Visceral Fat Area Measured by Abdominal Bioelectrical Impedance Analysis in School-Aged Japanese Children
Abstract
:1. Introduction
2. Materials and Methods
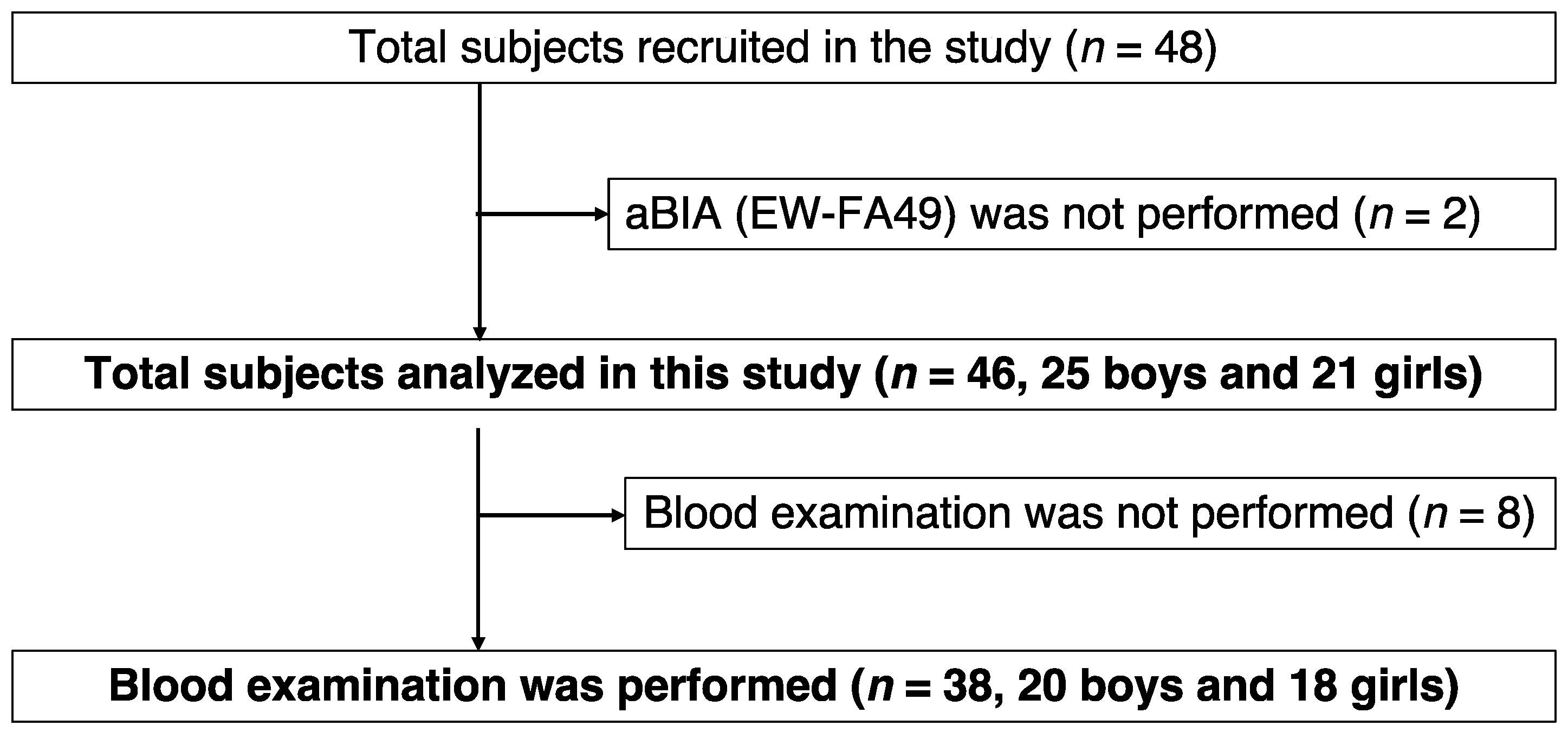
2.1. Study Design and Subjects
2.2. Anthropometry and Laboratory Tests
2.3. Bioelectrical Impedance Analysis “EW-FA90” for Measurement of Abdominal Fat Area
2.4. Abdominal CT Measurement of Visceral Fat Area and Subcutaneous Fat Area
2.5. Definition of Obesity
2.6. Non-Alcoholic Fatty Liver Disease
2.7. Statistical Analyses
3. Results
3.1. Characteristics of Participants
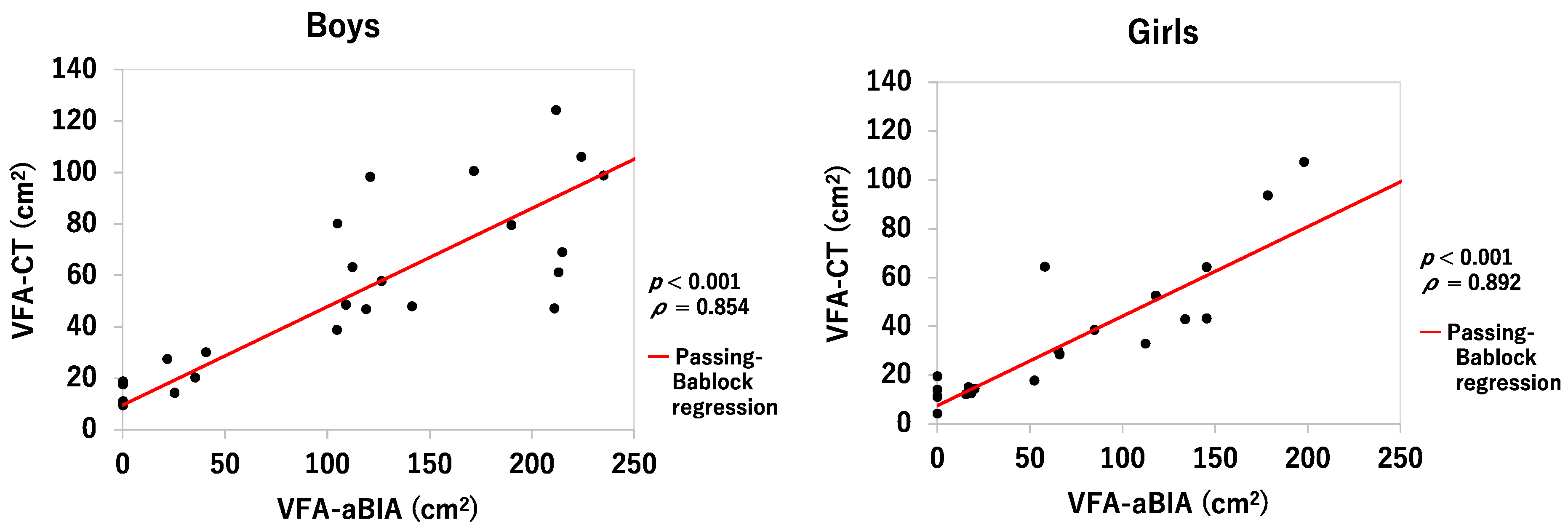
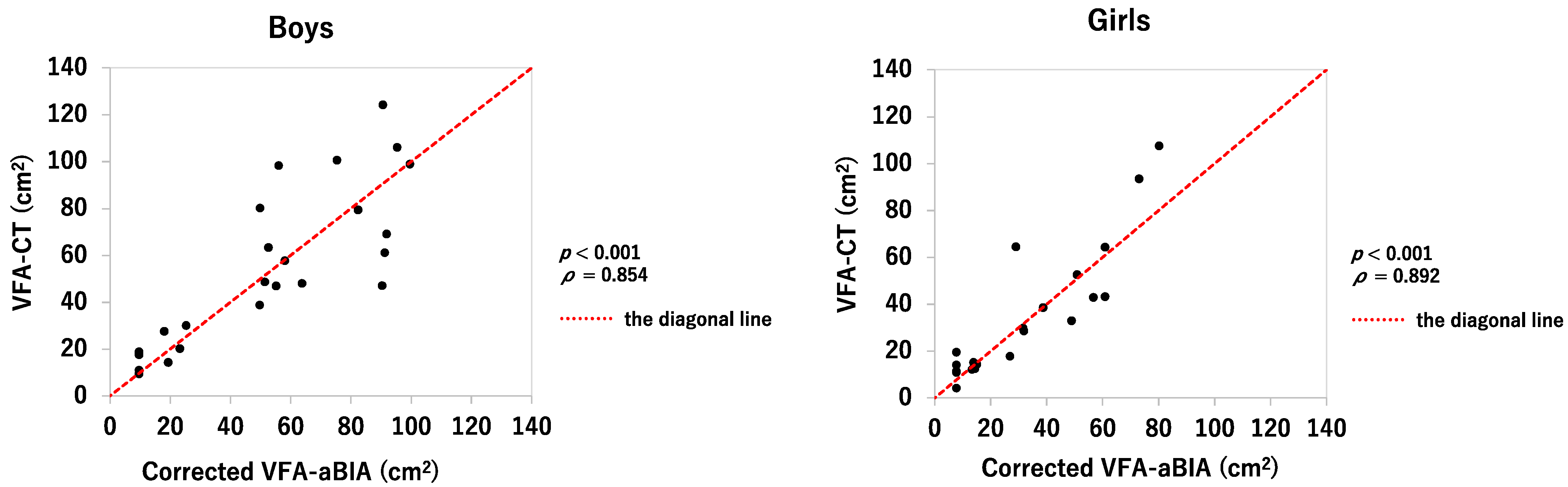
3.2. Relationship between VFA-aBIA or Corrected VFA-aBIA and VFA-CT
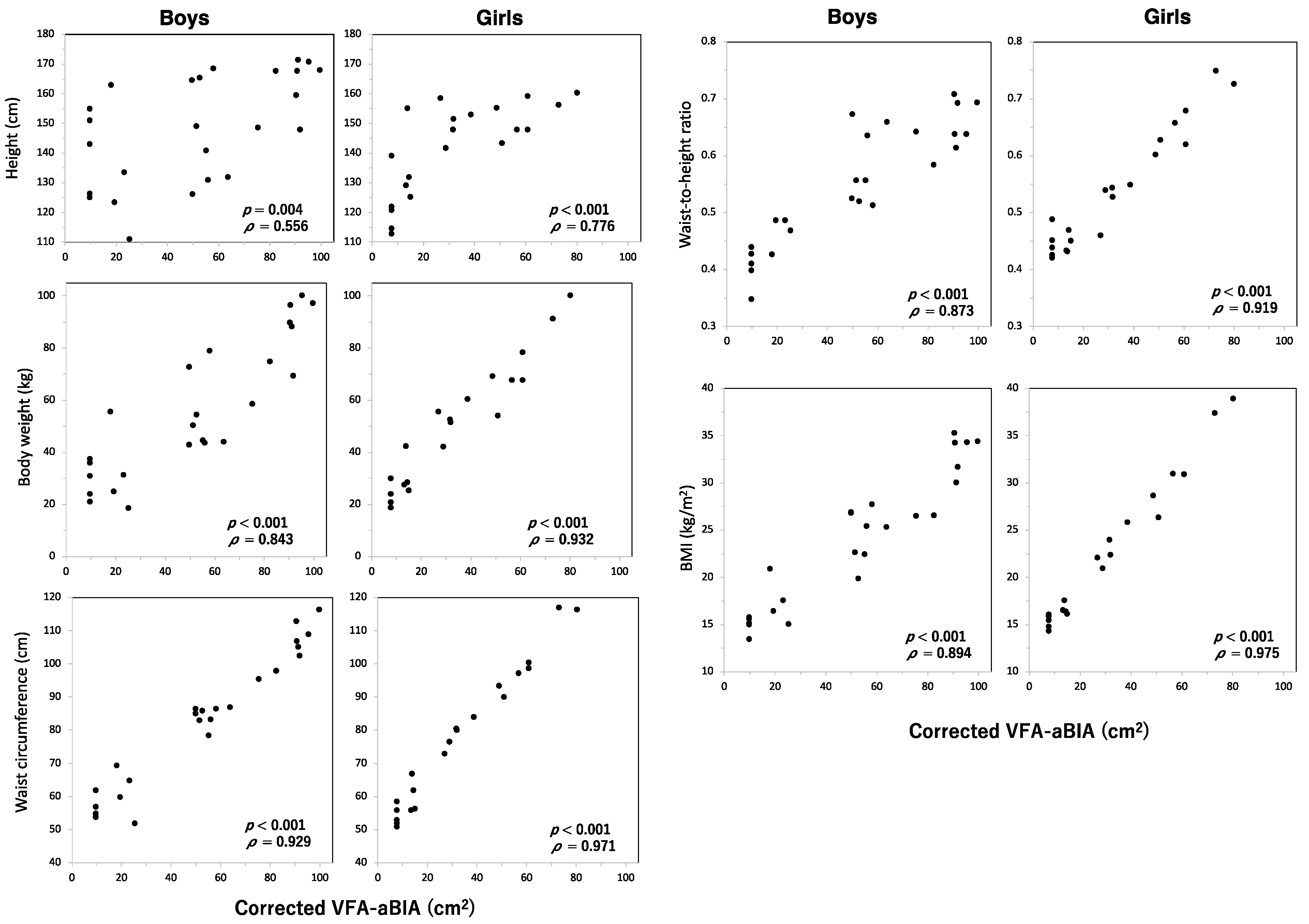
3.3. Relationship between VFA and Height, Body Weight, Waist Circumference, Waist-To-Height Ratio, or Body Mass Index
3.4. Relationship between VFA and NAFLD
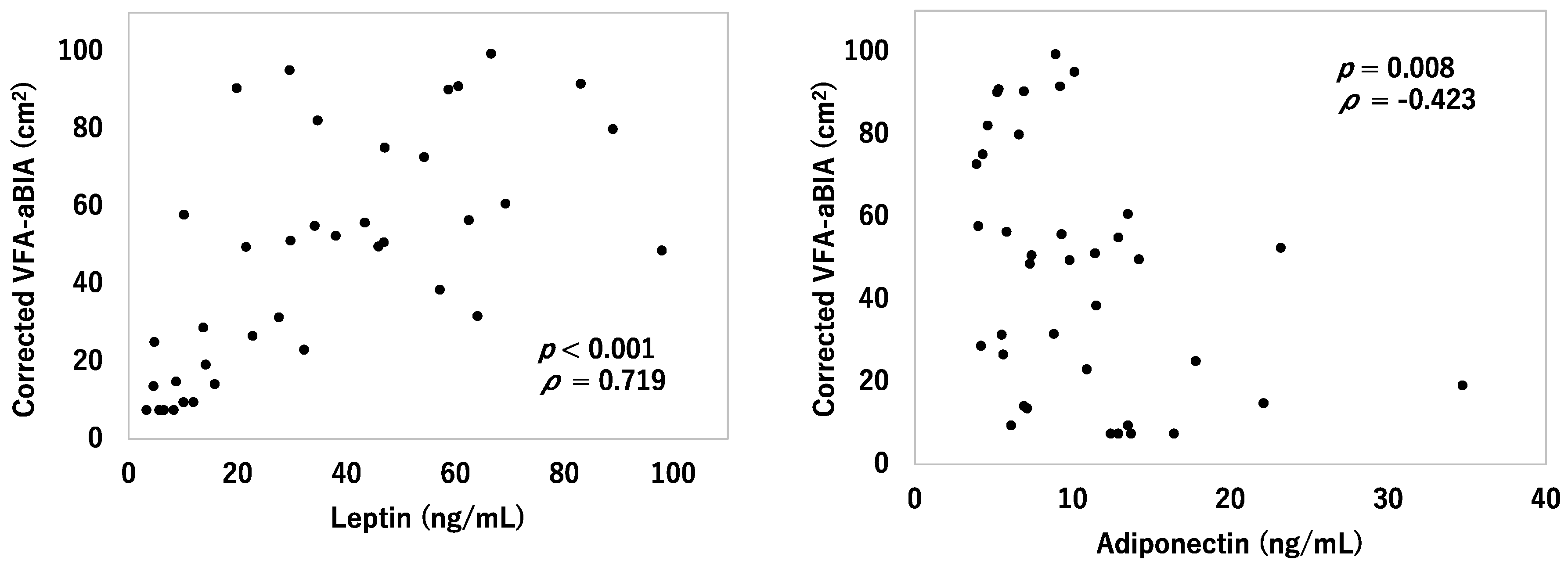
3.5. Relationship between VFA and Serum Leptin and Adiponectin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H. Obesity Management Task Force of the European Association for the Study of Obesity. European Guidelines for Obesity Management in Adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, Z.; Heshka, S.; Heo, M.; Faith, M.S.; Heymsfield, S.B. Waist circumference and obesity-associated risk factors among whites in the third National Health and Nutrition Examination Survey: Clinical action thresholds. Am. J. Clin. Nutr. 2002, 76, 743–749. [Google Scholar] [CrossRef]
- Niemirska, A.; Litwin, M.; Feber, J.; Jurkiewicz, E. Blood pressure rhythmicity and visceral fat in children with hypertension. Hypertension 2013, 62, 782–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, Y.; Urakami, T.; Hara, M.; Yoshida, K.; Mine, Y.; Aoki, M.; Suzuki, J.; Saito, E.; Yoshino, Y.; Iwata, F.; et al. The characteristics of abdominal fat distribution in Japanese adolescents with type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. 2019, 12, 2281–2288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Liu, J.; Zhao, X.; Cheng, H.; Huang, G.; Mi, J.; China Child and Adolescent Cardiovascular Health (CCACH) study research group. Abdominal visceral and subcutaneous adipose tissues in association with cardiometabolic risk in children and adolescents: The China Child and Adolescent Cardiovascular Health (CCACH) study. BMJ Open. Diabetes Res. Care 2019, 7, e000824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, H.; Berg, E.; Cheng, X.; Shen, W. How to best assess abdominal obesity. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 360–365. [Google Scholar] [CrossRef]
- Yoshizumi, T.; Nakamura, T.; Yamane, M.; Islam, A.H.; Menju, M.; Yamasaki, K.; Arai, T.; Kotani, K.; Funahashi, T.; Yamashita, S.; et al. Abdominal fat: Standardized technique for measurement at CT. Radiology 1999, 211, 283–286. [Google Scholar] [CrossRef]
- Wajchenberg, B.L. Subcutaneous and visceral adipose tissue: Their relation to the metabolic syndrome. Endocr. Rev. 2000, 21, 697–738. [Google Scholar] [CrossRef]
- Maurovich-Horvat, P.; Massaro, J.; Fox, C.S.; Moselewski, F.; O’Donnell, C.J.; Hoffmann, U. Comparison of anthropometric, area-and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int. J. Obes. 2007, 31, 500–506. [Google Scholar] [CrossRef] [Green Version]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Matsushita, Y.; Nakagawa, T.; Shinohara, M.; Yamamoto, S.; Takahashi, Y.; Mizoue, T.; Yokoyama, T.; Noda, M. How can waist circumference predict the body composition? Diabetol. Metab. Syndr. 2014, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Ryo, M.; Maeda, K.; Onda, T.; Katashima, M.; Okumiya, A.; Nishida, M.; Yamaguchi, T.; Funahashi, T.; Matsuzawa, Y.; Nakamura, T.; et al. A new simple method for the measurement of visceral fat accumulation by bioelectrical impedance. Diabetes Care 2005, 28, 451–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoji, K.; Maeda, K.; Nakamura, T.; Funahashi, T.; Matsuzawa, Y.; Shimomura, I. Measurement of visceral fat by abdominal bioelectrical impedance analysis is beneficial in medical checkup. Obes. Res. Clin. Pract. 2008, 2, 269–275. [Google Scholar] [CrossRef] [PubMed]
- de-Mateo-Silleras, B.; de-la-Cruz-Marcos, S.; Alonso-Izquierdo, L.; Camina-Martín, M.A.; Marugán-de-Miguelsanz, J.M.; Redondo-Del-Río, M.P. Bioelectrical impedance vector analysis in obese and overweight children. PLoS ONE 2019, 14, e0211148. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Takimoto, H.; Sudo, N. The Cubic Functions for Spline Smoothed L, S and M Values for BMI Reference Data of Japanese Children. Clin. Pediatr. Endocrinol. 2011, 20, 47–49. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, A.; Sawai, T. Determination of adiponectin in serum using a latex particle-enhanced turbidimetric immunoassay with an automated analyzer. Clin. Chim. Acta 2006, 371, 163–168. [Google Scholar] [CrossRef]
- Ozet, A.; Arpaci, F.; Yilmaz, M.I.; Ayta, H.; Ozturk, B.; Komurcu, S.; Yavuz, A.A.; Tezcan, Y.; Acikel, C. Effects of tamoxifen on the serum leptin level in patients with breast cancer. Jpn. J. Clin. Oncol. 2001, 31, 424–427. [Google Scholar] [CrossRef] [Green Version]
- Barlow, S.E.; Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics 2007, 120 (Suppl. S4), 164–192. [Google Scholar] [CrossRef] [Green Version]
- Vos, M.B.; Abrams, S.H.; Barlow, S.E.; Caprio, S.; Daniels, S.R.; Kohli, R.; Mouzaki, M.; Sathya, P.; Schwimmer, J.B.; Sundaram, S.S.; et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J. Pediatr. Gastroenterol. Nutr. 2017, 64, 319–334. [Google Scholar]
- Nishikawa, T.; Omura, M.; Kawaguchi, M.; Takatsu, A.; Satoh, F.; Ito, S.; Kurihara, I.; Itoh, H.; Yanase, T.; Shibata, H.; et al. Calibration and evaluation of routine methods by serum certified reference material for aldosterone measurement in blood. Endocr. J. 2016, 63, 1065–1080. [Google Scholar] [CrossRef] [Green Version]
- Styne, D.M.; Arslanian, S.A.; Connor, E.L.; Farooqi, I.S.; Murad, M.H.; Silverstein, J.H.; Yanovski, J.A. Pediatric Obesity-Assessment, Treatment, and Prevention: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 709–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berker, D.; Koparal, S.; Işik, S.; Paşaoğlu, L.; Aydin, Y.; Erol, K.; Delibaşi, T.; Güler, S. Compatibility of different methods for the measurement of visceral fat in different body mass index strata. Diagn. Interv. Radiol. 2010, 16, 99–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Browning, L.M.; Mugridge, O.; Chatfield, M.D.; Dixon, A.K.; Aitken, S.W.; Joubert, I.; Prentice, A.M.; Jebb, S.A. Validity of a new abdominal bioelectrical impedance device to measure abdominal and visceral fat: Comparison with MRI. Obesity 2010, 18, 2385–2391. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Lee, D.H.; Lee, J.; Kim, Y.J.; Jung, K.Y.; Kim, K.M.; Kwak, S.H.; Choi, S.H.; Park, K.S.; Jang, H.C.; et al. Comparison between two methods of bioelectrical impedance analyses for accuracy in measuring abdominal visceral fat area. J. Diabetes Complicat. 2016, 30, 343–349. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; González-Crespo, I.; Catalán, V.; Rodríguez, A.; Moncada, R.; Valentí, V.; Romero, S.; Ramírez, B.; Silva, C.; Gil, M.J.; et al. Clinical usefulness of abdominal bioimpedance (ViScan) in the determination of visceral fat and its application in the diagnosis and management of obesity and its comorbidities. Clin. Nutr. 2018, 37, 580–589. [Google Scholar] [CrossRef]
- Mattoo, T.K.; Lu, H.; Ayers, E.; Thomas, R. Total body water by BIA in children and young adults with normal and excessive weight. PLoS ONE 2020, 15, e0239212. [Google Scholar]
- Boyraz, M.; Hatipoğlu, N.; Sarı, E.; Akçay, A.; Taşkın, N.; Ulucan, K.; Akçay, T. Non-alcoholic fatty liver disease in obese children and the relationship between metabolic syndrome criteria. Obes. Res. Clin. Pract. 2014, 8, e356–e363. [Google Scholar] [CrossRef]
- Martin, S.S.; Qasim, A.; Reilly, M.P. Leptin resistance: A possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J. Am. Coll. Cardiol. 2008, 52, 1201–1210. [Google Scholar] [CrossRef] [Green Version]
- Landecho, M.F.; Tuero, C.; Valenti, V.; Bilbao, I.; de la Higuera, M.; Frühbeck, G. Relevance of leptin and other adipokines in obesity-associated cardiovascular risk. Nutrients 2019, 11, 2664. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.S.; Blaha, M.J.; Muse, E.D.; Qasim, A.N.; Reilly, M.P.; Blumenthal, R.S.; Nasir, K.; Criqui, M.H.; McClelland, R.L.; Hughes-Austin, J.M.; et al. Leptin and incident cardiovascular disease: The Multi-ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2015, 239, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, T.; Kadowaki, T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab. 2013, 17, 185–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.J.; Boyko, E.J.; Fujimoto, W.Y.; Kahn, S.E.; Leonetti, D.L. Low plasma adiponectin concentrations predict increases in visceral adiposity and insulin resistance. J. Clin. Endocrinol. Metab. 2017, 102, 4626–4633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauli, A.M.; Matin, S. Why Do Men Accumulate Abdominal Visceral Fat? Front. Physiol. 2019, 10, 1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urakami, T. Clinical characteristics in Japanese children with nonobese type 2 diabetes. Ann. Pediatr. Endocrinol. Metab. 2018, 23, 113–118. [Google Scholar] [CrossRef] [Green Version]


| Boys, n = 25 | Girls, n = 21 | p-Value | |
|---|---|---|---|
| Age (year) | 12 (6–17) | 10 (7–17) | 0.632 |
| Height (cm) | 149.1 (111.0–171.4) | 147.9 (112.9–160.4) | 0.142 |
| Body weight (kg) | 50.5 (18.6–100.2) | 51.6 (18.9–100.3) | 0.372 |
| Waist circumference (cm) | 85 (52.0–116.5) | 76.5 (51.0–117.0) | 0.384 |
| Waist-to-height ratio | 0.56 (0.35–0.71) | 0.53 (0.42–0.75) | 0.662 |
| BMI (kg/m2) | 25.4 (13.5–35.3) | 22.1 (14.4–39.0) | 0.674 |
| BMI percentile (%) | 95.1 (4.2–99.9) | 90.0 (17.6–99.9) | 0.861 |
| Impedance-aBIA (Ω) | 1495 (619–2969) | 1586 (645–2140) | 0.972 |
| VFA-aBIA (cm2) | 112.3 (0.0–235.0) | 58.0 (0.0–198.0) | 0.110 |
| Corrected VFA-aBIA (cm2) | 52.6 (9.6–99.5) | 28.8 (7.6–80.1) | 0.035 * |
| VFA-CT (cm2) | 48.1 (9.6–124.4) | 28.7 (4.3–107.6) | 0.042 * |
| NAFLD (%) | 36 | 24 | 0.522 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abe, Y.; Tonouchi, R.; Hara, M.; Okada, T.; Jego, E.H.; Taniguchi, T.; Koshinaga, T.; Morioka, I. Visceral Fat Area Measured by Abdominal Bioelectrical Impedance Analysis in School-Aged Japanese Children. J. Clin. Med. 2022, 11, 4148. https://doi.org/10.3390/jcm11144148
Abe Y, Tonouchi R, Hara M, Okada T, Jego EH, Taniguchi T, Koshinaga T, Morioka I. Visceral Fat Area Measured by Abdominal Bioelectrical Impedance Analysis in School-Aged Japanese Children. Journal of Clinical Medicine. 2022; 11(14):4148. https://doi.org/10.3390/jcm11144148
Chicago/Turabian StyleAbe, Yuriko, Ryousuke Tonouchi, Mitsuhiko Hara, Tomoo Okada, Eric H. Jego, Tetsuya Taniguchi, Tsugumichi Koshinaga, and Ichiro Morioka. 2022. "Visceral Fat Area Measured by Abdominal Bioelectrical Impedance Analysis in School-Aged Japanese Children" Journal of Clinical Medicine 11, no. 14: 4148. https://doi.org/10.3390/jcm11144148
APA StyleAbe, Y., Tonouchi, R., Hara, M., Okada, T., Jego, E. H., Taniguchi, T., Koshinaga, T., & Morioka, I. (2022). Visceral Fat Area Measured by Abdominal Bioelectrical Impedance Analysis in School-Aged Japanese Children. Journal of Clinical Medicine, 11(14), 4148. https://doi.org/10.3390/jcm11144148






