Cell Salvage at the ICU
Abstract
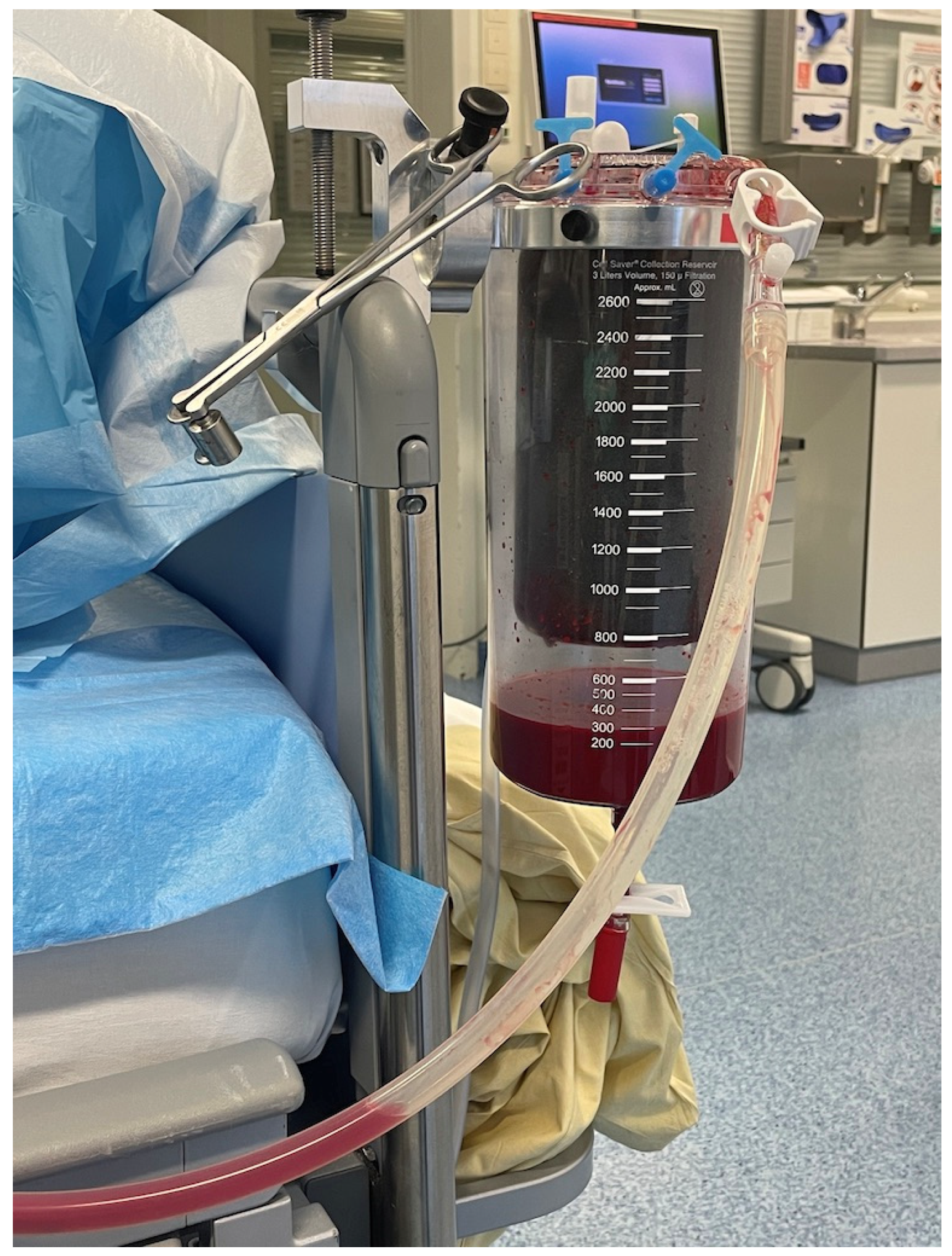
:1. Introduction
2. Legal Aspects and Problems of Collection Time
3. Logistics and Practical Procedure
- Training and competency evaluations of cell salvage personnel are mandatory (documented in the form of a training record);
- An individual’s identification number and unique case number must be written on every bag of cell salvage blood;
- Whenever intraoperative cell salvage (ICS) or postoperative cell salvage (PCS) is performed, SHOT should be notified of any adverse events;
- As important as monitoring patients for the reinfusion of allogeneic red cells is monitoring patients for red cells collected via ICS or PCS;
- The SHOT Reports from previous years should be reexamined by practitioners, in particular those that deal with autologous transfusion, to ensure that historic incidents do not repeat themselves. Action: Cell salvage teams.
4. Costs
5. Discussion
6. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Vincent, J.L.; Baron, J.F.; Reinhart, K.; Gattinoni, L.; Thijs, L.; Webb, A.; Meier-Hellmann, A.; Nollet, G.; Peres-Bota, D. Anemia and blood transfusion in critically ill patients. JAMA 2002, 288, 1499–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shander, A.; Hardy, J.F.; Ozawa, S.; Farmer, S.L.; Hofmann, A.; Frank, S.M.; Kor, D.J.; Faraoni, D.; Freedman, J. A Global Definition of Patient Blood Management. Anesth. Analg. 2022. [Google Scholar] [CrossRef] [PubMed]
- Althoff, F.C.; Neb, H.; Herrmann, E.; Trentino, K.M.; Vernich, L.; Füllenbach, C.; Freedman, J.; Waters, J.H.; Farmer, S.; Leahy, M.F.; et al. Multimodal Patient Blood Management Program Based on a Three-pillar Strategy: A Systematic Review and Meta-analysis. Ann. Surg. 2019, 269, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Meybohm, P.; Choorapoikayil, S.; Wessels, A.; Herrmann, E.; Zacharowski, K.; Spahn, D.R. Washed cell salvage in surgical patients: A review and meta-analysis of prospective randomized trials under PRISMA. Medicine 2016, 95, e4490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karkouti, K.; Wijeysundera, D.N.; Yau, T.M.; Beattie, W.S.; Abdelnaem, E.; McCluskey, S.A.; Ghannam, M.; Yeo, E.; Djaiani, G.; Karski, J. The independent association of massive blood loss with mortality in cardiac surgery. Transfusion 2004, 44, 1453–1462. [Google Scholar] [CrossRef]
- Dalrymple-Hay, M.J.; Pack, L.; Deakin, C.D.; Shephard, S.; Ohri, S.K.; Haw, M.P.; Livesey, S.A.; Monro, J.L. Autotransfusion of washed shed mediastinal fluid decreases the requirement for autologous blood transfusion following cardiac surgery: A prospective randomized trial. Eur. J. Cardiothorac. Surg. 1999, 15, 830–834. [Google Scholar] [CrossRef] [Green Version]
- Huët, C.; Salmi, L.R.; Fergusson, D.; Koopman-van Gemert, A.W.; Rubens, F.; Laupacis, A. A meta-analysis of the effectiveness of cell salvage to minimize perioperative allogeneic blood transfusion in cardiac and orthopedic surgery. International Study of Perioperative Transfusion (ISPOT) Investigators. Anesth. Analg. 1999, 89, 861–869. [Google Scholar] [CrossRef]
- Folkersen, L.; Tang, M.; Grunnet, N.; Jakobsen, C.-J. Transfusion of shed mediastinal blood reduces the use of allogenic blood transfusion without increasing complications. Perfusion 2010, 26, 145–150. [Google Scholar] [CrossRef]
- Davies, L.; Brown, T.J.; Haynes, S.; Payne, K.; Elliott, R.A.; McCollum, C. Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: A systematic review and economic model. Health Technol. Assess. 2006, 10, iii–iv, ix–x, 1–210. [Google Scholar] [CrossRef] [Green Version]
- Innerhofer, P.; Klingler, A.; Klimmer, C.; Fries, D.; Nussbaumer, W. Risk for postoperative infection after transfusion of white blood cell-filtered allogeneic or autologous blood components in orthopedic patients undergoing primary arthroplasty. Transfusion 2005, 45, 103–110. [Google Scholar] [CrossRef]
- Duffy, G.; Neal, K.R. Differences in post-operative infection rates between patients receiving autologous and allogeneic blood transfusion: A meta-analysis of published randomized and nonrandomized studies. Transfus. Med. 1996, 6, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Brander, L.; Reil, A.; Bux, J.; Taleghani, B.M.; Regli, B.; Takala, J. Severe transfusion-related acute lung injury. Anesth. Analg. 2005, 101, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Spiess, B.D.; Ley, C.; Body, S.C.; Siegel, L.C.; Stover, E.P.; Maddi, R.; D’Ambra, M.; Jain, U.; Liu, F.; Herskowitz, A.; et al. Hematocrit value on intensive care unit entry influences the frequency of Q-wave myocardial infarction after coronary artery bypass grafting. The Institutions of the Multicenter Study of Perioperative Ischemia (McSPI) Research Group. J. Thorac. Cardiovasc. Surg. 1998, 116, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Koch, C.G.; Li, L.; Duncan, A.I.; Mihaljevic, T.; Cosgrove, D.M.; Loop, F.D.; Starr, N.J.; Blackstone, E.H. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit. Care Med. 2006, 34, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Bundesärztekammer. Querschnitts-Leitlinien zur Therapie mit Blutkomponenten und Plasmaderivaten: Mit 19 Tabellen; Deutscher Ärzteverlag: Cologne, Germany, 2020. [Google Scholar]
- Joint United Kingdom (UK) Blood Transfusion and Tissue Transplantation Services Professional Advisory Committee. Guidelines for the Blood Transfusion Services; Joint United Kingdom (UK) Blood Transfusion and Tissue Transplantation Services Professional Advisory Committee: London, UK, 2021. [Google Scholar]
- Kozek-Langenecker, S.A.; Afshari, A.; Albaladejo, P.; Santullano, C.A.; De Robertis, E.; Filipescu, D.C.; Fries, D.; Görlinger, K.; Haas, T.; Imberger, G.; et al. Management of severe perioperative bleeding: Guidelines from the European Society of Anaesthesiology. Eur. J. Anaesthesiol. 2013, 30, 270–382. [Google Scholar] [CrossRef] [Green Version]
- Frietsch, T.; Steinbicker, A.U.; Hackbusch, M.; Nguyen, X.D.; Dietrich, G. Sicherheit der maschinellen Autotransfusion in der Tumorchirurgie. Der Anaesthesist 2020, 69, 331–351. [Google Scholar] [CrossRef] [Green Version]
- Ashworth, A.; Klein, A.A. Cell salvage as part of a blood conservation strategy in anaesthesia. Br. J. Anaesth. 2010, 105, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Fowler, R.A.; Berenson, M. Blood conservation in the intensive care unit. Crit. Care Med. 2003, 31, S715–S720. [Google Scholar] [CrossRef]
- Tinmouth, A.T.; McIntyre, L.A.; Fowler, R.A. Blood conservation strategies to reduce the need for red blood cell transfusion in critically ill patients. Can. Med. Assoc. J. 2008, 178, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Hansen, E.; Hansen, M.P. Reasons against the retransfusion of unwashed wound blood. Transfusion 2004, 44, 45S–53S. [Google Scholar] [CrossRef]
- Die Österreichische Gesellschaft für Blutgruppenserologie und Transfusionsmedizin. Die Österreichische Gesellschaft für Blutgruppenserologie und Transfusionsmedizin. Die Leitlinie Transfusion von Blut und Blutprodukten ist ein Produkt der Arbeitsgruppe Transusion. In Regenerative Medizin und Immungenetik; Die Österreichische Gesellschaft für Blutgruppenserologie und Transfusionsmedizin: Vienna, Austria, 2005. [Google Scholar]
- Schweizerische Arbeitsgruppe zur Qualitätssicherung in derAnwendung von Blutprodukten. Leitfaden Für Die Qualitätssicherung in der Transfusionspraxis; Schweizerische Arbeitsgruppe zur Qualitätssicherung in derAnwendung von Blutprodukten: Bern, Switzerland, 2017. [Google Scholar]
- National Clinical Guideline Center. National Clinical Guideline Center. National Institute for Health and Care Excellence: Clinical Guidelines. In Blood Transfusion; National Institute for Health and Care Excellence: London, UK, 2015. [Google Scholar]
- Ezzedine, H.; Baele, P.; Robert, A. Bacteriologic quality of intraoperative autotransfusion. Surgery 1991, 109, 259–264. [Google Scholar] [PubMed]
- Carson, J.L.; Guyatt, G.; Heddle, N.M.; Grossman, B.J.; Cohn, C.S.; Fung, M.K.; Gernsheimer, T.; Holcomb, J.B.; Kaplan, L.J.; Katz, L.M.; et al. Clinical Practice Guidelines from the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA 2016, 316, 2025–2035. [Google Scholar] [CrossRef] [PubMed]
- Bolcato, M.; Russo, M.; Trentino, K.; Isbister, J.; Rodriguez, D.; Aprile, A. Patient Blood Management: The best approach to transfusion medicine risk management. Transfus. Apher. Sci. 2020, 59, 102779. [Google Scholar] [CrossRef]
- Cholette, J.M.; Powers, K.S.; Alfieris, G.M.; Angona, R.; Henrichs, K.F.; Masel, D.; Swartz, M.F.; Daugherty, L.E.; Belmont, K.; Blumberg, N. Transfusion of Cell Saver Salvaged Blood in Neonates and Infants Undergoing Open Heart Surgery Significantly Reduces RBC and Coagulant Product Transfusions and Donor Exposures. Pediatric Crit. Care Med. 2013, 14, 137–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertl, D.; Kaltenecker, G. Minimizing allogeneic blood transfusion in knee prosthesis implantation. Unfallchirurg 2001, 104, 808–812. [Google Scholar] [CrossRef]
- Sebastián, C.; Romero, R.; Olalla, E.; Ferrer, C.; García-Vallejo, J.J.; Muñoz, M. Postoperative blood salvage and reinfusion in spinal surgery: Blood quality, effectiveness and impact on patient blood parameters. Eur. Spine J. 2000, 9, 458–465. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Feng, X.; Ma, J.; Kang, P.; Shen, B.; Yang, J.; Zhou, Z.; Pei, F. Is postoperative cell salvage necessary in total hip or knee replacement? A meta-analysis of randomized controlled trials. Int. J. Surg. 2015, 21, 135–144. [Google Scholar] [CrossRef]
- Klein, A.A.; Arnold, P.; Bingham, R.M.; Brohi, K.; Clark, R.; Collis, R.; Gill, R.; McSporran, W.; Moor, P.; Rao Baikady, R.; et al. AAGBI guidelines: The use of blood components and their alternatives 2016. Anaesthesia 2016, 71, 829–842. [Google Scholar] [CrossRef]
- de Varennes, B.; Nguyen, D.; Denis, F.; Ergina, P.; Latter, D.; Morin, J.E. Reinfusion of mediastinal blood in CABG patients: Impact on homologous transfusions and rate of re-exploration. J. Card. Surg. 1996, 11, 387–395. [Google Scholar] [CrossRef]
- Martin, J.; Robitaille, D.; Perrault, L.P.; Pellerin, M.; Pagé, P.; Searle, N.; Cartier, R.; Hébert, Y.; Pelletier, L.C.; Thaler, H.T.; et al. Reinfusion of mediastinal blood after heart surgery. J. Thorac. Cardiovasc. Surg. 2000, 120, 499–504. [Google Scholar] [CrossRef] [Green Version]
- Seyfried, T.F.; Streithoff, F.; Gruber, M.; Unterbuchner, C.; Zech, N.; Kieninger, M.; Hansen, E. Platelet sequestration with a new-generation autotransfusion device. Transfusion 2018, 58, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Bolton-Maggs, P.H. SHOT Conference Report 2020: Serious Hazards of Transfusion—Human Factors Continue to Cause Most Transfusion-Related Incidents; Transfusion Medicine: Oxford, UK, 2020. [Google Scholar]
- Kleinerüschkamp, A.G.; Zacharowski, K.; Ettwein, C.; Müller, M.M.; Geisen, C.; Weber, C.F.; Meybohm, P. Cost analysis of Patient Blood Management. Der Anaesthesist 2016, 65, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Wie das Rote Kreuz den Preis für Blut bestimmt. Available online: https://www.addendum.org/blutspenden/blutmarkt/ (accessed on 25 May 2022).
- Strumper, D.; Weber, E.W.; Gielen-Wijffels, S.; Van Drumpt, R.; Bulstra, S.; Slappendel, R.; Durieux, M.E.; Marcus, M.A. Clinical efficacy of postoperative autologous transfusion of filtered shed blood in hip and knee arthroplasty. Transfusion 2004, 44, 1567–1571. [Google Scholar] [CrossRef]
- Weltert, L.; Nardella, S.; Rondinelli, M.B.; Pierelli, L.; De Paulis, R. Reduction of allogeneic red blood cell usage during cardiac surgery by an integrated intra- and postoperative blood salvage strategy: Results of a randomized comparison. Transfusion 2013, 53, 790–797. [Google Scholar] [CrossRef]
- NHSBT Board July 2019 Pricing Proposals for 2019-20 and 2020-21. Available online: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/16611/ncg-pricing-proposals-board-paper-19_62.pdf (accessed on 25 May 2022).
- McQuilten, Z.K.; Higgins, A.M.; Burns, K.; Chunilal, S.; Dunstan, T.; Haysom, H.E.; Kaplan, Z.; Rushford, K.; Saxby, K.; Tahiri, R.; et al. The cost of blood: A study of the total cost of red blood cell transfusion in patients with β-thalassemia using time-driven activity-based costing. Transfusion 2019, 59, 3386–3395. [Google Scholar] [CrossRef]
- Rigal, J.C.; Riche, V.P.; Tching-Sin, M.; Fronteau, C.; Huon, J.F.; Cadiet, J.; Boukhari, R.; Vourc’h, M.; Rozec, B. Cost of red blood cell transfusion; evaluation in a French academic hospital. Transfus. Clin. Biol. 2020, 27, 222–228. [Google Scholar] [CrossRef]
- Bateman, R.M.; Sharpe, M.D.; Jagger, J.E.; Ellis, C.G.; Solé-Violán, J.; López-Rodríguez, M.; Herrera-Ramos, E.; Ruíz-Hernández, J.; Borderías, L.; Horcajada, J.; et al. 36th International Symposium on Intensive Care and Emergency Medicine. Crit. Care 2016, 20, 94. [Google Scholar] [CrossRef] [Green Version]
- aG-DRG-Version 2021 und Pflegeerlöskatalog 2021 Fallpauschalen-Katalog gem. § 17b Abs. 1 S. 4 KHG. Available online: https://www.g-drg.de/aG-DRG-System_2021/Fallpauschalen-Katalog/Fallpauschalen-Katalog_2021 (accessed on 25 May 2022).
- Regan, F.; Taylor, C. Blood transfusion medicine. BMJ 2002, 325, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Moonen, A.F.; Knoors, N.T.; van Os, J.J.; Verburg, A.D.; Pilot, P. Retransfusion of filtered shed blood in primary total hip and knee arthroplasty: A prospective randomized clinical trial. Transfusion 2007, 47, 379–384. [Google Scholar] [CrossRef]
- Sarkanović, M.L.; Gvozdenović, L.; Savić, D.; Ilić, M.P.; Jovanović, G. Autologous blood transfusion in total knee replacement surgery. Vojnosanit. Pregl. 2013, 70, 274–278. [Google Scholar] [CrossRef]
- Practice guidelines for perioperative blood management: An updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management. Anesthesiology 2015, 122, 241–275. [CrossRef]
- Horstmann, W.G.; Kuipers, B.M.; Slappendel, R.; Castelein, R.M.; Kollen, B.J.; Verheyen, C.C.P.M. Postoperative autologous blood transfusion drain or no drain in primary total hip arthroplasty? A randomised controlled trial. Int. Orthop. 2012, 36, 2033–2039. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidbauer, S.L.; Seyfried, T.F. Cell Salvage at the ICU. J. Clin. Med. 2022, 11, 3848. https://doi.org/10.3390/jcm11133848
Schmidbauer SL, Seyfried TF. Cell Salvage at the ICU. Journal of Clinical Medicine. 2022; 11(13):3848. https://doi.org/10.3390/jcm11133848
Chicago/Turabian StyleSchmidbauer, Stephan L., and Timo F. Seyfried. 2022. "Cell Salvage at the ICU" Journal of Clinical Medicine 11, no. 13: 3848. https://doi.org/10.3390/jcm11133848
APA StyleSchmidbauer, S. L., & Seyfried, T. F. (2022). Cell Salvage at the ICU. Journal of Clinical Medicine, 11(13), 3848. https://doi.org/10.3390/jcm11133848




