Nonsubjective Assessment of Shape, Volume and Symmetry during Breast Augmentation with Handheld 3D Device
Abstract
:1. Introduction
2. Patients, Materials and Methods
2.1. Study Population
2.2. Surgical Consultation and Implant Size
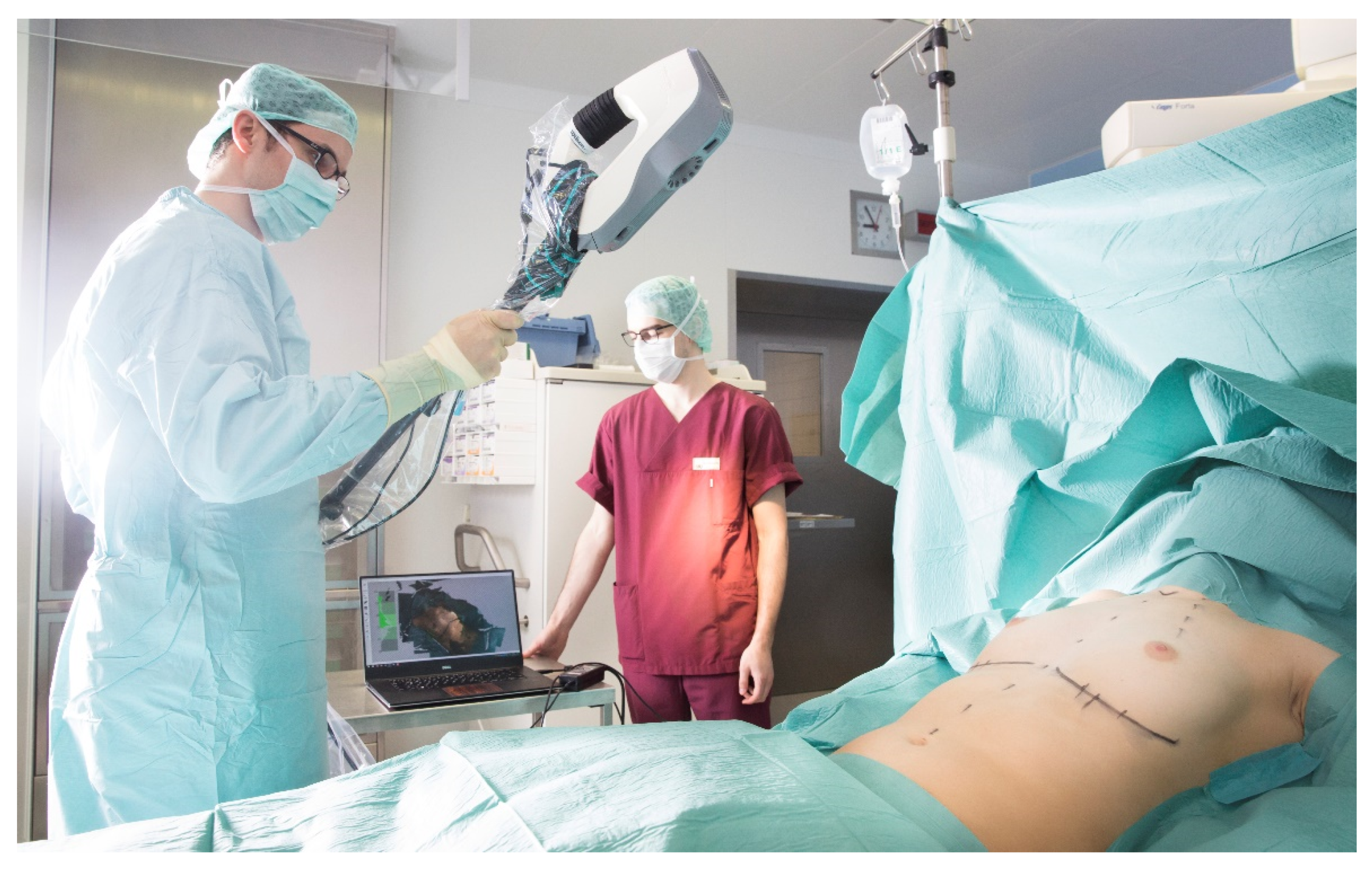
2.3. Intraoperative 3D Surface Imaging
2.4. Breast Distance Measurements
2.5. Breast Volume Measurements
2.6. Breast Symmetry Assessment
2.7. Statistical Analysis
3. Results
3.1. Subject Demographics
3.2. The Duration of Operation and Scan
3.3. Comparison of Digital and Manual Breast Distances
3.4. Comparison of Digital Volume Change and Implant/Sizer Volume
3.5. Digital Breast Symmetry Assessment
3.6. Surface and Volumetric Changes in Different Sizers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clearinghouse, A.N.; Statistics, P.S.P. Plastic Surgery Statistics Report 2019; American Society of Plastic Surgeons: Arlington Heights, IL, USA, 2015; pp. 1–27. [Google Scholar]
- Broer, P.N.; Juran, S.; Walker, M.E.; Ng, R.; Weichman, K.; Tanna, N.; Liu, Y.J.; Shah, A.; Patel, A.; Persing, J.A.; et al. Aesthetic breast shape preferences among plastic surgeons. Ann. Plast. Surg. 2015, 74, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Asplund, O.; Nilsson, B. Interobserver variation and cosmetic result of submuscular breast reconstruction. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1984, 18, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Rohrich, R.J.; Hartley, W.; Brown, S. Incidence of breast and chest wall asymmetry in breast augmentation: A retrospective analysis of 100 patients. Plast. Reconstr. Surg. 2003, 111, 1513. [Google Scholar] [CrossRef] [PubMed]
- Howes, B.H.L.; Fosh, B.; Watson, D.I.; Yip, J.M.; Eaton, M.; Smallman, A.; Dean, N.R. Autologous fat grafting for whole breast reconstruction. Plast. Reconstr. Surg. 2014, 2, e124. [Google Scholar] [CrossRef] [Green Version]
- Roostaeian, J.; Adams, W.P.J. Three-Dimensional Imaging for Breast Augmentation: Is This Technology Providing Accurate Simulations? Aesthetic Surg. J. 2014, 34, 857–875. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, R.L.; Stevens, R.J.G.; Harris, P.A.; Rusby, J.E. Review of three-dimensional (3D) surface imaging for oncoplastic, reconstructive and aesthetic breast surgery. Breast 2015, 24, 331–342. [Google Scholar] [CrossRef]
- Losken, A.; Seify, H.; Denson, D.D.; Paredes, A.A.; Carlson, G.W. Validating three-dimensional imaging of the breast. Ann. Plast. Surg. 2005, 54, 471–476. [Google Scholar] [CrossRef]
- Lee, W.Y.; Kim, M.J.; Lew, D.H.; Song, S.Y.; Lee, D.W. Three-dimensional surface imaging is an effective tool for measuring breast volume: A validation study. Arch. Plast. Surg. 2016, 43, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Chae, M.P.; Rozen, W.M.; Spychal, R.T.; Hunter-Smith, D.J. Breast volumetric analysis for aesthetic planning in breast reconstruction: A literature review of techniques. Gland Surg. 2016, 5, 212–226. [Google Scholar] [CrossRef]
- Eder, M.; Waldenfels, F.v.; Swobodnik, A.; Klöppel, M.; Pape, A.K.; Schuster, T.; Raith, S.; Kitzler, E.; Papadopulos, N.A.; Machens, H.G.; et al. Objective breast symmetry evaluation using 3-D surface imaging. Breast 2012, 21, 152–158. [Google Scholar] [CrossRef]
- Donfrancesco, A.; Montemurro, P.; Hedén, P. Three-dimensional simulated images in breast augmentation surgery: An investigation of patients’ satisfaction and the correlation between prediction and actual outcome. Plast. Reconstr. Surg. 2013, 132, 810–822. [Google Scholar] [CrossRef] [PubMed]
- de Runz, A.; Boccara, D.; Bertheuil, N.; Claudot, F.; Brix, M.; Simon, E. Three-dimensional imaging, an important factor of decision in breast augmentation. Ann. Chir. Plast. Esthet. 2018, 63, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Overschmidt, B.; Qureshi, A.A.; Parikh, R.P.; Yan, Y.; Tenenbaum, M.M.; Myckatyn, T.M. A prospective evaluation of three-dimensional image simulation: Patient-reported outcomes and mammometrics in primary breast augmentation. Plast. Reconstr. Surg. 2018, 142, 133E–144E. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, D.A. SIEF-simultaneous implant exchange with fat: A new option in revision breast implant surgery. Plast. Reconstr. Surg. 2012, 130, 1187–1196. [Google Scholar] [CrossRef]
- Camison, L.; Bykowski, M.; Lee, W.W.; Carlson, J.C.; Roosenboom, J.; Goldstein, J.A.; Losee, J.E.; Weinberg, S.M. Validation of the Vectra H1 portable three-dimensional photogrammetry system for facial imaging. Int. J. Oral Maxillofac. Surg. 2018, 47, 403–410. [Google Scholar] [CrossRef]
- Mitsuno, D.; Ueda, K.; Itamiya, T.; Nuri, T.; Otsuki, Y. Intraoperative Evaluation of Body Surface Improvement by an Augmented Reality System That a Clinician Can Modify. Plast. Reconstr. Surg.-Glob. Open 2017, 5, e1432. [Google Scholar] [CrossRef]
- Koban, K.C.; Etzel, L.; Li, Z.; Pazos, M.; Schönecker, S.; Belka, C.; Giunta, R.E.; Schenck, T.L.; Corradini, S. Three-dimensional surface imaging in breast cancer: A new tool for clinical studies? Radiat. Oncol. 2020, 15, 52. [Google Scholar] [CrossRef] [Green Version]
- Esme, D.L.; Bucksch, A.; Beekman, W.H. Three-dimensional laser imaging as a valuable tool for specifying changes in breast shape after augmentation mammaplasty. Aesthetic Plast. Surg. 2009, 33, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Tanabe, Y.N.; Honda, T.; Nakajima, Y.; Sakurai, H.; Nozaki, M. Intraoperative application of three-dimensional imaging for breast surgery. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2005, 39, 349–352. [Google Scholar] [CrossRef]
- Maxwell, G.P.; Gabriel, A. Breast Augumentation:Implant selection. In Plastic Surgery Volume 5 Breast; Grotting, J.C., Ed.; Elsevier Saunder: Philadelphia, PA, USA, 2012; pp. 24–30. [Google Scholar]
- Hidalgo, D.A.; Weinstein, A.L. Intraoperative Comparison of Anatomical versus Round Implants in Breast Augmentation: A Randomized Controlled Trial. Plast. Reconstr. Surg. 2017, 139, 587–596. [Google Scholar] [CrossRef]
- Mallucci, P. Discussion: Intraoperative Comparison of Anatomical versus Round Implants in Breast Augmentation: A Randomized Controlled Trial. Plast. Reconstr. Surg. 2017, 139, 599–600. [Google Scholar] [CrossRef] [PubMed]
- Calobrace, M.B. Teaching Breast Augmentation: A Focus on Critical Intraoperative Techniques and Decision Making to Maximize Results and Minimize Revisions. Clin. Plast. Surg. 2015, 42, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Weck Roxo, A.C.; Nahas, F.X.; Salin, R.; de Castro, C.C.; Aboudib, J.H.; Marques, R.G. Volumetric Evaluation of the Mammary Gland and Pectoralis Major Muscle following Subglandular and Submuscular Breast Augmentation. Plast. Reconstr. Surg. 2016, 137, 62–69. [Google Scholar] [CrossRef]
- Modabber, A.; Peters, F.; Kniha, K.; Goloborodko, E.; Ghassemi, A.; Lethaus, B.; Hölzle, F.; Möhlhenrich, S.C. Evaluation of the accuracy of a mobile and a stationary system for three-dimensional facial scanning. J. Cranio-Maxillofac. Surg. 2016, 44, 1719–1724. [Google Scholar] [CrossRef]
- Koban, K.C.; Cotofana, S.; Frank, K.; Green, J.B.; Etzel, L.; Li, Z.; Giunta, R.E.; Schenck, T.L. Precision in 3-Dimensional Surface Imaging of the Face: A Handheld Scanner Comparison Performed in a Cadaveric Model. Aesthetic Surg. J. 2019, 39, NP36–NP44. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Lista, F. Discussion: Intraoperative Comparison of Anatomical versus Round Implants in Breast Augmentation: A Randomized Controlled Trial. Plast. Reconstr. Surg. 2017, 139, 597–598. [Google Scholar] [CrossRef]
- Hersant, B.; SidAhmed-Mezi, M.; Aboud, C.; Niddam, J.; Levy, S.; Mernier, T.; La Padula, S.; Meningaud, J.-P. Synergistic Effects of Autologous Platelet-Rich Plasma and Hyaluronic Acid Injections on Facial Skin Rejuvenation. Aesthetic Surg. J. 2021, 41, NP854–NP865. [Google Scholar] [CrossRef]
- La Padula, S.; Hersant, B.; Pizza, C.; Chesné, C.; Jamin, A.; Ben Mosbah, I.; Errico, C.; D’Andrea, F.; Rega, U.; Persichetti, P.; et al. Striae Distensae: In Vitro Study and Assessment of Combined Treatment with Sodium Ascorbate and Platelet-Rich Plasma on Fibroblasts. Aesthetic Plast. Surg. 2021, 45, 1282–1293. [Google Scholar] [CrossRef]
- La Padula, S.; Hersant, B.; Meningaud, J.P. Intraoperative use of indocyanine green angiography for selecting the more reliable perforator of the anterolateral thigh flap: A comparison study. Microsurgery 2018, 38, 738–744. [Google Scholar] [CrossRef]
- Weinberg, S.M.; Naidoo, S.; Govier, D.P.; Martin, R.A.; Kane, A.A.; Marazita, M.L. Anthropometric precision and accuracy of digital three-dimensional photogrammetry: Comparing the Genex and 3dMD imaging systems with one another and with direct anthropometry. J. Craniofac. Surg. 2006, 17, 477–483. [Google Scholar] [CrossRef]
- Jayaratne, Y.S.N.; Deutsch, C.K.; Zwahlen, R.A. Nasal Morphology of the Chinese: Three-Dimensional Reference Values for Rhinoplasty. Otolaryngol. Head Neck Surg. 2014, 150, 956–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.B.; Small, K.H.; Choi, M.; Karp, N.S. Three-dimensional surface imaging in plastic surgery: Foundation, practical applications, and beyond. Plast. Reconstr. Surg. 2015, 135, 1295–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wesselius, T.S.; Verhulst, A.C.; Vreeken, R.D.; Xi, T.; Maal, T.J.J.; Ulrich, D.J.O. Accuracy of three software applications for breast volume calculations from three-dimensional surface images. Plast. Reconstr. Surg. 2018, 142, 858–865. [Google Scholar] [CrossRef]
- Kovacs, L.; Eder, M.; Hollweck, R.; Zimmermann, A.; Settles, M.; Schneider, A.; Udosic, K.; Schwenzer-Zimmerer, K.; Papadopulos, N.A.; Biemer, E. New aspects of breast volume measurement using 3-dimensional surface imaging. Ann. Plast. Surg. 2006, 57, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Eder, M.; Schneider, A.; Feussner, H.; Zimmermann, A.; Höhnke, C.; Papadopulos, N.A.; Kovacs, L. Brustvolumenbestimmung anhand der 3-D-oberflächengeometrie: Verifizierung der methode mit hilfe der kernspintomographie. Biomed. Tech. 2008, 53, 112–121. [Google Scholar] [CrossRef]
- Creasman, C.N.; Mordaunt, D.; Liolios, T.; Chiu, C.; Gabriel, A.; Maxwell, G.P. Four-dimensional breast imaging, part I: Introduction of a technology-driven, evidence-based approach to breast augmentation planning. Aesthetic Surg. J. 2011, 31, 914–924. [Google Scholar] [CrossRef]
- Yip, J.M.; Mouratova, N.; Jeffery, R.M.; Veitch, D.E.; Woodman, R.J.; Dean, N.R. Accurate assessment of breast volume: A study comparing the volumetric gold standard (direct water displacement measurement of mastectomy specimen) with a 3D laser scanning technique. Ann. Plast. Surg. 2012, 68, 135–141. [Google Scholar] [CrossRef]
- Campaigne, B.N.; Katch, V.L.; Freedson, P.; Sady, S.; Katch, F.I. Measurement of breast volume in females: Description of a reliable method. Ann. Hum. Biol. 1979, 6, 363–367. [Google Scholar] [CrossRef]
- Kayar, R.; Civelek, S.; Cobanoglu, M.; Gungor, O.; Catal, H.; Emiroglu, M. Five methods of breast volume measurement: A comparative study of measurements of specimen volume in 30 mastectomy cases. Breast Cancer Basic Clin. Res. 2011, 5, 43–52. [Google Scholar] [CrossRef]
- Fowler, P.A.; Casey, C.E.; Cameron, G.G.; Foster, M.A.; Knight, C.H. Cyclic changes in composition and volume of the breast during the menstrual cycle, measured by magnetic resonance imaging. Br. J. Obstet. Gynaecol. 1990, 97, 595–602. [Google Scholar] [CrossRef]
- Swanson, E. Prospective photographic measurement study of 196 cases of breast augmentation, Mastopexy, augmentation/Mastopexy, and breast reduction. Plast. Reconstr. Surg. 2013, 131, 802–819. [Google Scholar] [CrossRef] [PubMed]
- Losken, A.; Fishman, I.; Denson, D.D.; Moyer, H.R.; Carlson, G.W. An objective evaluation of breast symmetry and shape differences using 3-dimensional images. Ann. Plast. Surg. 2005, 55, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Mailey, B.; Freel, A.; Wong, R.; Pointer, D.T.; Khoobehi, K. Clinical accuracy and reproducibility of Portrait 3D Surgical Simulation Platform in breast augmentation. Aesthetic Surg. J. 2013, 33, 84–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tepper, O.M.; Small, K.; Rudolph, L.; Choi, M.; Karp, N. Virtual 3-dimensional modeling as a valuable adjunct to aesthetic and reconstructive breast surgery. Am. J. Surg. 2006, 192, 548–551. [Google Scholar] [CrossRef]
- Kovacs, L.; Eder, M.; Zimmermann, A.; Müller, D.; Schuster, T.; Papadopulos, N.A.; Biemer, E.; Klöppel, M.; MacHens, H.-G.G. Three-dimensional evaluation of breast augmentation and the influence of anatomic and round implants on operative breast shape changes. Aesthetic Plast. Surg. 2012, 36, 879–887. [Google Scholar] [CrossRef]
- Rha, E.Y.; Choi, I.K.; Yoo, G. Accuracy of the method for estimating breast volume on three-dimensional simulated magnetic resonance imaging scans in breast reconstruction. Plast. Reconstr. Surg. 2014, 133, 14–20. [Google Scholar] [CrossRef]
- Gentile, P. Breast Silicone Gel Implants versus Autologous Fat Grafting: Biomaterials and Bioactive Materials in Comparison. J. Clin. Med. 2021, 10, 3310. [Google Scholar] [CrossRef]
- Gentile, P.; De Angelis, B.; Di Pietro, V.; Amorosi, V.; Scioli, M.G.; Orlandi, A.; Cervelli, V. Gentle Is Better: The Original “Gentle Technique” for Fat Placement in Breast Lipofilling. J. Cutan. Aesthet. Surg. 2018, 11, 120–126. [Google Scholar] [CrossRef]
- Gentile, P.; Kothari, A.; Casella, D.; Calabrese, C. Fat Graft Enhanced with Adipose-Derived Stem Cells in Aesthetic Breast Augmentation: Clinical, Histological, and Instrumental Evaluation. Aesthetic Surg. J. 2020, 40, 962–977. [Google Scholar] [CrossRef]
- Gentile, P.; Casella, D.; Palma, E.; Calabrese, C. Engineered fat graft enhanced with adipose-derived stromal vascular fraction cells for regenerative medicine: Clinical, histological and instrumental evaluation in breast reconstruction. J. Clin. Med. 2019, 8, 504. [Google Scholar] [CrossRef] [Green Version]



| Distance | Manual | 3D | Delta | Range | p | R |
|---|---|---|---|---|---|---|
| Sn-N pre | 180.4 ± 14.8 | 180.8 ± 14.7 | −0.4 ± 1.6 | −6.3 to 2.2 | 0.217 | 0.994 |
| Sn-N post | 189.3 ± 16.9 | 184.8 ± 18.1 | 0.5 ± 2.00 | −4.9 to 5.7 | 0.120 | 0.991 |
| N-M pre | 64.5 ± 9.9 | 64.7 ± 9.6 | −0.2 ± 1.4 | −2.7 to 3.1 | 0.560 | 0.990 |
| N-M post | 91.1 ± 10.1 | 89.9 ± 11.2 | 1.2 ± 3.8 | −11.5 to 8.9 | 0.090 | 0.939 |
| Volume | Actual | 3D | Delta | Range | p | R |
|---|---|---|---|---|---|---|
| Implant | 294.67 ± 75.56 | 292.11 ± 76.87 | 0.67 ± 9.4 | −33.1 to 39.3 | 0.837 | 0.965 |
| Sizer | 276.85 ± 81.52 | 274.38 ± 84.47 | 0.42 ± 12.31 | −41.3 to 38.5 | 0.860 | 0.995 |
| Volume | Right | Left | Delta | Range | p | RMSE |
|---|---|---|---|---|---|---|
| Pre-Op | 189.94 ± 112 | 172.65 ± 98.73 | 33.93 ± 30.6 | −33.1 to 39.3 | 0.157 | 2.64 ± 1.29 |
| Post-op | 479.10 ± 147.1 | 471.65 ± 139.88 | 35.66 ± 29.4 | −41.3 to 38.5 | 0.593 | 2.16 ± 0.89 |
| Right-Left Breast Difference | Manual/Actual | 3D | Delta | Range | p | R |
|---|---|---|---|---|---|---|
| Sn-N distance pre | 4.3 ± 2.1 | 3.9 ± 1.8 | 0.4 ± 0.7 | −1.1 to 0.9 | 0.35 | 0.992 |
| Sn-N distance post | 4.5 ± 1.9 | 4.2 ± 2.0 | 0.3 ± 0.5 | −1.3 to 0.8 | 0.41 | 0.992 |
| N-M distance pre | 7.1 ± 1.5 | 6.6 ± 1.9 | 1.3 ± 0.6 | −1.9 to 1.2 | 0.47 | 0.976 |
| N-M distance post | 7.4 ± 1.8 | 7.0 ± 2.1 | 1.1 ± 0.9 | −1.7 to 1.3 | 0.58 | 0.989 |
| Sizer Volume | 92.37 ± 76.87 | 91.72 ± 75.54 | 0.38 ± 2.3 | −9.4 to 7.8 | 0.51 | 0.985 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Schenck, T.L.; Giunta, R.E.; Etzel, L.; Koban, K.C. Nonsubjective Assessment of Shape, Volume and Symmetry during Breast Augmentation with Handheld 3D Device. J. Clin. Med. 2022, 11, 4002. https://doi.org/10.3390/jcm11144002
Li Z, Schenck TL, Giunta RE, Etzel L, Koban KC. Nonsubjective Assessment of Shape, Volume and Symmetry during Breast Augmentation with Handheld 3D Device. Journal of Clinical Medicine. 2022; 11(14):4002. https://doi.org/10.3390/jcm11144002
Chicago/Turabian StyleLi, Zhouxiao, Thilo Ludwig Schenck, Riccardo Enzo Giunta, Lucas Etzel, and Konstantin Christoph Koban. 2022. "Nonsubjective Assessment of Shape, Volume and Symmetry during Breast Augmentation with Handheld 3D Device" Journal of Clinical Medicine 11, no. 14: 4002. https://doi.org/10.3390/jcm11144002






