[18F]Fluoride Positron-Emission Tomography (PET) and [18F]FDG PET for Assessment of Osteomyelitis of the Jaw in Comparison to Computed Tomography (CT) and Magnetic Resonance Imaging (MRI): A Prospective PET/CT and PET/MRI Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Study Protocol
2.3. [18F]FDG-PET/MRI
2.4. [18F]Fluoride PET/CT
2.5. Qualitative Image Analysis
2.6. Quantitative Image Analysis
2.7. Statistics
3. Results
3.1. Clinical Findings
3.2. Qualitative Analysis
3.3. Quantitative Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baltensperger, M.; Eyrich, G. Osteomyelitis of the Jaws: Definition and Classification. In Osteomyelitis of the Jaws; Baltensperger, M.M., Eyrich, G.K.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 5–56. [Google Scholar] [CrossRef]
- Prasad, K.C.; Rao, S.C.P.; Mouli, N.; Agarwal, S. Osteomyelitis in the head and neck. Acta Oto-Laryngol. 2007, 127, 194–205. [Google Scholar] [CrossRef]
- Malmström, M.; Fyhrquist, F.; Kosunen, T.U.; Tasanen, A. Immunological features of patients with chronic sclerosing osteomyelitis of the mandible. Int. J. Oral Surg. 1983, 12, 6–13. [Google Scholar] [CrossRef]
- Baltensperger, M.; Grätz, K.; Bruder, E.; Lebeda, R.; Makek, M.; Eyrich, G. Is primary chronic osteomyelitis a uniform disease? Proposal of a classification based on a retrospective analysis of patients treated in the past 30 years. J. Cranio-Maxillofac. Surg. 2004, 32, 43–50. [Google Scholar] [CrossRef]
- Guhlmann, A.; Brecht-Krauss, D.; Suger, G.; Glatting, G.; Kotzerke, J.; Kinzl, L.; Reske, S.N. Chronic osteomyelitis: Detection with FDG PET and correlation with histopathologic findings. Radiology 1998, 206, 749–754. [Google Scholar] [CrossRef]
- Jacobsson, S.; Heyden, G. Chronic sclerosing osteomyelitis of the mandible: Histologic and histochemical findings. Oral Surg. Oral Med. Oral Pathol. 1977, 43, 357–364. [Google Scholar] [CrossRef]
- Hakim, S.; Bruecker, C.; Jacobsen, H.; Hermes, D.; Lauer, I.; Eckerle, S.; Froehlich, A.; Sieg, P. The value of FDG-PET and bone scintigraphy with SPECT in the primary diagnosis and follow-up of patients with chronic osteomyelitis of the mandible. Int. J. Oral Maxillofac. Surg. 2006, 35, 809–816. [Google Scholar] [CrossRef]
- Shin, J.W.; Kim, J.-E.; Huh, K.-H.; Yi, W.-J.; Heo, M.-S.; Lee, S.-S.; Choi, S.-C. Computed tomography imaging features of osteomyelitis of the jaw: Comparison between antiresorptive medication-related conditions and medication-unrelated conditions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 129, 629–634. [Google Scholar] [CrossRef]
- Belcher, R.; Boyette, J.; Pierson, T.; Siegel, E.; Bartel, T.B.; Aniasse, E.; Stack, B., Jr. What Is the Role of Positron Emission Tomography in Osteonecrosis of the Jaws? J. Oral Maxillofac. Surg. 2014, 72, 306–310. [Google Scholar] [CrossRef]
- Stumpe, K.D.M.; Strobel, K. 18F FDG-PET imaging in musculoskeletal infection. Q. J. Nucl. Med. Mol. Imaging 2006, 50, 131–142. [Google Scholar]
- Crymes, W.B.; Demos, H.; Gordon, L. Detection of musculoskeletal infection with 18F-FDG PET: Review of the current literature. J. Nucl. Med. Technol. 2004, 32, 12–15. [Google Scholar]
- Meller, J.; Koster, G.; Liersch, T.; Siefker, U.; Lehmann, K.; Meyer, I.; Schreiber, K.; Altenvoerde, G.; Becker, W. Chronic bacterial osteomyelitis: Prospective comparison of 18F-FDG imaging with a dual-head coincidence camera and 111In-labelled autologous leucocyte scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 53–60. [Google Scholar] [CrossRef] [PubMed]
- van der Bruggen, W.; Bleeker-Rovers, C.P.; Boerman, O.C.; Gotthardt, M.; Oyen, W.J. PET and SPECT in Osteomyelitis and Prosthetic Bone and Joint Infections: A Systematic Review. Semin. Nucl. Med. 2010, 40, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Mukherjee, A.; Karunanithi, S.; Bal, C.; Kumar, R. Potential Role of 18F-FDG PET/CT in Patients with Fungal Infections. Am. J. Roentgenol. 2014, 203, 180–189. [Google Scholar] [CrossRef]
- Wilde, F.; Steinhoff, K.; Frerich, B.; Schulz, T.; Winter, K.; Hemprich, A.; Sabri, O.; Kluge, R. Positron-emission tomography imaging in the diagnosis of bisphosphonate-related osteonecrosis of the jaw. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2009, 107, 412–419. [Google Scholar] [CrossRef]
- Even-Sapir, E.; Metser, U.; Flusser, G.; Zuriel, L.; Kollender, Y.; Lerman, H.; Lievshitz, G.; Ron, I.; Mishani, E. Assessment of Malignant Skeletal Disease: Initial Experience with 18F-Fluoride PET/CT and Comparison between 18F-Fluoride PET and 18F-Fluoride PET/CT. J. Nucl. Med. 2004, 45, 272–278. [Google Scholar]
- Even-Sapir, E.; Metser, U.; Mishani, E.; Lievshitz, G.; Lerman, H.; Leibovitch, I. The Detection of Bone Metastases in Patients with High-Risk Prostate Cancer: 99mTc-MDP Planar Bone Scintigraphy, Single- and Multi-Field-of-View SPECT, 18F-Fluoride PET, and 18F-Fluoride PET/CT. J. Nucl. Med. 2006, 47, 287. [Google Scholar]
- Even-Sapir, E.; Mishani, E.; Flusser, G.; Metser, U. 18F-Fluoride Positron Emission Tomography and Positron Emission Tomography/Computed Tomography. Semin. Nucl. Med. 2007, 37, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Rahmim, A.; Zaidi, H. PET versus SPECT: Strengths, limitations and challenges. Nucl. Med. Commun. 2008, 29, 193–207. [Google Scholar] [CrossRef] [Green Version]
- Raynor, W.; Houshmand, S.; Gholami, S.; Emamzadehfard, S.; Rajapakse, C.S.; Blomberg, B.A.; Werner, T.J.; Høilund-Carlsen, P.F.; Baker, J.F.; Alavi, A. Evolving Role of Molecular Imaging with (18)F-Sodium Fluoride PET as a Biomarker for Calcium Metabolism. Curr. Osteoporos. Rep. 2016, 14, 115–125. [Google Scholar] [CrossRef]
- Ma, L.D.; Frassica, F.J.; Bluemke, D.; Fishman, E.K. CT and MRI evaluation of musculoskeletal infection. Crit. Rev. Diagn. Imaging 1997, 38, 535–568. [Google Scholar]
- Coviello, V.; Stevens, M.R. Contemporary Concepts in the Treatment of Chronic Osteomyelitis. Oral Maxillofac. Surg. Clin. N. Am. 2007, 19, 523–534. [Google Scholar] [CrossRef]
- Job-Deslandre, C.; Krebs, S.; Kahan, A. Chronic recurrent multifocal osteomyelitis: Five-year outcomes in 14 pediatric cases. Jt. Bone Spine 2001, 68, 245–251. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, K.; Shang, W.; Song, K. Oral administration of alendronate and vitamin D3 for the treatment of chronic non-bacterial osteomyelitis of the jaw. Int. J. Oral Maxillofac. Surg. 2020, 49, 1595–1598. [Google Scholar] [CrossRef] [PubMed]
- Timme, M.; Bohner, L.; Huss, S.; Kleinheinz, J.; Hanisch, M. Response of Different Treatment Protocols to Treat Chronic Non-Bacterial Osteomyelitis (CNO) of the Mandible in Adult Patients: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 1737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, P.A.; Mintz, A.; Perry, B.; Trout, A.; Vergara-Wentland, P. Clinical Radiopharmaceuticals. In Specialty Imaging: PET; Elsevier: Amsterdam, The Netherlands, 2018; pp. 24–29. [Google Scholar]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef] [PubMed]
- Krakowiak, P.A. Alveolar Osteitis and Osteomyelitis of the Jaws. Oral Maxillofac. Surg. Clin. N. Am. 2011, 23, 401–413. [Google Scholar] [CrossRef]
- Kruskal, J.B. Can USPIO-enhanced spinal MR imaging help distinguish acute infectious osteomyelitis from chronic infectious and inflammatory processes? Radiology 2008, 248, 1–3. [Google Scholar] [CrossRef]
- Grant, F.D.; Fahey, F.H.; Packard, A.B.; Davis, R.T.; Alavi, A.; Treves, S.T. Skeletal PET with18F-Fluoride: Applying New Technology to an Old Tracer. J. Nucl. Med. 2007, 49, 68–78. [Google Scholar] [CrossRef] [Green Version]
- Gholamrezanezhad, A.; Basques, K.; Batouli, A.; Matcuk, G.; Alavi, A.; Jadvar, H. Clinical Nononcologic Applications of PET/CT and PET/MRI in Musculoskeletal, Orthopedic, and Rheumatologic Imaging. Am. J. Roentgenol. 2018, 210, W245–W263. [Google Scholar] [CrossRef]
- Schirrmeister, H.; Guhlmann, A.; Kotzerke, J.; Santjohanser, C.; Kühn, T.; Kreienberg, R.; Messer, P.; Nüssle, K.; Elsner, K.; Glatting, G.; et al. Early Detection and Accurate Description of Extent of Metastatic Bone Disease in Breast Cancer With Fluoride Ion and Positron Emission Tomography. J. Clin. Oncol. 1999, 17, 2381. [Google Scholar] [CrossRef]
- Piert, M.; Zittel, T.T.; Becker, G.A.; Jahn, M.; Stahlschmidt, A.; Maier, G.; Machulla, H.-J.; Bares, R. Assessment of Porcine Bone Metabolism by Dynamic [18F]Fluoride Ion PET: Correlation with Bone Histomorphometry. J. Nucl. Med. 2001, 42, 1091. [Google Scholar]
- Dasa, V.; Adbel-Nabi, H.; Anders, M.J.; Mihalko, W.M. F-18 Fluoride Positron Emission Tomography of the Hip for Osteonecrosis. Clin. Orthop. Relat. Res. 2008, 466, 1081–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laverick, S.; Bounds, G.; Wong, W.L. [18F]-fluoride positron emission tomography for imaging condylar hyperplasia. Br. J. Oral Maxillofac. Surg. 2009, 47, 196–199. [Google Scholar] [CrossRef]
- Temmerman, O.; Raijmakers, P.; Heyligers, I.; Comans, E.; Lubberink, M.; Teule, G.; Lammertsma, A. Bone Metabolism after Total Hip Revision Surgery with Impacted Grafting: Evaluation using H215O and [18F]fluoride PET; A Pilot Study. Mol. Imaging Biol. 2008, 10, 288–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, D.R.; Maquieira, G.J.; Espinosa, N.; Zanetti, M.; Hesselmann, R.; Johayem, A.; Hany, T.F.; von Schulthess, G.K.; Strobel, K. Therapeutic impact of [18F]fluoride positron-emission tomography/computed tomography on patients with unclear foot pain. Skelet. Radiol. 2010, 39, 987–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Hou, T.; Luo, F.; Wu, X.; Xie, Z.; Xu, J. Involvement of Toll-Like Receptor 2 and Pro-Apoptotic Signaling Pathways in Bone Remodeling in Osteomyelitis. Cell. Physiol. Biochem. 2014, 34, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Dym, H.; Zeidan, J. Microbiology of Acute and Chronic Osteomyelitis and Antibiotic Treatment. Dent. Clin. N. Am. 2017, 61, 271–282. [Google Scholar] [CrossRef]
- Pincus, D.J.; Armstrong, M.B.; Thaller, S.R. Osteomyelitis of the Craniofacial Skeleton. Semin. Plast. Surg. 2009, 23, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Malina-Altzinger, J.; Klaeser, B.; Suter, V.G.; Schriber, M.; Vollnberg, B.; Schaller, B. Comparative evaluation of SPECT/CT and CBCT in patients with mandibular osteomyelitis and osteonecrosis. Clin. Oral Investig. 2019, 23, 4213–4222. [Google Scholar] [CrossRef]
- Marx, R.; Tursun, R. Suppurative osteomyelitis, bisphosphonate induced osteonecrosis, osteoradionecrosis: A blinded histopathologic comparison and its implications for the mechanism of each disease. Int. J. Oral Maxillofac. Surg. 2012, 41, 283–289. [Google Scholar] [CrossRef]
- Zhuang, H.; Duarte, P.; Pourdehand, M.; Shnier, D.; Alavi, A. Exclusion of Chronic Osteomyelitis with F-18 Fluorodeoxyglucose Positron Emission Tomographic Imaging. Clin. Nucl. Med. 2000, 25, 281–284. [Google Scholar] [CrossRef] [PubMed]
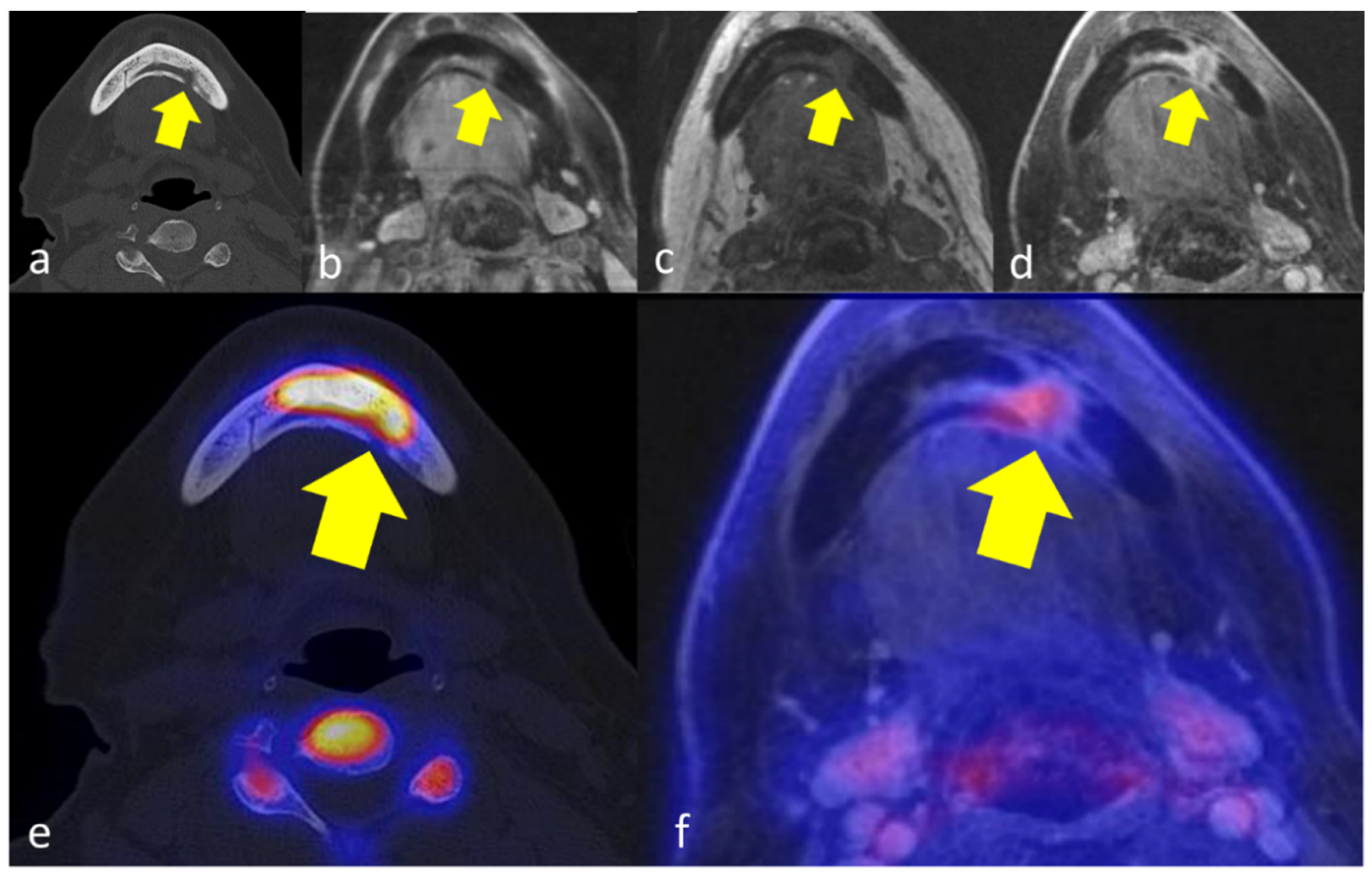


| CT | MRI | [18F]FDG PET | [18F]Fluorid PET | |||||
|---|---|---|---|---|---|---|---|---|
| Trabecular Sclerosis (Regions) | Cortical Erosion (Regions) | Bone Marrow Edema (Regions) | Gadolinium Enhancement (Regions) | Regions | SUVmean | Regions | SUVmean | |
| Patient 1 | 34–42 | 32–33 | 31–33 | 31–33 | 31 | 3.7 | / | / |
| Patient 2 | LMR–42 | n.o. | n.o. | n.o. | / | / | 41, 36–37 | 10.6 |
| Patient 3 | 43–47 | n.o. | 44–47 | 43–48 | 46 | 0.7 | 45–47 | 18.8 |
| Patient 4 | 14–15, 46–47 | n.o. | 14–15, 46–47 | 14–15, 46–47 | 14–15 | 0.9 | 14–15, 47 | 13.0 |
| Patient 5 | 43–RMC | RMC | RMC | RMC | RMC | 2.3 | RMR–RMC | 37.7 |
| Patient 6 | 37–48 | 32–33 | 33–43 | 33–43 | 33 | 2.0 | 33–42 | 14.5 |
| Imaging Markers | Affected Bone | Healthy Bone | SQI | p Value |
|---|---|---|---|---|
| T2w | 113 ± 101 | 37 ± 16 | 3.2 ± 2.1 | >0.05 |
| T1w | 211 ± 127 | 296 ± 127 | 0.9 ± 0.5 | >0.05 |
| T1w post-contrast (bone) | 464 ± 174 | 237 ± 98 | 2.4 ± 1.3 | <0.05 |
| 18F-FDG PET SUVmean (bone) | 1.9 ± 0.7 | 0.7 ± 0.2 | 2.6 ± 0.6 | <0.05 |
| 18F-FDG PET SUVmean (soft tissue) | 2.4 ± 0.8 | 1.9 ± 0.4 | 1.3 ± 0.3 | >0.05 |
| 18F-fluoride PET SUVmean (bone) | 15.4 ± 4.2 | 2.1 ± 0.6 | 7.4 ± 1.3 | <0.01 |
| Hounsfield Units | 560 ± 328 | 282 ± 211 | 2.2 ± 0.9 | >0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reinert, C.P.; Pfannenberg, C.; Dittmann, H.; Gückel, B.; la Fougère, C.; Nikolaou, K.; Hoefert, S. [18F]Fluoride Positron-Emission Tomography (PET) and [18F]FDG PET for Assessment of Osteomyelitis of the Jaw in Comparison to Computed Tomography (CT) and Magnetic Resonance Imaging (MRI): A Prospective PET/CT and PET/MRI Pilot Study. J. Clin. Med. 2022, 11, 3998. https://doi.org/10.3390/jcm11143998
Reinert CP, Pfannenberg C, Dittmann H, Gückel B, la Fougère C, Nikolaou K, Hoefert S. [18F]Fluoride Positron-Emission Tomography (PET) and [18F]FDG PET for Assessment of Osteomyelitis of the Jaw in Comparison to Computed Tomography (CT) and Magnetic Resonance Imaging (MRI): A Prospective PET/CT and PET/MRI Pilot Study. Journal of Clinical Medicine. 2022; 11(14):3998. https://doi.org/10.3390/jcm11143998
Chicago/Turabian StyleReinert, Christian Philipp, Christina Pfannenberg, Helmut Dittmann, Brigitte Gückel, Christian la Fougère, Konstantin Nikolaou, and Sebastian Hoefert. 2022. "[18F]Fluoride Positron-Emission Tomography (PET) and [18F]FDG PET for Assessment of Osteomyelitis of the Jaw in Comparison to Computed Tomography (CT) and Magnetic Resonance Imaging (MRI): A Prospective PET/CT and PET/MRI Pilot Study" Journal of Clinical Medicine 11, no. 14: 3998. https://doi.org/10.3390/jcm11143998







