Abstract
Background: There is a plethora of real-world data on the safety and effectiveness of direct-acting oral anticoagulants (DOACs); however, study heterogeneity has contributed to inconsistent findings. We compared the effectiveness and safety of apixaban with those of other direct-acting oral anticoagulants (DOACs) and vitamin K antagonists (VKA e.g., warfarin). Methods: A systematic review and meta-analysis was conducted retrieving data from PubMed, SCOPUS and Web of Science from January 2009 to December 2021. Studies that evaluated apixaban (intervention) prescribed for adults (aged 18 years or older) with AF for stroke prevention compared to other DOACs or VKAs were identified. Primary outcomes included stroke/systemic embolism (SE), all-cause mortality, and major bleeding. Secondary outcomes were intracranial haemorrhage (ICH) and ischaemic stroke. Randomised controlled trials and non-randomised trials were considered for inclusion. Results: In total, 67 studies were included, and 38 studies were meta-analysed. Participants taking apixaban had significantly lower stroke/SE compared to patients taking VKAs (relative risk (RR) 0.77, 95% confidence interval (CI) 0.64–0.93, I2 = 94%) and dabigatran (RR 0.84, 95% CI 0.74–0.95, I2 = 66%), but not to patients administered rivaroxaban. There was no statistical difference in mortality between apixaban and VKAs or apixaban and dabigatran. Compared to patients administered rivaroxaban, participants taking apixaban had lower mortality rates (RR 0.83, 95% CI 0.71–0.96, I2 = 96%). Apixaban was associated with a significantly lower risk of major bleeding compared to VKAs (RR 0.58, 95% CI 0.52–0.65, I2 = 90%), dabigatran (RR 0.79, 95% CI 0.70–0.88, I2 = 78%) and rivaroxaban (RR 0.61, 95% CI 0.53–0.70, I2 = 87%). Conclusions: Apixaban was associated with a better overall safety and effectiveness profile compared to VKAs and other DOACs.
Keywords:
apixaban; atrial fibrillation; stroke; major bleeding; anticoagulant; secondary prevention 1. Introduction
Anticoagulation is the fundamental priority for the prevention of stroke in people diagnosed with atrial fibrillation (AF) and is one of the pillars of guideline-recommended AF management [1,2]. The efficacy of direct-acting oral anticoagulants (DOACs) versus warfarin has received considerable attention in the last decade. DOACs have generally [3,4], although not always [5], demonstrated favourable outcomes for stroke and mortality. Typically, DOACs are now favoured over warfarin for stroke prevention in AF due to their superior safety profile regarding intracranial haemorrhage (all DOACs) and major bleeding (some DOACs) [1,6]. However, agreement on the most favourable DOAC in terms of effectiveness and safety is challenging, particularly given that there are no head-to-head comparison trials.
There is a plethora of real-world data on the safety, and to a lesser extent, the effectiveness of DOACs, but the disparity in study populations, inclusion and exclusion criteria, and statistical analyses, has resulted in inconsistent findings and several unanswered questions. Further, our understanding of the potential impact of geographical region, study design and age on such outcomes is lacking. Addressing these gaps in the current evidence base will allow clinicians and patients to make better, evidence-informed decisions when selecting a DOAC to prevent stroke in people with AF.
Given apixaban is the most commonly used DOAC [7], the aims of this systematic review and meta-analysis were to compare the effectiveness and safety of apixaban with those of other DOACs and vitamin K antagonists (VKA e.g., warfarin). First, we sought to determine if apixaban was more effective (reduced stroke and mortality) and safer (reduced major bleeding) than dabigatran, rivaroxaban, edoxaban, and VKAs for patients with AF. Second, we investigated how geographical region (Asia, Europe, North America) and age (≥75/<75 years of age) may impact the effectiveness and safety of apixaban.
2. Methods
This systematic review was registered in the International Prospective Register of Systematic Reviews—PROSPERO (CRD42021236826) and conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [8].
2.1. Study Inclusion Criteria
We included studies carried out in any setting that evaluated apixaban (intervention) prescribed for adults (aged 18 years or older) with AF for stroke prevention compared to other DOACs or VKAs (e.g., warfarin). Primary outcomes included stroke or any thromboembolic event (stroke/SE composite), all-cause mortality, and major bleeding. Secondary outcomes included intracranial haemorrhage (ICH) and ischaemic stroke. Definitions used for each outcome were employed by the primary trials and may not be consistent between studies. All randomised controlled trials and non-randomised studies, pre–post studies and interrupted time series were considered for inclusion. Cross-sectional studies, case reports and qualitative studies were excluded.
2.2. Search Strategy
The search strategy was developed by the review team who selected all key terms. Medical subject headings (MesH) terms and synonyms for the different terms such as “atrial fibrillation”, “apixaban” and “stroke” were used and combined with Boolean operators, proximity operators, truncations and wildcards. PubMed, SCOPUS and Web of Science were searched from 1 January 2009 to 21 December 2021 for relevant studies (refer to Supplement S1 for full search strategies). Database searches were initiated from 2009 rather than inception, because the first DOAC trial (RE-LY) was published in 2009 [9]. There were no language restrictions; however, availability of the full text was a requirement for inclusion. Search results were managed using EndNote X9.3.3.
2.3. Study Selection
Two reviewers (B.J.R.B., J.Z.), independently and in duplicate, screened the titles and abstracts of the studies retrieved by the databases against the search criteria. The full texts of all potentially relevant articles were retrieved and independently assessed by the reviewers (B.J.R.B., P.C., D.A.L., M.C., D.G.). Any disagreement was resolved through discussion with the first author (B.J.R.B.).
2.4. Data Extraction
Data extraction was conducted independently by five reviewers (B.J.R.B., P.C., D.A.L., M.C., D.G.), with at least 20% checked by (B.J.R.B.) to ensure consistency/accuracy. The following information was extracted: (i) authors, year, country, reference; (ii) study design with inclusion/exclusion criteria; (iii) study aim; (iv) intervention and comparator characteristics (n = age, sex, CHA2DS2-VASc, HAS-BLED); (v) outcomes (effectiveness and safety); (vi) follow-up time points; (vii) results; (viii) study conclusions; (ix) risk of bias assessment.
2.5. Risk of Bias Assessment
Five authors (B.J.R.B., P.C., D.A.L., M.C., D.G.) independently assessed the individual studies for risk of bias in duplicate, and any discrepancies were resolved via discussion or referral to a third reviewer, as required. The Cochrane Risk of Bias v.2 (RoB2) tool [10] was used to assess the risk of bias for randomised controlled trials (RCTs). The Risk Of Bias In Non-randomised Studies—of Interventions (ROBINS-I) [11] was used to assess the risk of bias for non-randomised studies.
2.6. Data Synthesis
Meta-analyses were conducted for comparable studies. Primary and secondary outcome effect measures with 95% confidence intervals were pooled using RevMan software [12]. Results are presented visually using Forest plots. For studies where quantitative data were too few or too heterogeneous, a narrative synthesis approach was used. Effect measures for dichotomous outcomes were analysed using the number of events and total sample size as reported in the included studies. Results of the selected studies were combined using the Mantel–Haenszel method. Effect sizes were expressed as relative risk and 95% confidence intervals. Heterogeneity was quantitatively assessed using Higgins’s index (I2), with 25%, 50% and 75% considered moderate, substantial and considerable heterogeneity, respectively. Random-effect models were applied allowing for between-study variability by weighting studies using a combination of intra- and inter-study variance.
2.7. Sub-Group and Sensitivity Analyses
Sub-group analyses were conducted (if sufficient data) to explore any impact of cohort age (≥75 and <75 years) and geographical region (North America, Asia, Europe) on the safety and effectiveness of apixaban compared to VKAs, dabigatran, and rivaroxaban. Sensitivity analyses were planned to explore the impact of studies deemed as ‘serious risk of bias’ on the safety and effectiveness of apixaban.
3. Results
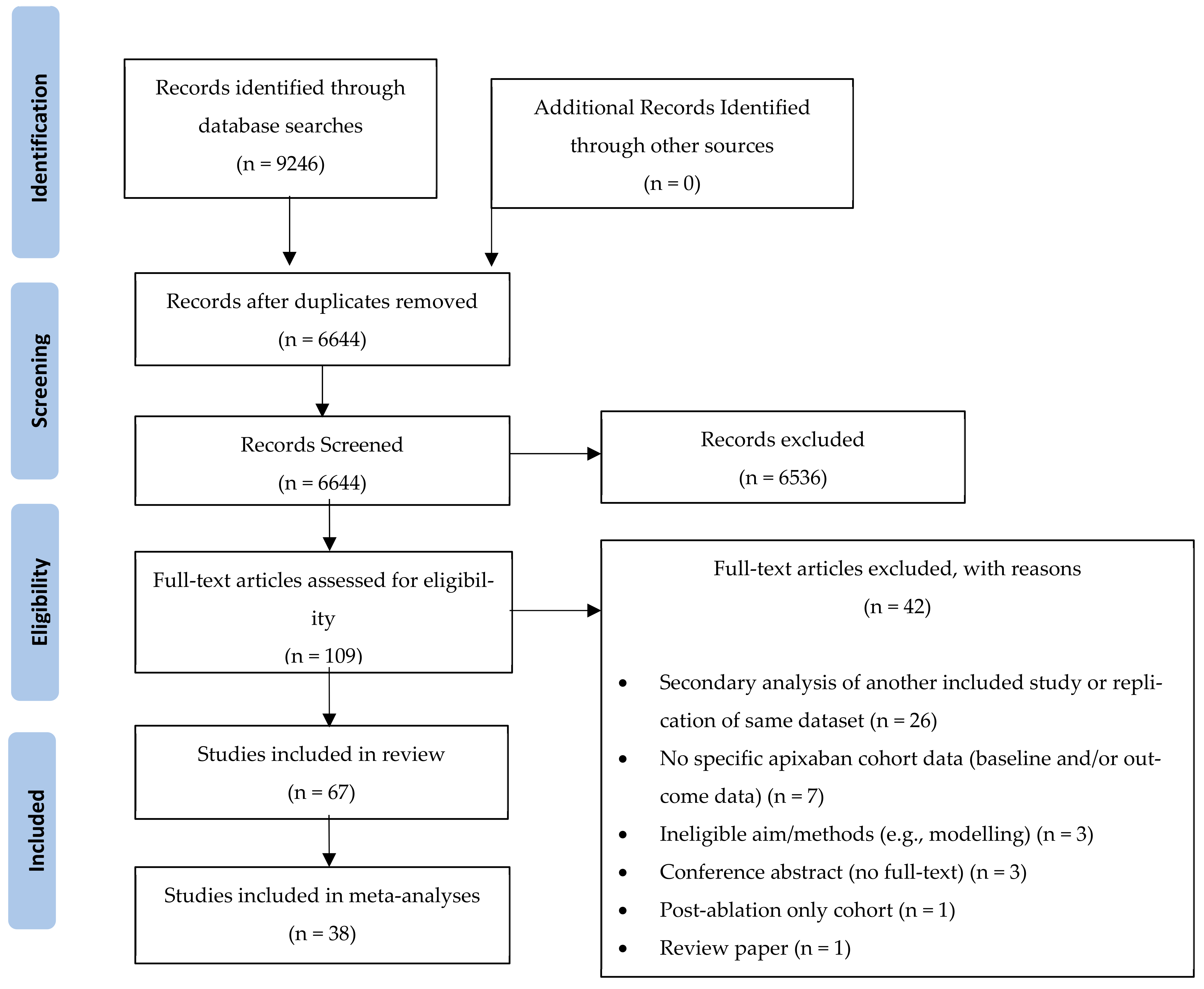
The database searches identified 9246 papers. After removal of duplicates, 6644 papers were included in the title and abstract screening, which resulted in 109 papers retrieved for full-text screening against the inclusion/exclusion criteria. Of these, 67 (61%) studies were included in the systematic review, and 38 (35%) studies were included in meta-analyses (Figure 1).
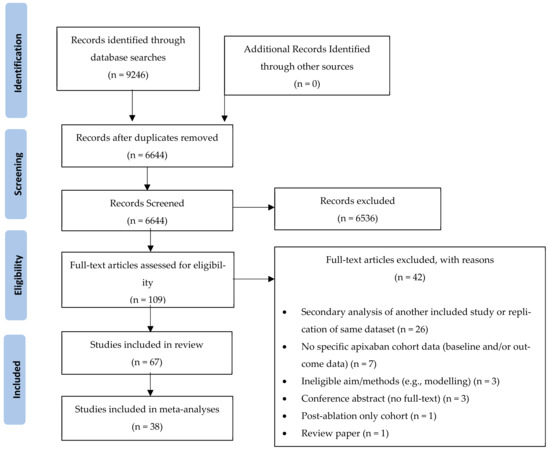
Figure 1.
PRISMA Diagram depicting the screening and study selection process.
3.1. Characteristics of the Included Studies
The included studies were published between 2009 and 2021; two of them were randomised controlled trials [13,14], two were prospective cohort studies [15,16], and the remaining 63 were retrospective cohort studies (Supplementary File; Table S1). The sample size for the apixaban-treated cohorts ranged between n = 98 [17] and n = 353,897 patients [18]. The total sample of patients included in the review was 3,911,894, of which, 1,292,620 patients (33%) were taking apixaban. Mean/median patient age ranged from 62 [19] to 86 [20] years, and the proportion of females ranged between 15% [14] and 69% [21]. Of the 67 included studies, 34 were conducted in the U.S.A., 7 in Denmark, 3 each in Sweden, Spain, Norway, Japan, and Germany, 2 each in the UK and South Korea, 1 each in Taiwan, Canada, Thailand, France, and Singapore, 1 was international, with 39 participating countries, and 1 included data from Canada, the U.S.A. and the UK.
For the two included randomised controlled trials, one was deemed ‘low risk of bias’ [13], and one was deemed ‘some concerns’ [14], with the latter due to non-blinded participants. Overall, 64/65 real-world studies were deemed ‘moderate risk of bias‘, with one study deemed to be at serious risk of bias [22]. For all included real-world studies, the risk of bias was elevated due to confounding which was inherent in the study design. Further study-level detail regarding the risk of bias is reported within the Supplementary File, Table S2 and Figure S1.
3.2. Primary Outcomes
Meta-analyses for stroke/SE (Figure 2), mortality (Figure 3) and major bleeding (Figure 4) are presented below. Each analysis includes all eligible studies and compared apixaban with VKAs, dabigatran and rivaroxaban for each outcome. A comparison of apixaban with edoxaban was not possible due to only one eligible study including data for both drugs [23].
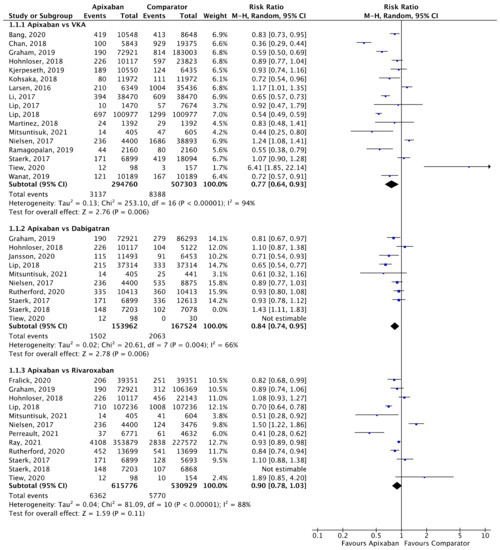
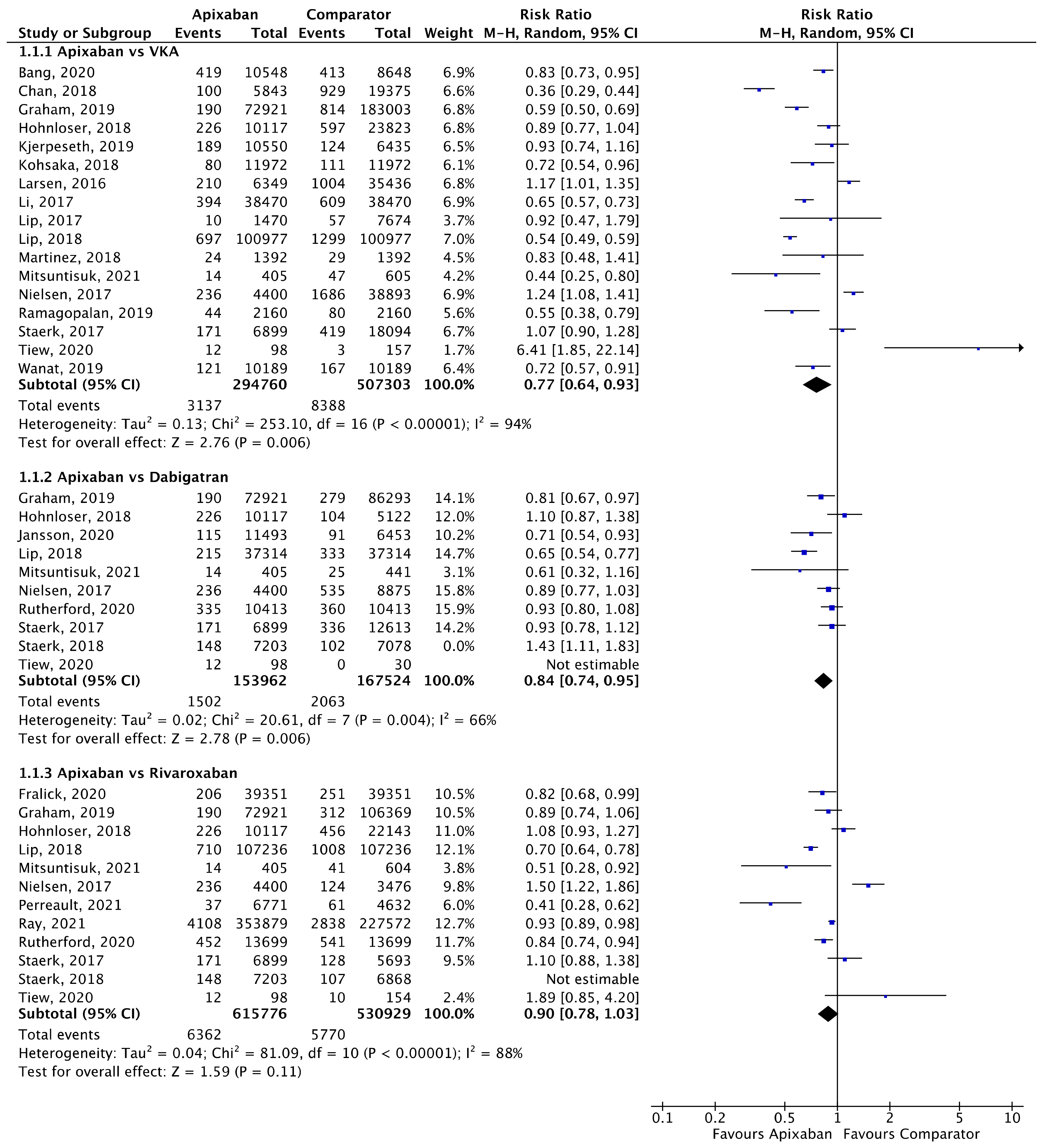
Figure 2.
Comparison of apixaban to VKAs (1.1.1), dabigatran (1.1.2) and rivaroxaban (1.1.3) for stroke/SE [17,18,20,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43].
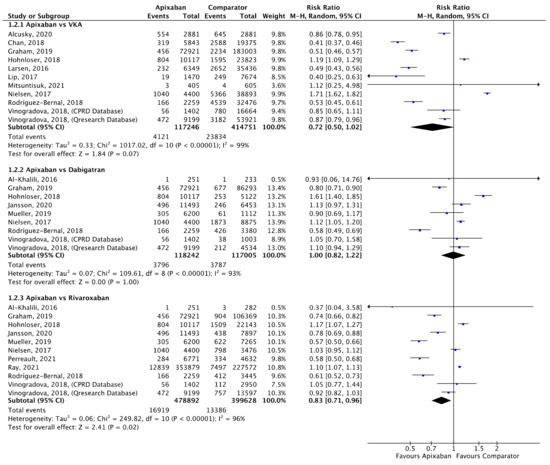
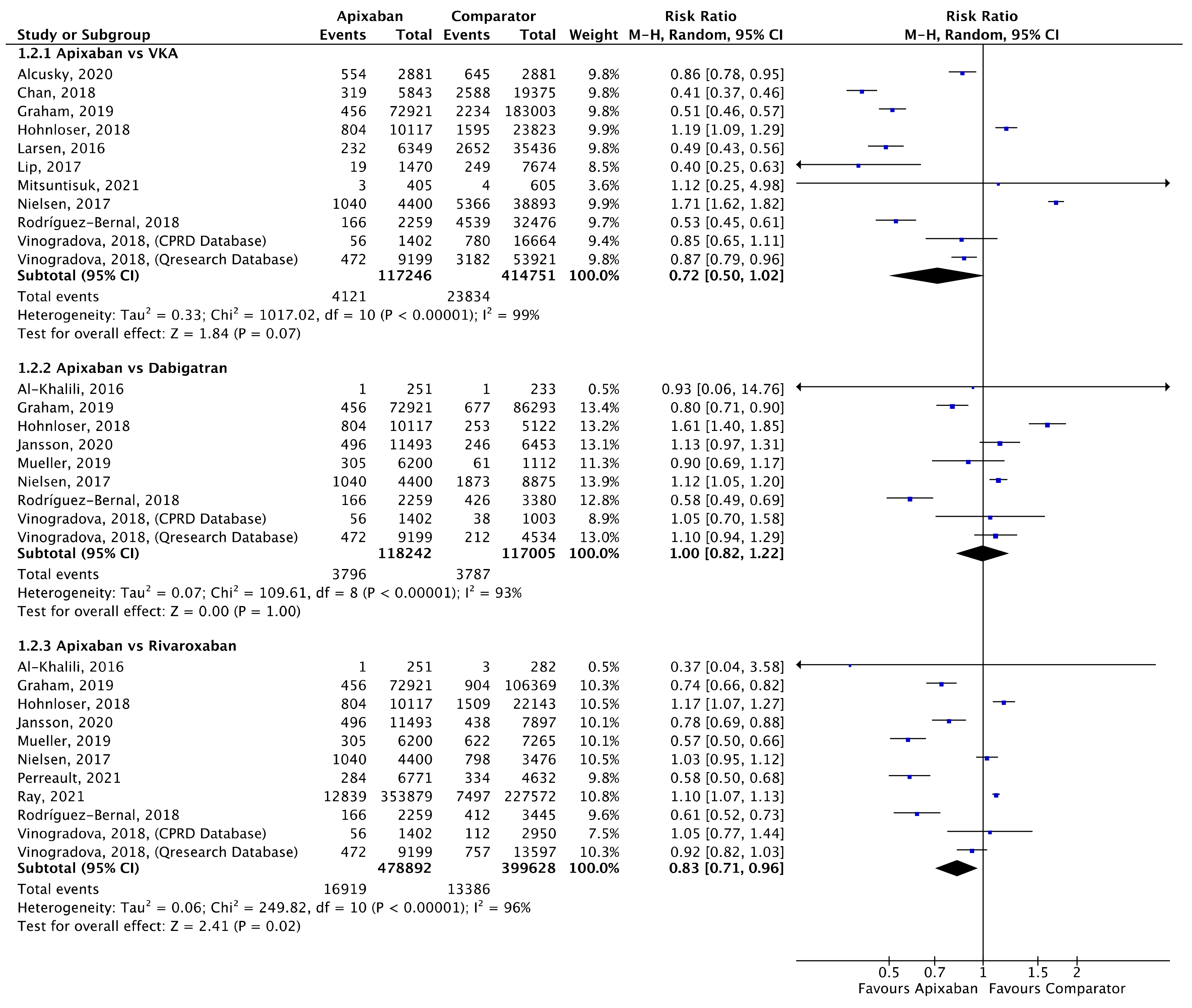
Figure 3.
Comparison of apixaban to VKAs (1.2.1), dabigatran (1.2.2) and rivaroxaban (1.2.3) for mortality [16,18,21,25,26,27,30,32,34,35,39,44,45,46,47].
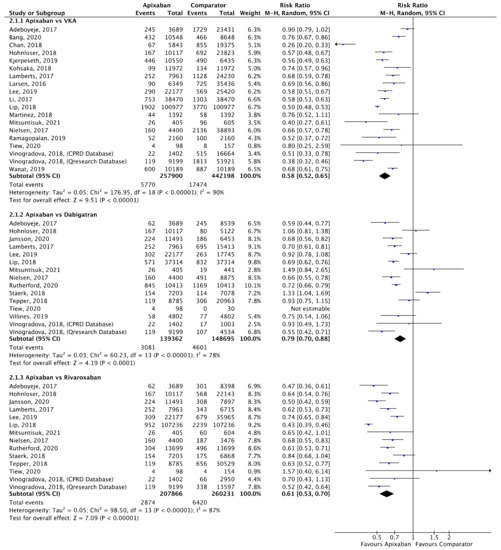
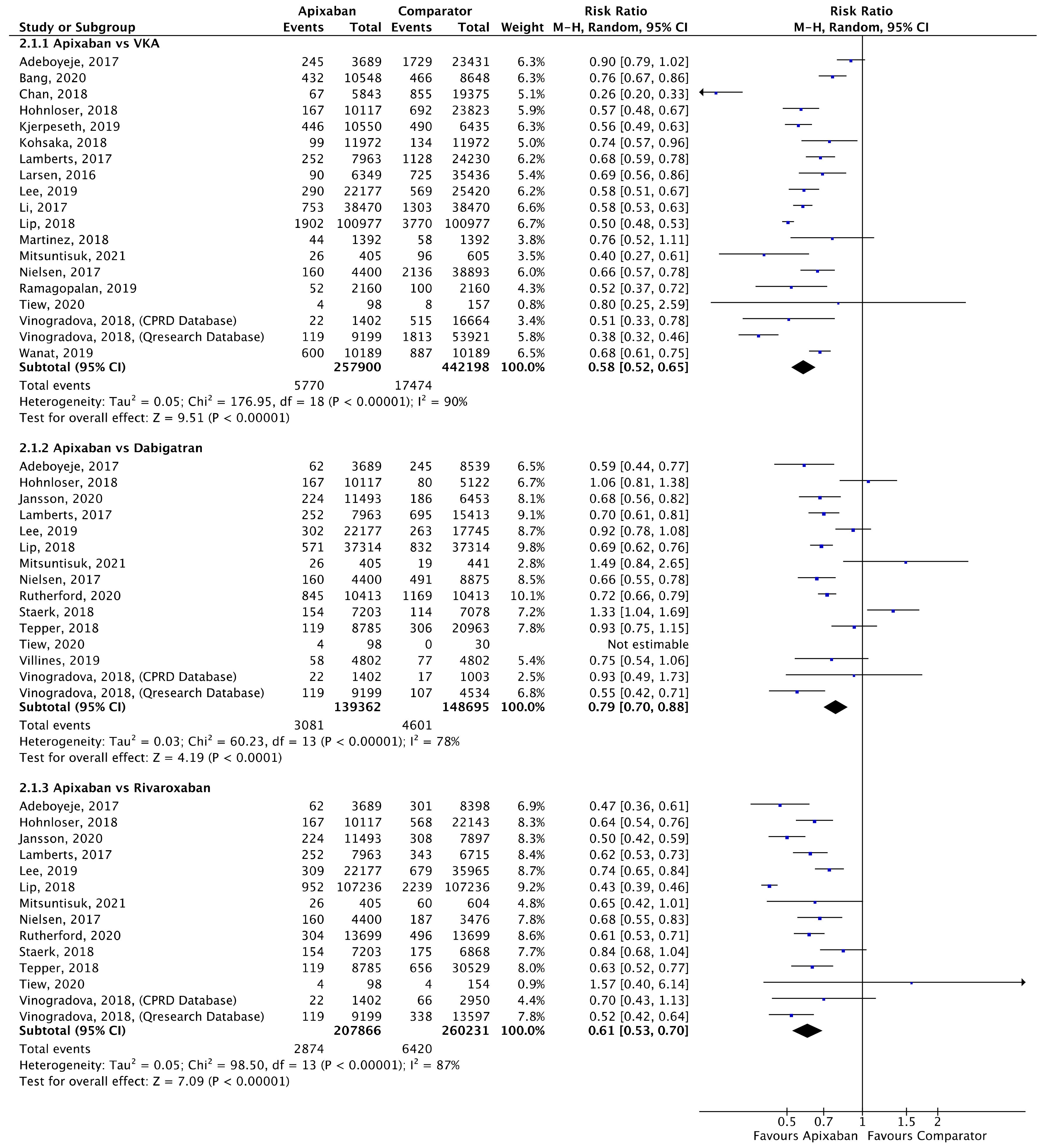
Figure 4.
Comparison of apixaban to VKAs (2.1.1), dabigatran (2.1.2) and rivaroxaban (2.1.3) for major bleeding [16,17,20,23,24,25,27,28,29,30,31,33,34,35,36,38,39,40,41,48,49,50,51].
A total of 17 (n = 802,063), 10 (n = 321,486), and 12 (n = 1,146,705) studies compared apixaban to VKAs, dabigatran and rivaroxaban, respectively, and were included in meta-analyses investigating stroke/SE (Figure 2). Compared to VKAs, apixaban was associated with a significantly lower risk of stroke/SE (relative risk (RR) 0.77, 95% confidence interval (CI) 0.64–0.93, I2 = 94%). Compared to dabigatran, apixaban was associated with a significantly lower risk of stroke/SE (RR 0.84, 95% CI 0.74–0.95, I2 = 66%). There was no statistical difference in risk of stroke/SE between apixaban and rivaroxaban (RR 0.90, 95% CI 0.78–1.03, I2 = 88%).
A total of 10 (n = 533,997), 8 (n = 235,247) and 10 (n = 878,520) studies compared apixaban to VKAs, dabigatran and rivaroxaban, respectively, and were included in meta-analyses investigating mortality (Figure 3). There was no statistical difference in mortality between apixaban and VKAs (RR 0.72, 95% CI 0.50–1.00, I2 = 99%) or apixaban and dabigatran (RR 1.00, 95% CI 0.82–1.22, I2 = 93%). Compared to rivaroxaban, apixaban was associated with a significantly lower risk of mortality (RR 0.83, 95% CI 0.71–0.96, I2 = 96%).
A total of 18 (n = 700,098), 14 (n = 288,057) and 13 (n = 468,097) studies compared apixaban to VKAs, dabigatran and rivaroxaban, respectively, and were included in meta-analyses investigating major bleeding (Figure 4). Apixaban was associated with a significantly lower risk of major bleeding compared to VKAs (RR 0.58, 95% CI 0.52–0.65, I2 = 90%), dabigatran (RR 0.79, 95% CI 0.70–0.88, I2 = 78%) and rivaroxaban (RR 0.61, 95% CI 0.53–0.70, I2 = 87%).
3.3. Secondary Outcomes
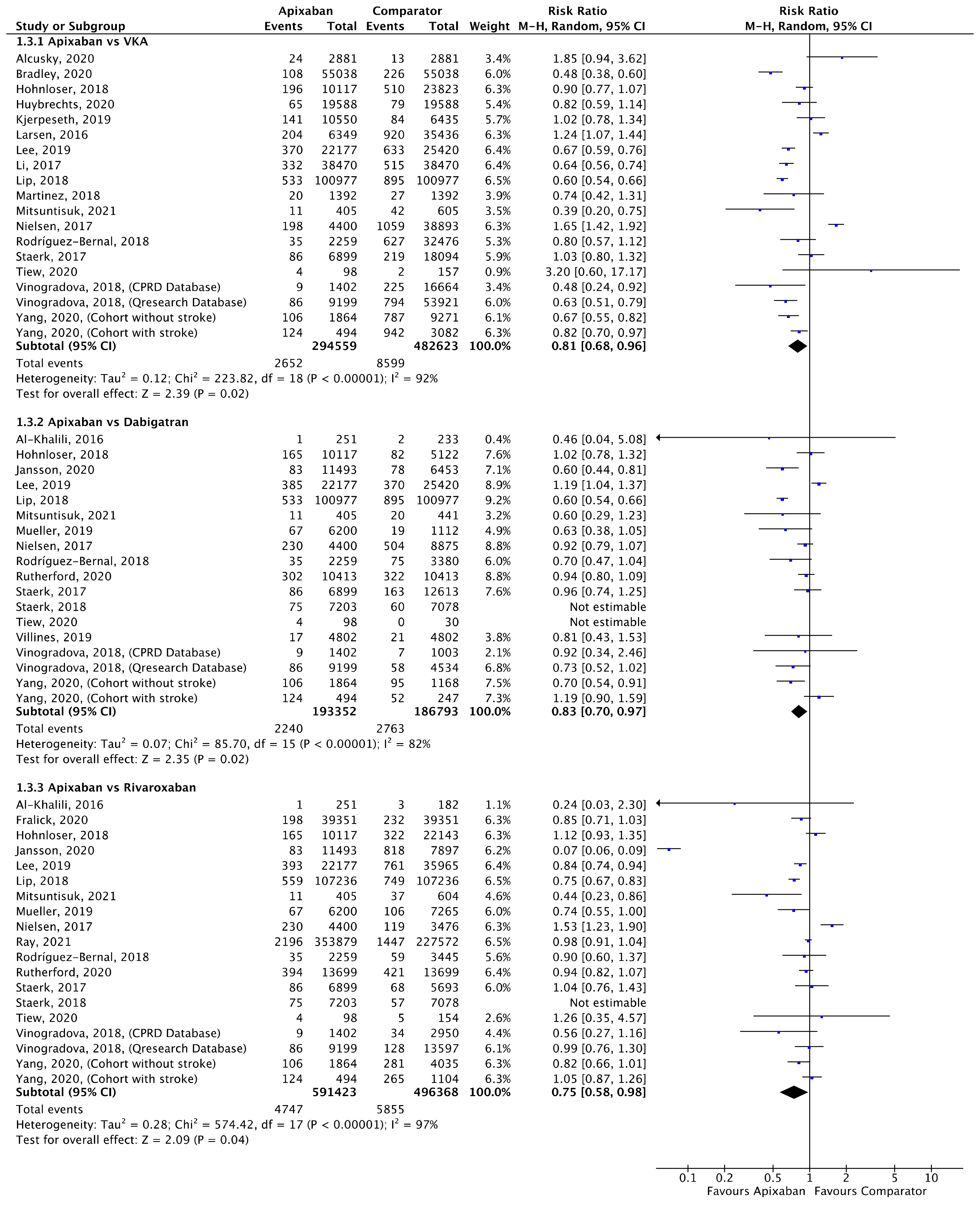
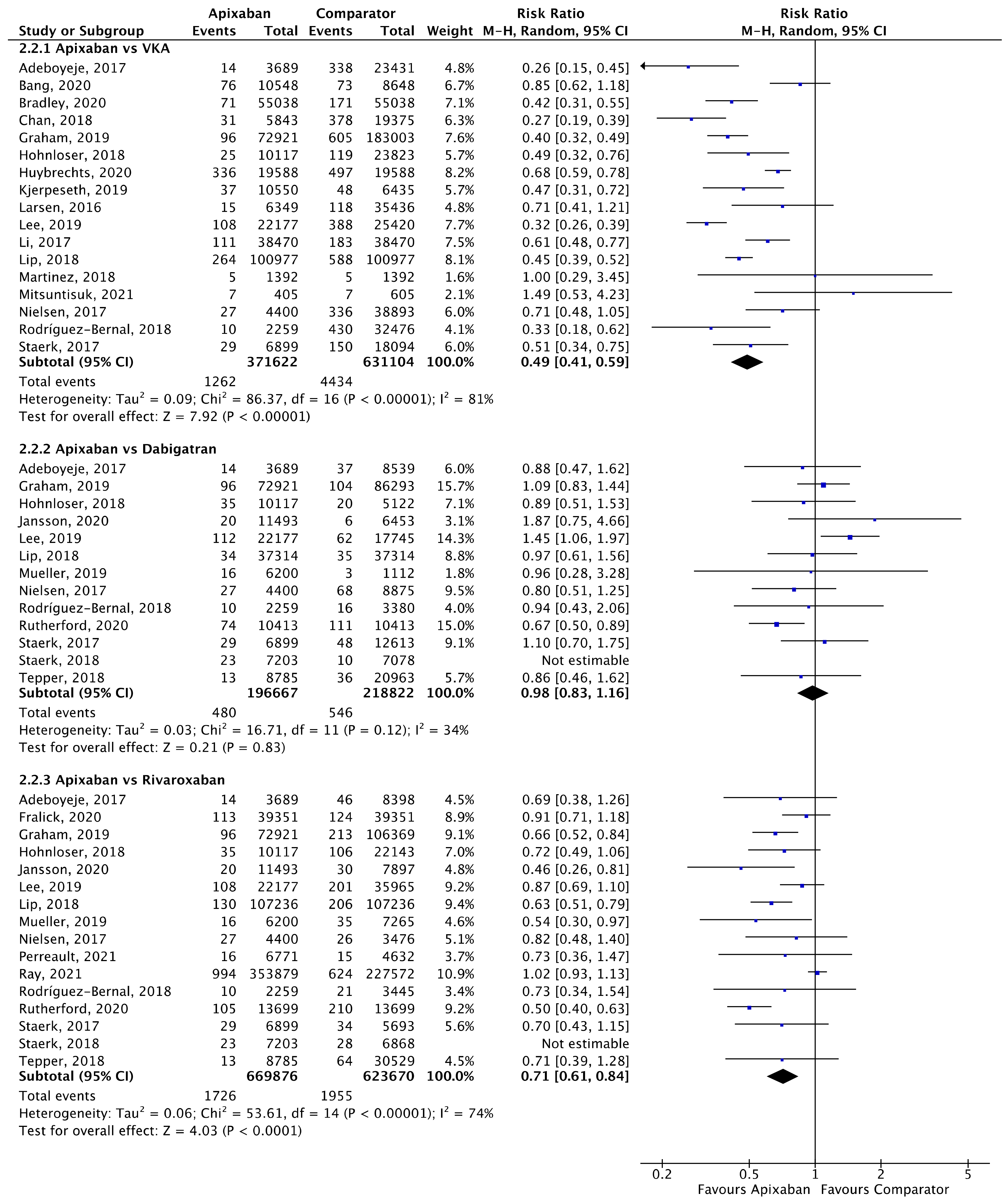
Meta-analyses for ischaemic stroke (Figure 5) and ICH (Figure 6) are presented below. Each analysis included all eligible studies and compared apixaban with VKAs, dabigatran and rivaroxaban for each outcome.
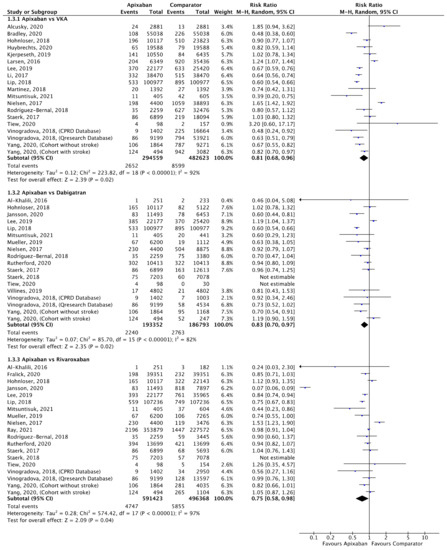
Figure 5.
Comparison of apixaban to VKAs (1.3.1), dabigatran (1.3.2) and rivaroxaban (1.3.3) for ischaemic stroke [16,17,18,20,21,23,27,28,30,31,33,34,35,37,38,39,40,41,42,44,45,46,52,53,54].
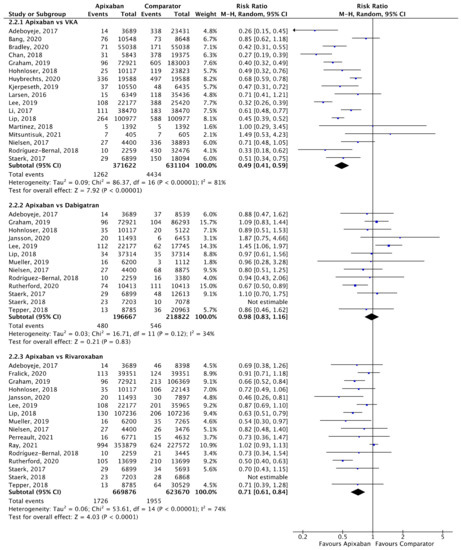
Figure 6.
Comparison of apixaban to VKAs (2.2.1), dabigatran (2.2.2) and rivaroxaban (2.2.3) for ICH [18,20,23,24,25,26,27,28,30,31,33,34,35,37,39,40,41,42,44,46,47,48,50,52,53].
A total of 19 (n = 777,182), 16 (n = 380,145) and 17 (n = 1,087,791) studies compared apixaban to VKAs, dabigatran and rivaroxaban, respectively, and were included in meta-analyses investigating ischaemic stroke (Figure 5). Apixaban was associated with a significantly lower risk of ischaemic stroke compared to VKAs (RR 0.81, 95% CI 0.68–0.96, I2 = 92%), dabigatran (RR 0.83, 95% CI 0.70–0.97, I2 = 82%) and rivaroxaban (RR 0.75, 95% CI 0.58–0.98, I2 = 97%).
A total of 17 (n = 1,002,726), 13 (n = 415,489) and 16 (n = 1,293,546) studies compared apixaban to VKAs, dabigatran and rivaroxaban, respectively, and were included in meta-analyses investigating ICH (Figure 6). Apixaban was associated with a significantly lower rates of ICH compared to VKAs (RR 0.49, 95% CI 0.41–0.59, I2 = 81%) and rivaroxaban (RR 0.71, 95% CI 0.61–0.84, I2 = 74%), but not dabigatran (RR 0.98, 95% CI 0.83–1.16, I2 = 34%).
3.4. Sub-Group and Sensitivity Analyses
Sub-group meta-analyses exploring the impact of participants’ age (≥75 and <75 years) were not possible due to insufficient sub-group data stratified by consistent age boundaries within the primary trials. Sub-group analyses for the impact of geographic region (North America, Asia, Europe) on the safety and effectiveness of apixaban are presented in Figures S2–S4 (Supplement S3). Meta-analyses are presented for the primary outcomes stroke/SE, mortality and major bleeding comparing apixaban to VKAs, apixaban to dabigatran and apixaban to rivaroxaban stratified by geographic region.
3.5. Stratification by Geographic Region
Regarding the apixaban vs VKA comparisons, the relative risk of stroke/SE was significantly lower with apixaban in North America, but not in Asia or Europe. There was no difference in the relative risk of mortality across the geographic sub-groups, and the relative risk of major bleeding was lower with apixaban across all geographic sub-groups (Figure S2; Supplement S4). For the apixaban vs dabigatran comparisons, the relative risk of stroke/SE and mortality were significantly lower with apixaban in North America only. The relative risk of major bleeding was significantly lower with apixaban in North America and Europe (Figure S3; Supplement S4). For the apixaban vs rivaroxaban comparisons, the relative risk of stroke/SE was significantly lower with apixaban in North America only. The relative risk for mortality was significantly lower with apixaban in North America and Europe and could not be estimated in Asia (due to no eligible studies). The relative risk for major bleeding was significantly lower with apixaban across all geographic subgroups (Figure S4; Supplement S4).
Sensitivity analyses were not necessary to explore the impact of studies deemed ‘serious risk of bias’ on the safety and effectiveness of apixaban, as no studies with ‘serious risk of bias’ were included in the meta-analyses.
4. Discussion
Our systematic review and meta-analyses show that the use of apixaban was associated with improved effectiveness (reduced stroke/SE and ischaemic stroke) and safety profile (major bleeding and ICH) when compared with the use of VKAs. Compared with dabigatran, apixaban was associated with significantly lower stroke or systemic embolism, major bleeding events and ischaemic stroke but not mortality or ICH. Compared to rivaroxaban, apixaban was associated with significantly lower mortality, major bleeding, ischaemic stroke, and ICH but not stroke/SE. Some results varied when stratified by geographic region.
In the ARISTOTLE randomised controlled trial (n = 18,201 people with AF), apixaban was superior to warfarin for the prevention of stroke or systemic embolism [13]. A phase II randomised controlled trial, ARISTOTLE-J, showed that in Japanese patients with AF, apixaban was well tolerated, with lower rates of major bleeding than warfarin over 12 weeks. However, to determine the effectiveness and safety of apixaban with those of OACs other than VKAs (i.e., warfarin), namely, dabigatran, rivaroxaban and edoxaban, real-world studies are needed.
Our findings extend those of a previous systematic review and meta-analysis (16 studies with up to n = 266,598 people with AF included in the meta-analysis), which showed that the use of apixaban in cohort studies was associated with an overall similar effectiveness in reducing stroke and any thromboembolic events when compared with the use of warfarin (odds ratio 0.92, 95% CI 0.72–1.10) [4]. However, the previous review demonstrated a better safety profile for apixaban compared to warfarin, dabigatran, and rivaroxaban. A more recent systematic review and network meta-analysis (21 studies with 605,771 people with AF) [55] found that apixaban was associated with a lower risk of major bleeding compared to rivaroxaban (hazard ratio 1.8, 95% CI 1.6–2.1) and dabigatran (hazard ratio 1.4, 95% CI 1.2–1.6), which is in agreement with the findings of the present study. Menichelli et al. [55] did, however, showed a higher risk of stroke or systemic embolism with rivaroxaban compared to apixaban (hazard ratio 1.4, 95% CI 1.00–1.80) but not with dabigatran compared with apixaban, which is contrary to our findings, although their work included fewer studies and participants. The authors also did not find a mortality benefit for apixaban, whereas the present study found a mortality benefit for apixaban vs. rivaroxaban. Thus, the analysis of randomised controlled trials and cohort studies demonstrated differing (and not yet established) effectiveness and safety profiles in DOAC–DOAC comparisons.
A recent retrospective cohort study (published after the searches for this systematic review), including >580,000 US Medicare beneficiaries, found that rivaroxaban was associated with a higher adjusted risk for ischaemic or haemorrhagic events (hazard ratio 1.18 95% CI 1.12–1.24) compared to apixaban [18]. This present study was updated to include this work in the appropriate meta-analyses. The findings in the present review demonstrate an overall beneficial association for apixaban over rivaroxaban for stroke/SE, mortality, major bleeding, ischaemic stroke and ICH. These findings add to the body of evidence suggesting that apixaban is associated with a lower bleeding risk and greater thromboembolic protection compared with rivaroxaban. Further and more broadly, our findings provide evidence that, although apixaban was not associated with an improvement in all outcomes across all DOAC comparisons, none of the outcomes we investigated favoured either dabigatran or rivaroxaban when compared to apixaban.
Limitations
The generally high heterogeneity in several meta-analyses makes it challenging to ascertain definitive conclusions. Furthermore, there was insufficient evidence from real-world studies to compare edoxaban with other commonly used DOACs. Other factors may have also influenced our findings, including inappropriate DOAC dosing and individual patient OAC adherence and associated comorbidities. Similarly, we did not investigate differences in DOAC dosage and associated study outcomes within this systematic review. Despite the reduced data on the number of participants receiving the standard or a lower dose apixaban, Proietti et al. previously demonstrated that the standard dose may be superior to a reduced dose of apixaban for the reduction of any thromboembolic event [4]. Indeed, OAC is only one aspect of holistic or integrated care management of AF [2], whereby adherence to such an approach and appropriate characterisation of AF patients have been associated with improved clinical outcomes [56,57]. Further, the addition of statin therapy to OAC has been shown to improve in-hospital prognosis of patients with acute ischaemic stroke [43] and reduce long-term major adverse cardiovascular events in patients with embolic stroke of undetermined source [58]. This is also important when considering the use of apixaban, given the need for twice-daily dosing compared to once-daily dosing for rivaroxaban, for example, and should be considered on a patient-by-patient and shared decision-making process. Finally, most real-world studies included in this review were of a retrospective cohort design, with innate and well-known limitations, although, adjusted effect measures or propensity score matched populations were used where possible.
5. Conclusions
In this systematic review and meta-analysis combining data from clinical trials and real-world studies with >3.9 million participants, apixaban was associated with a better overall safety and effectiveness profile compared to VKAs and other DOACs. Despite the use of random-effect models to estimate overall effect estimates, considerable heterogeneity was present in most meta-analyses, and this should be considered when interpreting the results.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11133788/s1, Supplement S1. Full search strategies. Supplement S2. Characteristics of the included studies. Supplement S3. Risk of bias of the included studies. Supplement S4. Sub-group meta-analyses. References [59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84] are cited in Supplementary Materials.
Author Contributions
Conceptualization, B.J.R.B., D.A.L. and G.Y.H.L.; formal analysis, B.J.R.B.; investigation, B.J.R.B., D.A.L., J.Z., P.C. and D.G.; data curation, B.J.R.B., D.A.L., J.Z., P.C. and D.G.; writing—original draft preparation, B.J.R.B.; writing—review and editing, B.J.R.B., D.A.L., P.C., J.Z., D.G., C.D.M., P.D., S.K., S.H.H. and G.Y.H.L., visualization, B.J.R.B. and P.C.; supervision, B.J.R.B., D.A.L. and G.Y.H.L.; funding acquisition, B.J.R.B., D.A.L. and G.Y.H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by a research grant from Pfizer Inc and Bristol-Myers Squibb.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to it being a systematic review and meta-analysis of published data.
Informed Consent Statement
Not applicable as a systematic review and meta-analysis of published data.
Data Availability Statement
Not applicable as data is already published.
Conflicts of Interest
B.J.R.B. has received research funding from Bristol Myers Squibb (BMS)/Pfizer. D.A.L. has received investigator-initiated educational grants from BMS, has been a speaker for Boehringer Ingeheim, and BMS/Pfizer and has consulted for BMS, Boehringer Ingelheim, and Daiichi-Sankyo. C.D.M.: Consultant for AstraZeneca, Bayer Pharmaceuticals, Incyte, Merck, Pfizer, Sanofi, and Takeda and grant funding from Merck. P.D.: Honoraria and consulting fees and research support from Bayer, BMS, Pfizer, and Servier. S.K.: investigator-initiated grants from Novartis and Bristol-Myers Squibb (BMS), and has been a speaker for BMS/Pfizer. S.H.H.: consulting fees from Bayer Healthcare, BI, BMS, Boston Scientific, Daiichi Sankyo, Gilead, Johnson & Johnson, Medtronic, Pfizer, Sanofi Aventis, Servier, Zoll and lecture fees from Bayer Healthcare, BI, BMS, Daiichi Sankyo, Pfizer, Sanofi Aventis, and Medtronic. G.Y.H.L.: Consultant and speaker fees from BMS/Pfizer, Boehringer Ingelheim and Daiichi-Sankyo. No fees are received personally.
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.H. The ABC pathway: An integrated approach to improve AF management. Nat. Rev. Cardiol. 2017, 14, 627–628. [Google Scholar] [CrossRef] [PubMed]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
- Proietti, M.; Romanazzi, I.; Romiti, G.F.; Farcomeni, A.; Lip, G.Y. Real-World Use of Apixaban for Stroke Prevention in Atrial Fibrillation: A Systematic Review and Meta-Analysis. Stroke 2018, 49, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Outes, A.A.; Terleira-Fernández, A.; Rojas, G.C.; Suárez-Gea, M.L.; Vargas-Castrillón, E. Direct oral anticoagulants for stroke prevention in patients with atrial fibrillation: Meta-analysis by geographic region with a focus on European patients. Br. J. Clin. Pharmacol. 2016, 82, 633–644. [Google Scholar] [CrossRef] [Green Version]
- Chao, T.-F.; Joung, B.; Takahashi, Y.; Lim, T.W.; Choi, E.-K.; Chan, Y.-H.; Guo, Y.; Sriratanasathavorn, C.; Oh, S.; Okumura, K.; et al. 2021 Focused Update Consensus Guidelines of the Asia Pacific Heart Rhythm Society on Stroke Prevention in Atrial Fibrillation: Executive Summary. Thromb. Haemost. 2021, 122, 020–047. [Google Scholar] [CrossRef]
- Afzal, S.; Zaidi, S.T.R.; Merchant, H.A.; Babar, Z.-U.; Hasan, S.S. Prescribing trends of oral anticoagulants in England over the last decade: A focus on new and old drugs and adverse events reporting. J. Thromb. Thrombolysis 2021, 52, 646–653. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 88, 105906. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Review Manager (Rev.Man.) [Program]; 5.3 Version; The Cochrane Collaboration: Copenhagen, Denmark, 2014.
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Ave-zum, A.; et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, S.; Shinohara, Y.; Kanmuri, K. Safety and Efficacy of the Oral Direct Factor Xa Inhibitor Apixaban in Japanese Patients with Non-Valvular Atrial Fibrillation—The ARISTOTLE-J study. Circ. J. 2011, 75, 1852–1859. [Google Scholar] [CrossRef] [Green Version]
- Halvorsen, S.; Atar, D.; Yang, H.; De Caterina, R.; Erol, C.; Garcia, D.; Granger, C.B.; Hanna, M.; Held, C.; Husted, S.; et al. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: Observations from the ARISTOTLE trial. Eur. Heart J. 2014, 35, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, Y.; Coupland, C.; Hill, T.; Hippisley-Cox, J. Risks and benefits of direct oral anticoagulants versus warfarin in a real world setting: Cohort study in primary care. BMJ 2018, 362, k2505. [Google Scholar] [CrossRef] [Green Version]
- Tiew, W.J.; Wong, V.L.; Tan, V.H.; Tan, Y.C.; Lee, E.M. A Real-world Experience of the Safety and Efficacy of Non-vitamin K Oral Anticoagulants Versus Warfarin in Patients with Non-valvular Atrial Fibrillation—A Single-centre Retrospective Cohort Study in Singapore. Ann. Acad. Med. Singap. 2020, 49, 838–847. [Google Scholar] [CrossRef]
- Ray, W.A.; Chung, C.P.; Stein, C.M.; Smalley, W.; Zimmerman, E.; Dupont, W.D.; Hung, A.M.; Daugherty, J.R.; Dickson, A.; Murray, K.T. Association of Rivaroxaban vs Apixaban with Major Ischemic or Hemorrhagic Events in Patients With Atrial Fibrillation. JAMA 2021, 326, 2395–2404. [Google Scholar] [CrossRef]
- Lin, J.; Trocio, J.; Gupta, K.; Mardekian, J.; Lingohr-Smith, M.; Menges, B.; You, M.; Nadkarni, A. Major bleeding risk and healthcare economic outcomes of non-valvular atrial fibrillation patients newly-initiated with oral anticoagulant therapy in the real-world setting. J. Med. Econ. 2017, 20, 952–961. [Google Scholar] [CrossRef]
- Martinez, B.K.; Sood, N.A.; Bunz, T.J.; Coleman, C.I. Effectiveness and Safety of Apixaban, Dabigatran, and Rivaroxaban Versus Warfarin in Frail Patients with Nonvalvular Atrial Fibrillation. J. Am. Heart Assoc. 2018, 7, e008643. [Google Scholar] [CrossRef]
- Alcusky, M.; Tjia, J.; McManus, D.D.; Hume, A.L.; Fisher, M.; Lapane, K.L. Comparative Safety and Effectiveness of Direct-Acting Oral Anticoagulants Versus Warfarin: A National Cohort Study of Nursing Home Residents. J. Gen. Intern. Med. 2020, 35, 2329–2337. [Google Scholar] [CrossRef]
- Noseworthy, P.A.; Yao, X.; Gersh, B.J.; Hargraves, I.; Shah, N.D.; Montori, V.M. Long-term stroke and bleeding risk in patients with atrial fibrillation treated with oral anticoagulants in contemporary practice: Providing evidence for shared decision-making. Int. J. Cardiol. 2017, 245, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-R.; Choi, E.-K.; Kwon, S.; Han, K.-D.; Jung, J.-H.; Cha, M.-J.; Oh, S.; Lip, G.Y. Effectiveness and Safety of Contemporary Oral Anticoagulants Among Asians with Nonvalvular Atrial Fibrillation. Stroke 2019, 50, 2245–2249. [Google Scholar] [CrossRef] [PubMed]
- Bang, O.Y.; On, Y.K.; Lee, M.-Y.; Jang, S.-W.; Han, S.; Han, S.; Won, M.-M.; Park, Y.-J.; Lee, J.-M.; Choi, H.-Y.; et al. The risk of stroke/systemic embolism and major bleeding in Asian patients with non-valvular atrial fibrillation treated with non-vitamin K oral anticoagulants compared to warfarin: Results from a real-world data analysis. PLoS ONE 2020, 15, e0242922. [Google Scholar] [CrossRef]
- Chan, Y.; See, L.; Tu, H.; Yeh, Y.; Chang, S.; Wu, L.; Lee, H.; Wang, C.; Kuo, C.; Kuo, C. Efficacy and Safety of Apixaban, Dabigatran, Rivaroxaban, and Warfarin in Asians with Nonvalvular Atrial Fibrillation. J. Am. Heart Assoc. 2018, 7, e008150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, D.J.; Baro, E.; Zhang, R.; Liao, J.; Wernecke, M.; Reichman, M.E.; Hu, M.; Illoh, O.; Wei, Y.; Goulding, M.R.; et al. Comparative Stroke, Bleeding, and Mortality Risks in Older Medicare Patients Treated with Oral Anticoagulants for Nonvalvular Atrial Fibrillation. Am. J. Med. 2019, 132, 596–604.e11. [Google Scholar] [CrossRef] [PubMed]
- Hohnloser, S.H.; Basic, E.; Hohmann, C.; Nabauer, M. Effectiveness and Safety of Non–Vitamin K Oral Anticoagulants in Comparison to Phenprocoumon: Data from 61,000 Patients with Atrial Fibrillation. Thromb. Haemost. 2018, 118, 526–538. [Google Scholar] [CrossRef]
- Kjerpeseth, L.J.; Selmer, R.; Ariansen, I.; Karlstad, Ø.; Ellekjær, H.; Skovlund, E. Comparative effectiveness of warfarin, dabigatran, rivaroxaban and apixaban in non-valvular atrial fibrillation: A nationwide pharmacoepidemiological study. PLoS ONE 2019, 14, e0221500. [Google Scholar] [CrossRef] [Green Version]
- Kohsaka, S.; Katada, J.; Saito, K.; Terayama, Y. Safety and effectiveness of apixaban in comparison to warfarin in patients with nonvalvular atrial fibrillation: A propensity-matched analysis from Japanese administrative claims data. Curr. Med. Res. Opin. 2018, 34, 1627–1634. [Google Scholar] [CrossRef]
- Larsen, T.B.; Skjøth, F.; Nielsen, P.B.; Kjældgaard, J.N.; Lip, G.Y.H. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: Propensity weighted nationwide cohort study. BMJ 2016, 353, i3189. [Google Scholar] [CrossRef] [Green Version]
- Li, X.S.; Deitelzweig, S.; Keshishian, A.; Hamilton, M.; Horblyuk, R.; Gupta, K.; Luo, X.; Mardekian, J.; Friend, K.; Nadkarni, A.; et al. Effectiveness and safety of apixaban versus warfarin in non-valvular atrial fibrillation patients in “real-world” clinical practice. Thromb. Haemost. 2017, 117, 1072–1082. [Google Scholar] [CrossRef] [Green Version]
- Lip, G.Y.H.; Skjøth, F.; Nielsen, P.B.; Kjældgaard, J.N.; Larsen, T.B. Effectiveness and Safety of Standard-Dose Nonvitamin K Antagonist Oral Anticoagulants and Warfarin Among Patients with Atrial Fibrillation with a Single Stroke Risk Factor. JAMA Cardiol. 2017, 2, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Keshishian, A.; Li, X.; Hamilton, M.; Masseria, C.; Gupta, K.; Luo, X.; Mardekian, J.; Friend, K.; Nadkarni, A.; et al. Effectiveness and Safety of Oral Anticoagulants Among Nonvalvular Atrial Fibrillation Patients. Stroke 2018, 49, 2933–2944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsuntisuk, P.; Nathisuwan, S.; Junpanichjaroen, A.; Wongcharoen, W.; Phrommintikul, A.; Wattanaruengchai, P.; Rattanavipanon, W.; Chulavatnatol, S.; Chaiyakunapruk, N.; Likittanasombat, K.; et al. Real-World Comparative Effectiveness and Safety of Non-Vitamin K Antagonist Oral Anticoagulants vs. Warfarin in a Developing Country. Clin. Pharmacol. Ther. 2020, 109, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.B.; Skjøth, F.; Søgaard, M.; Kjældgaard, J.N.; Lip, G.Y.H.; Larsen, T.B. Effectiveness and safety of reduced dose non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: Propensity weighted nationwide cohort study. BMJ 2017, 356, j510. [Google Scholar] [CrossRef] [Green Version]
- Ramagopalan, S.V.; Sicras-Mainar, A.; Polanco-Sanchez, C.; Carroll, R.; De Bobadilla, J.F. Patient characteristics and stroke and bleeding events in nonvalvular atrial fibrillation patients treated with apixaban and vitamin K antagonists: A Spanish real-world study. J. Comp. Eff. Res. 2019, 8, 1201–1212. [Google Scholar] [CrossRef] [Green Version]
- Staerk, L.; Gerds, T.A.; Lip, G.Y.H.; Ozenne, B.; Bonde, A.N.; Lamberts, M.; Fosbøl, E.; Torp-Pedersen, C.; Gislason, G.; Olesen, J.B. Standard and reduced doses of dabigatran, rivaroxaban and apixaban for stroke prevention in atrial fibrillation: A nationwide cohort study. J. Intern. Med. 2017, 283, 45–55. [Google Scholar] [CrossRef]
- Wanat, M.A.; Wang, X.; Ms, R.P.; Chen, H.; Johnson, M.L.; Fleming, M.L.; Abughosh, S.M. Warfarin vs. apixaban in nonvalvular atrial fibrillation, and analysis by concomitant antiarrhythmic medication use: A national retrospective study. Res. Pract. Thromb. Haemost. 2019, 3, 674–683. [Google Scholar] [CrossRef] [Green Version]
- Jansson, M.; Själander, S.; Sjögren, V.; Renlund, H.; Norrving, B.; Själander, A. Direct comparisons of effectiveness and safety of treatment with Apixaban, Dabigatran and Rivaroxaban in atrial fibrillation. Thromb. Res. 2020, 185, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Rutherford, O.-C.W.; Jonasson, C.; Ghanima, W.; Söderdahl, F.; Halvorsen, S. Comparison of dabigatran, rivaroxaban, and apixaban for effectiveness and safety in atrial fibrillation: A nationwide cohort study. Eur. Heart J.-Cardiovasc. Pharmacother. 2020, 6, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Staerk, L.; Fosbøl, E.; Lip, G.Y.; Lamberts, M.; Bonde, A.N.; Torp-Pedersen, C.; Ozenne, B.; Gerds, T.A.; Gislason, G.H.; Olesen, J.B. Ischaemic and haemorrhagic stroke associated with non-vitamin K antagonist oral anticoagulants and warfarin use in patients with atrial fibrillation: A nationwide cohort study. Eur. Heart J. 2016, 38, 907–915. [Google Scholar] [CrossRef] [Green Version]
- Fralick, M.; Colacci, M.; Schneeweiss, S.; Huybrechts, K.F.; Lin, K.J.; Gagne, J.J. Effectiveness and Safety of Apixaban Compared with Rivaroxaban for Patients with Atrial Fibrillation in Routine Practice. Ann. Intern. Med. 2020, 172, 463. [Google Scholar] [CrossRef] [PubMed]
- Wańkowicz, P.; Staszewski, J.; Dębiec, A.; Nowakowska-Kotas, M.; Szylińska, A.; Turoń-Skrzypińska, A.; Rotter, I. Pre-Stroke Statin Therapy Improves In-Hospital Prognosis Following Acute Ischemic Stroke Associated with Well-Controlled Nonvalvular Atrial Fibrillation. J. Clin. Med. 2021, 10, 3036. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Bernal, C.L.; Santa-Ana-Téllez, Y.; García-Sempere, A.; Hurtado, I.; Peiró, S.; Sanfélix-Gimeno, G. Clinical outcomes of nonvitamin K oral anticoagulants and acenocoumarol for stroke prevention in contemporary practice: A population-based propensity-weighted cohort study. Br. J. Clin. Pharmacol. 2020, 87, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalili, F.; Lindström, C.; Benson, L. The safety and persistence of non-vitamin-K-antagonist oral anticoagulants in atrial fibrillation patients treated in a well structured atrial fibrillation clinic. Curr. Med. Res. Opin. 2016, 32, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.; Alvarez-Madrazo, S.; Robertson, C.; Wu, O.; Bennie, M. Comparative safety and effectiveness of direct oral anticoagulants in patients with atrial fibrillation in clinical practice in Scotland. Br. J. Clin. Pharmacol. 2018, 85, 422–431. [Google Scholar] [CrossRef]
- Perreault, S.; Dragomir, A.; Côté, R.; Lenglet, A.; White-Guay, B.; de Denus, S.; Schnitzer, M.E.; Dubé, M.; Brophy, J.M.; Dorais, M.; et al. Comparative effectiveness and safety of high-dose rivaroxaban and apixaban for atrial fibrillation: A propensity score-matched cohort study. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2021, 41, 379–393. [Google Scholar] [CrossRef]
- Adeboyeje, G.; Sylwestrzak, G.; Barron, J.J.; White, J.; Rosenberg, A.; Abarca, J.; Crawford, G.; Redberg, R. Major Bleeding Risk During Anticoagulation with Warfarin, Dabigatran, Apixaban, or Rivaroxaban in Patients with Nonvalvular Atrial Fibrillation. J. Manag. Care Spéc. Pharm. 2017, 23, 968–978. [Google Scholar] [CrossRef]
- Lamberts, M.; Staerk, L.; Olesen, J.B.; Fosbøl, E.L.; Hansen, M.L.; Harboe, L.; Lefevre, C.; Evans, D.; Gislason, G.H. Major Bleeding Complications and Persistence with Oral Anticoagulation in Non-Valvular Atrial Fibrillation: Contemporary Findings in Real-Life Danish Patients. J. Am. Heart Assoc. 2017, 6, e004517. [Google Scholar] [CrossRef] [Green Version]
- Tepper, P.G.; Mardekian, J.; Masseria, C.; Phatak, H.; Kamble, S.; Abdulsattar, Y.; Petkun, W.; Lip, G.Y.H. Real-world comparison of bleeding risks among non-valvular atrial fibrillation patients prescribed apixaban, dabigatran, or rivaroxaban. PLoS ONE 2018, 13, e0205989. [Google Scholar] [CrossRef]
- Villines, T.C.; Ahmad, A.; Petrini, M.; Tang, W.; Evans, A.; Rush, T.; Thompson, D.; Oh, K.; Schwartzman, E. Comparative safety and effectiveness of dabigatran vs. rivaroxaban and apixaban in patients with non-valvular atrial fibrillation: A retrospective study from a large healthcare system. Eur. Heart J.-Cardiovasc. Pharmacother. 2018, 5, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Bradley, M.; Welch, E.C.; Eworuke, E.; Graham, D.J.; Zhang, R.; Huang, T.-Y. Risk of Stroke and Bleeding in Atrial Fibrillation Treated with Apixaban Compared with Warfarin. J. Gen. Intern. Med. 2020, 35, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Huybrechts, K.F.; Gopalakrishnan, C.; Bartels, D.B.; Zint, K.; Gurusamy, V.K.; Landon, J.; Schneeweiss, S. Safety and Effectiveness of Dabigatran and Other Direct Oral Anticoagulants Compared with Warfarin in Patients with Atrial Fibrillation. Clin. Pharmacol. Ther. 2019, 107, 1405–1419. [Google Scholar] [CrossRef]
- Yang, L.; Brooks, M.M.; Glynn, N.W.; Zhang, Y.; Saba, S.; Hernandez, I. Real-World Direct Comparison of the Effectiveness and Safety of Apixaban, Dabigatran, Rivaroxaban, and Warfarin in Medicare Beneficiaries with Atrial Fibrillation. Am. J. Cardiol. 2020, 126, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Menichelli, D.; Del Sole, F.; Di Rocco, A.; Farcomeni, A.; Vestri, A.; Violi, F.; Pignatelli, P.; Lip, G.Y.H.; Pastori, D. Real-world safety and efficacy of direct oral anticoagulants in atrial fibrillation: A systematic review and meta-analysis of 605 771 patients. Eur. Heart J.-Cardiovasc. Pharmacother. 2021, 7, f11–f19. [Google Scholar] [CrossRef] [PubMed]
- Romiti, G.F.; Pastori, D.; Rivera-Caravaca, J.M.; Ding, W.Y.; Gue, Y.X.; Menichelli, D.; Gumprecht, J.; Kozieł, M.; Yang, P.-S.; Guo, Y.; et al. Adherence to the ‘Atrial Fibrillation Better Care’ Pathway in Patients with Atrial Fibrillation: Impact on Clinical Outcomes—A Systematic Review and Meta-Analysis of 285,000 Patients. Thromb. Haemost. 2021, 122, 406–414. [Google Scholar] [CrossRef]
- Potpara, T.S.; Lip, G.Y.H.; Blomstrom-Lundqvist, C.; Boriani, G.; Van Gelder, I.C.; Heidbuchel, H.; Hindricks, G.; Camm, A.J. The 4S-AF Scheme (Stroke Risk; Symptoms; Severity of Burden; Substrate): A Novel Approach to In-Depth Characterization (Rather than Classification) of Atrial Fibrillation. Thromb. Haemost. 2020, 121, 270–278. [Google Scholar] [CrossRef]
- Sagris, D.; Perlepe, K.; Leventis, I.; Samara, S.; Manios, E.; Korompoki, E.; Makaritsis, K.; Milionis, H.; Vemmos, K.; Ntaios, G. Statin treatment and outcomes after embolic stroke of undetermined source. Intern. Emerg. Med. 2021, 16, 1261–1266. [Google Scholar] [CrossRef]
- Abraham, N.S.; Noseworthy, P.A.; Yao, X.; Sangaralingham, L.R.; Shah, N.D. Gastrointestinal Safety of Direct Oral Anticoagulants: A Large Population-Based Study. Gastroenterology 2017, 152, 1014–1022.e1. [Google Scholar] [CrossRef] [Green Version]
- Amin, A.; Keshishian, A.; Trocio, J.; Dina, O.; Le, H.; Rosenblatt, L.; Liu, X.; Mardekian, J.; Zhang, Q.; Baser, O.; et al. Risk of stroke/systemic embolism, major bleeding and associated costs in non-valvular atrial fibrillation patients who initiated apixaban, dabigatran or rivaroxaban compared with warfarin in the United States Medicare population. Curr. Med. Res. Opin. 2017, 33, 1595–1604. [Google Scholar] [CrossRef] [Green Version]
- Amin, A.; Keshishian, A.; Vo, L.; Zhang, Q.; Dina, O.; Patel, C.; Odell, K.; Trocio, J. Real-world comparison of all-cause hospitalizations, hospitalizations due to stroke and major bleeding, and costs for non-valvular atrial fibrillation patients prescribed oral anticoagulants in a US health plan. J. Med. Econ. 2017, 21, 244–253. [Google Scholar] [CrossRef]
- Amin, A.; Keshishian, A.; Dina, O.; Dhamane, A.; Nadkarni, A.; Carda, E.; Russ, C.; Rosenblatt, L.; Mardekian, J.; Yuce, H.; et al. Comparative clinical outcomes between direct oral anticoagulants and warfarin among elderly patients with non-valvular atrial fibrillation in the CMS medicare population. J. Thromb. Thrombolysis 2019, 48, 240–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, A.; Reeves, A.G.; Li, X.; Dhamane, A.; Luo, X.; Di Fusco, M.; Nadkarni, A.; Friend, K.; Rosenblatt, L.; Mardekian, J.; et al. Effectiveness and safety of oral anticoagulants in older adults with non-valvular atrial fibrillation and heart failure. PLoS ONE 2019, 14, e0213614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, N.W.; Svanström, H.; Lund, M.; Pasternak, B.; Melbye, M. Comparative effectiveness and safety of apixaban, dabigatran, and rivaroxaban in patients with non-valvular atrial fibrillation. Int. J. Cardiol. 2018, 268, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Coleman, C.I.; Antz, M.; Bowrin, K.; Evers, T.; Simard, E.P.; Bonnemeier, H.; Cappato, R. Real-world evidence of stroke prevention in patients with nonvalvular atrial fibrillation in the United States: The REVISIT-US study. Curr. Med. Res. Opin. 2016, 32, 2047–2053. [Google Scholar] [CrossRef]
- Coleman, C.I.; Peacock, W.F.; Bunz, T.J.; Alberts, M.J. Effectiveness and Safety of Apixaban, Dabigatran, and Rivaroxaban Versus Warfarin in Patients with Nonvalvular Atrial Fibrillation and Previous Stroke or Transient Ischemic Attack. Stroke 2017, 48, 2142–2149. [Google Scholar] [CrossRef]
- Coleman, C.I.; Peacock, W.F.; Antz, M. Comparative Effectiveness and Safety of Apixaban and Vitamin K Antagonist Therapy in Patients with Nonvalvular Atrial Fibrillation Treated in Routine German Practice. Heart Lung Circ. 2017, 27, 390–393. [Google Scholar] [CrossRef]
- Deitelzweig, S.; Bruno, A.; Trocio, J.; Tate, N.; Gupta, K.; Lin, J.; Lingohr-Smith, M. An early evaluation of bleeding-related hospital readmissions among hospitalized patients with nonvalvular atrial fibrillation treated with direct oral anticoagulants. Curr. Med. Res. Opin. 2016, 32, 573–582. [Google Scholar] [CrossRef]
- Deitelzweig, S.; Luo, X.; Gupta, K.; Trocio, J.; Mardekian, J.; Curtice, T.; Lingohr-Smith, M.; Menges, B.; Lin, J. Comparison of effectiveness and safety of treatment with apixaban vs. other oral anticoagulants among elderly nonvalvular atrial fibrillation patients. Curr. Med. Res. Opin. 2017, 33, 1745–1754. [Google Scholar] [CrossRef]
- Durand, M.; Schnitzer, M.E.; Pang, M.; Carney, G.; Eltonsy, S.; Filion, K.B.; Fisher, A.; Jun, M.; Kuo, I.F.; Matteau, A.; et al. Effectiveness and safety among direct oral anticoagulants in nonvalvular atrial fibrillation: A multi-database cohort study with meta-analysis. Br. J. Clin. Pharmacol. 2020, 87, 2589–2601. [Google Scholar] [CrossRef]
- Gupta, K.; Trocio, J.; Keshishian, A.; Zhang, Q.; Dina, O.; Mardekian, J.; Nadkarni, A.; Shank, T.C. Effectiveness and safety of direct oral anticoagulants compared to warfarin in treatment naïve non-valvular atrial fibrillation patients in the US Department of defense population. BMC Cardiovasc. Disord. 2019, 19, 142. [Google Scholar] [CrossRef] [Green Version]
- Halvorsen, S.; Ghanima, W.; Tvete, I.F.; Hoxmark, C.; Falck, P.; Solli, O.; Jonasson, C. A nationwide registry study to compare bleeding rates in patients with atrial fibrillation being prescribed oral anticoagulants. Eur. Heart J.-Cardiovasc. Pharmacother. 2016, 3, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, I.; Zhang, Y.; Saba, S. Comparison of the Effectiveness and Safety of Apixaban, Dabigatran, Rivaroxaban, and Warfarin in Newly Diagnosed Atrial Fibrillation. Am. J. Cardiol. 2017, 120, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Hohnloser, S.H.; Basic, E.; Nabauer, M. Comparative risk of major bleeding with new oral anticoagulants (NOACs) and phenprocoumon in patients with atrial fibrillation: A post-marketing surveillance study. Clin. Res. Cardiol. 2017, 15, 486–628. [Google Scholar] [CrossRef] [PubMed]
- Kohsaka, S.; Murata, T.; Izumi, N.; Katada, J.; Wang, F.; Terayama, Y. Bleeding risk of apixaban, dabigatran, and low-dose rivaroxaban compared with warfarin in Japanese patients with non-valvular atrial fibrillation: A propensity matched analysis of administrative claims data. Curr. Med. Res. Opin. 2017, 33, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.H.; Keshishian, A.; Kamble, S.; Pan, X.; Mardekian, J.; Horblyuk, R.; Hamilton, M. Real-world comparison of major bleeding risk among non-valvular atrial fibrillation patients initiated on apixaban, dabigatran, rivaroxaban, or warfarin. Thromb. Haemost. 2016, 116, 975–986. [Google Scholar] [CrossRef] [Green Version]
- Lip, G.Y.; Pan, X.; Kamble, S.; Kawabata, H.; Mardekian, J.; Masseria, C.; Bruno, A.; Phatak, H. Major bleeding risk among non-valvular atrial fibrillation patients initiated on apixaban, dabigatran, rivaroxaban or warfarin: A “real-world” observational study in the United States. Int. J. Clin. Pract. 2016, 70, 752–763. [Google Scholar] [CrossRef] [Green Version]
- Lip, G.Y.H.; Keshishian, A.; Kang, A.; Dhamane, A.D.; Luo, X.; Klem, C.; Rosenblatt, L.; Mardekian, J.; Jiang, J.; Yuce, H.; et al. Effectiveness and safety of oral anticoagulants among non-valvular atrial fibrillation patients with polypharmacy. Eur. Heart J. -Cardiovasc. Pharmacother. 2020, 7, 405–414. [Google Scholar] [CrossRef]
- Mentias, A.; Heller, E.; Sarrazin, M.V. Comparative Effectiveness of Rivaroxaban, Apixaban, and Warfarin in Atrial Fibrillation Patients with Polypharmacy. Stroke 2020, 51, 2076–2086. [Google Scholar] [CrossRef]
- Noseworthy, P.A.; Yao, X.; Abraham, N.S.; Sangaralingham, L.R.; McBane, R.; Shah, N.D. Direct Comparison of Dabigatran, Rivaroxaban, and Apixaban for Effectiveness and Safety in Nonvalvular Atrial Fibrillation. Chest 2016, 150, 1302–1312. [Google Scholar] [CrossRef]
- Ramagopalan, S.; Allan, V.; Saragoni, S.; Degli Esposti, L.; Alessandrini, D.; Perrone, V.; Buda, S.; Stynes, G.; Toma, C.; DeSolda, F.; et al. Patient characteristics and bleeding events in nonvalvular atrial fibrillation patients treated with apixaban or vitamin K antagonists: Real-world evidence from Italian administrative databases. J. Comp. Eff. Res. 2018, 7, 1063–1071. [Google Scholar] [CrossRef] [Green Version]
- Själander, S.; Sjögren, V.; Renlund, H.; Norrving, B.; Själander, A. Dabigatran, rivaroxaban and apixaban vs. high TTR warfarin in atrial fibrillation. Thromb. Res. 2018, 167, 113–118. [Google Scholar] [CrossRef]
- Van Ganse, E.; Danchin, N.; Mahé, I.; Hanon, O.; Jacoud, F.; Nolin, M.; Dalon, F.; Lefevre, C.; Cotté, F.-E.; Gollety, S.; et al. Comparative Safety and Effectiveness of Oral Anticoagulants in Nonvalvular Atrial Fibrillation. Stroke 2020, 51, 2066–2075. [Google Scholar] [CrossRef]
- Yao, X.; Abraham, N.S.; Sangaralingham, L.R.; Bellolio, M.F.; McBane, R.D.; Shah, N.D.; Noseworthy, P.A. Effectiveness and Safety of Dabigatran, Rivaroxaban, and Apixaban Versus Warfarin in Nonvalvular Atrial Fibrillation. J. Am. Heart Assoc. 2016, 5, e003725. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).