oliguria | Liver function tests i.e., SGOT, SGPT were improved with improved platelet count. Patient was hemodynamically stable during discharge. |
| 14. | 1 | Case Study | Dengue fever with septic shock and multiorgan failure admitted in the intensive care. | Survived |
| 15. | 1 | Case Study | SIRS and renal dysfunction after heart transplantation | Vasopressor was weaned completely post-CytoSorb therapy with reduction in serum lactate levels depicting clinical improvement in patient. |
| 16. | 1 | Case Study | Acute Kidney Injury due to Rhabdomyolysis | Patient showed hemodynamic stability post-CytoSorb® therapy and survived |
| 17. | 1 | Case Study | Sepsis complicated by typhoid fever | Survived |
| 18. | 1 | Case Report | Sepsis with multiple organ dysfunction syndrome with the manifestation of acute respiratory distress syndrome (ARDS) and acute renal failure (AKI) | Reduction in catecholamine demand with reduction in serum lactate levels. Patient survived |
| 19. | 1 | Case report | Sepsis with multiple organ dysfunction syndrome | Survived |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehta, Y.; Paul, R.; Rabbani, R.; Acharya, S.P.; Withanaarachchi, U.K. Sepsis Management in Southeast Asia: A Review and Clinical Experience. J. Clin. Med. 2022, 11, 3635. https://doi.org/10.3390/jcm11133635
Mehta Y, Paul R, Rabbani R, Acharya SP, Withanaarachchi UK. Sepsis Management in Southeast Asia: A Review and Clinical Experience. Journal of Clinical Medicine. 2022; 11(13):3635. https://doi.org/10.3390/jcm11133635
Chicago/Turabian StyleMehta, Yatin, Rajib Paul, Raihan Rabbani, Subhash Prasad Acharya, and Ushira Kapilani Withanaarachchi. 2022. "Sepsis Management in Southeast Asia: A Review and Clinical Experience" Journal of Clinical Medicine 11, no. 13: 3635. https://doi.org/10.3390/jcm11133635
APA StyleMehta, Y., Paul, R., Rabbani, R., Acharya, S. P., & Withanaarachchi, U. K. (2022). Sepsis Management in Southeast Asia: A Review and Clinical Experience. Journal of Clinical Medicine, 11(13), 3635. https://doi.org/10.3390/jcm11133635






