More than Antibiotics: Latest Therapeutics in the Treatment and Prevention of Ocular Surface Infections
Abstract
:1. Introduction
2. Probiotics
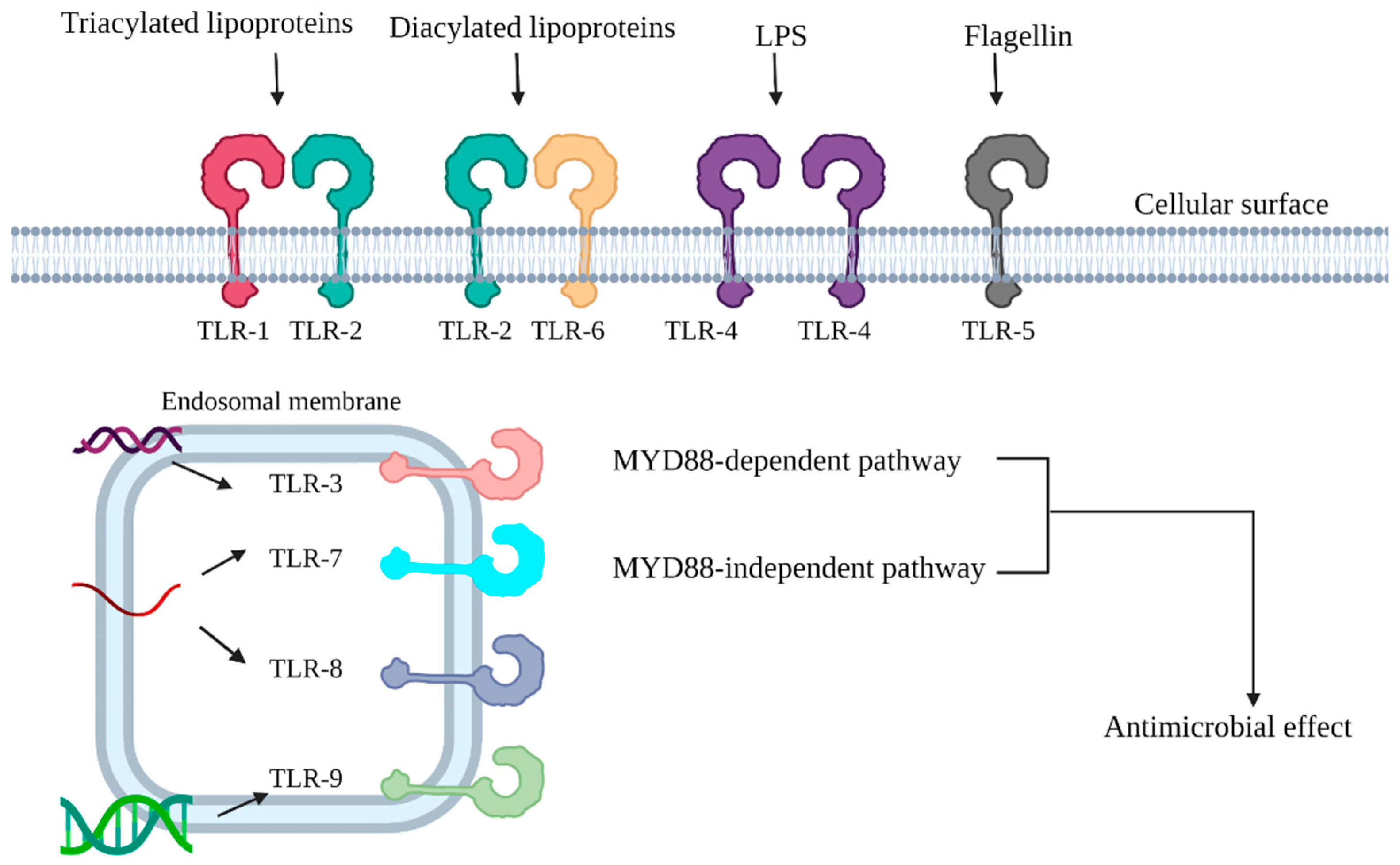
3. Toll-like Receptors (TLRs) and Prevention of Ocular Infection
4. Stem-Cell Therapy
4.1. MSCs—Tissue Repair
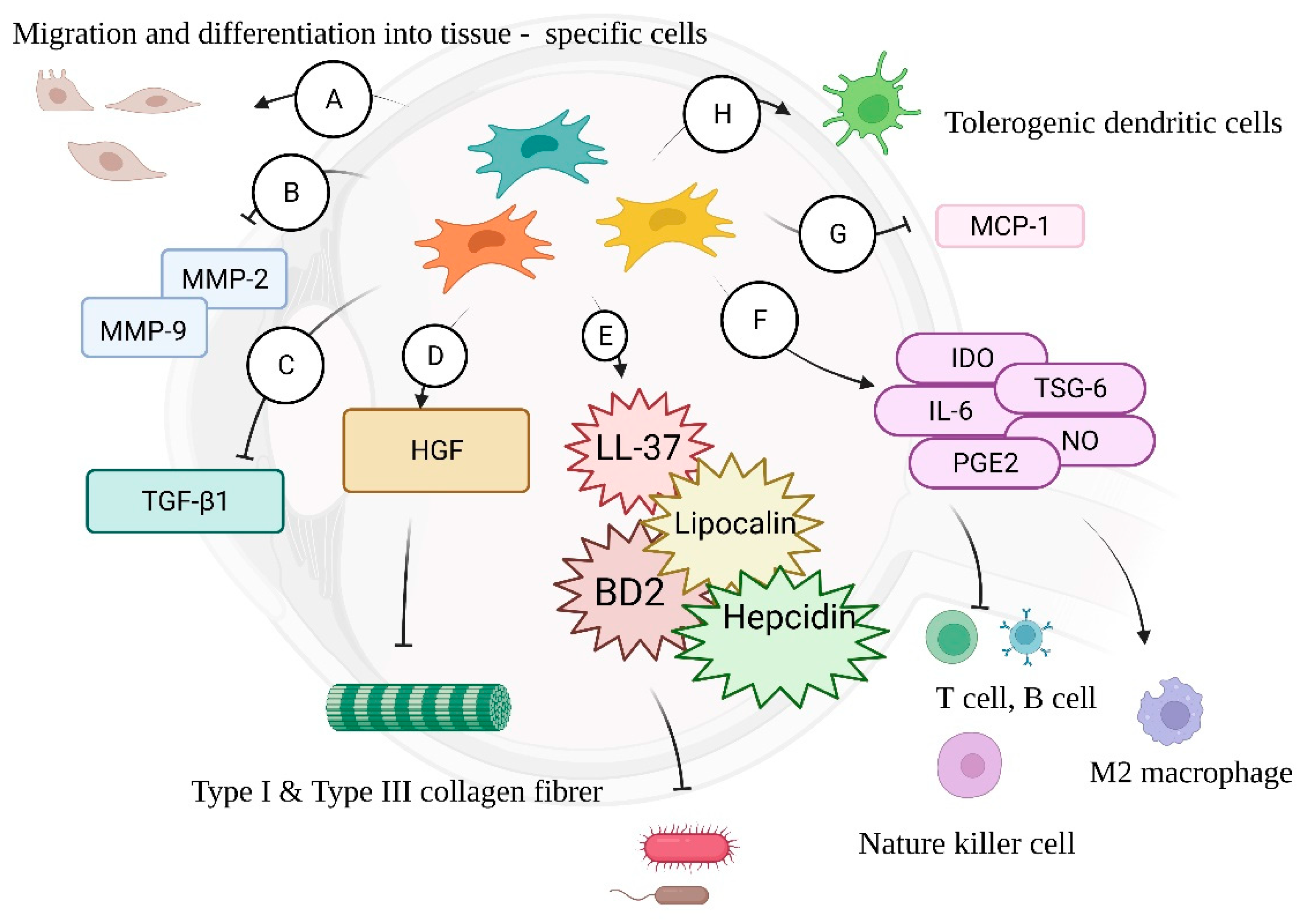
4.2. MSCs—Antimicrobial Properties
4.3. MSCs—Immunomodulation
4.4. MSCs—Limitations and Future Directions
5. siRNA
6. Cysteine Protease Inhibitors
7. Drug Delivery—In Situ Gels
7.1. pH-Sensitive In Situ Gels
7.2. Thermosensitive In Situ Gels
7.3. Ion-Sensitive In Situ Gels
8. Drug Delivery—Ocular Inserts
9. Drug Delivery—Nanofiber
10. Drug Delivery—Contact Lenses
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bispo, P.J.M.; Ung, L.; Chodosh, J.; Gilmore, M.S. Hospital-Associated Multidrug-Resistant MRSA Lineages Are Trophic to the Ocular Surface and Cause Severe Microbial Keratitis. Front. Public Health 2020, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Vazirani, J.; Wurity, S.; Ali, M.H. Multidrug-Resistant Pseudomonas aeruginosa Keratitis: Risk Factors, Clinical Characteristics, and Outcomes. Ophthalmology 2015, 122, 2110–2114. [Google Scholar] [CrossRef] [PubMed]
- Andrei, G.; Snoeck, R. Herpes simplex virus drug-resistance: New mutations and insights. Curr. Opin. Infect. Dis. 2013, 26, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Shum, C.; Martin, D.F.; Kuppermann, B.D.; Drew, W.L.; Margolis, T.P. Emergence of Drug-Resistant Cytomegalovirus Retinitis in the Contralateral Eyes of Patients with AIDS Treated with Ganciclovir. J. Infect. Dis. 2004, 189, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Rao, G.N. Corneal ulcer: Diagnosis and management. Community Eye Health 1999, 12, 21. [Google Scholar] [PubMed]
- El-Asrar, A.M.A.; Tabbara, K.F.; Al-Kharashi, S.A.; Geboes, K.; Missotten, L.; Desmet, V. Immunopathogenesis of conjunctival scarring in trachoma. Eye 1998, 12, 453–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedlander, M. Fibrosis and diseases of the eye. J. Clin. Investig. 2007, 117, 576–586. [Google Scholar] [CrossRef]
- Di Zazzo, A.; Gaudenzi, D.; Yin, J.; Coassin, M.; Fernandes, M.; Dana, R.; Bonini, S. Corneal angiogenic privilege and its failure. Exp. Eye Res. 2021, 204, 108457. [Google Scholar] [CrossRef]
- De Sordi, L.; Lourenço, M.; Debarbieux, L. The Battle Within: Interactions of Bacteriophages and Bacteria in the Gastrointestinal Tract. Cell Host Microbe 2019, 25, 210–218. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Oh, D.H.; Jung, J.Y.; Kim, J.C.; Jeon, C.O. Comparative Ocular Microbial Communities in Humans with and without Blepharitis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5585–5593. [Google Scholar] [CrossRef] [Green Version]
- Prashanthi, G.S.; Jayasudha, R.; Chakravarthy, S.K.; Padakandla, S.R.; SaiAbhilash, C.R.; Sharma, S.; Bagga, B.; Murthy, S.I.; Garg, P.; Shivaji, S. Alterations in the Ocular Surface Fungal Microbiome in Fungal Keratitis Patients. Microorganisms 2019, 7, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Holland, M.J.; Makalo, P.; Joof, H.; Roberts, C.H.; Mabey, D.C.; Bailey, R.L.; Burton, M.J.; Weinstock, G.M.; Burr, S.E. The conjunctival microbiome in health and trachomatous disease: A case control study. Genome Med. 2014, 6, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kugadas, A.; Christiansen, S.H.; Sankaranarayanan, S.; Surana, N.K.; Gauguet, S.; Kunz, R.; Fichorova, R.; Vorup-Jensen, T.; Gadjeva, M. Impact of Microbiota on Resistance to Ocular Pseudomonas aeruginosa-Induced Keratitis. PLoS Pathog. 2016, 12, e1005855. [Google Scholar] [CrossRef]
- Turner, P.V. The role of the gut microbiota on animal model reproducibility. Anim. Model Exp. Med. 2018, 1, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [Green Version]
- Jayasudha, R.; Chakravarthy, S.K.; Prashanthi, G.S.; Sharma, S.; Garg, P.; Murthy, S.I.; Shivaji, S. Alterations in gut bacterial and fungal microbiomes are associated with bacterial Keratitis, an inflammatory disease of the human eye. J. Biosci. 2018, 43, 835–856. [Google Scholar] [CrossRef]
- Williams, N.T. Probiotics. Am. J. Health-Syst. Pharm. 2010, 67, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeir, J.; De Vrese, M. Probiotics, prebiotics, and synbiotics—Approaching a definition. Am. J. Clin. Nutr. 2001, 73, 361s–364s. [Google Scholar] [CrossRef] [Green Version]
- Napolitano, P.; Filippelli, M.; Davinelli, S.; Bartollino, S.; Dell’Omo, R.; Costagliola, C. Influence of gut microbiota on eye diseases: An overview. Ann. Med. 2021, 53, 750–761. [Google Scholar] [CrossRef]
- Gálvez, A.; Abriouel, H.; López, R.L.; Omar, N.B. Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef]
- Fayol-Messaoudi, D.; Berger, C.N.; Coconnier-Polter, M.-H.; Moal, V.L.-L.; Servin, A.L. pH-, Lactic Acid-, and Non-Lactic Acid-Dependent Activities of Probiotic Lactobacilli against Salmonella enterica Serovar Typhimurium. Appl. Environ. Microbiol. 2005, 71, 6008–6013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aragona, P.; Baudouin, C.; Benitez Del Castillo, J.M.; Messmer, E.; Barabino, S.; Merayo-Lloves, J.; Brignole-Baudouin, F.; Inferrera, L.; Rolando, M.; Mencucci, R.; et al. The ocular microbiome and microbiota and their effects on ocular surface pathophysiology and disorders. Surv. Ophthalmol. 2021, 66, 907–925. [Google Scholar] [CrossRef] [PubMed]
- Akova, B.; Kıvanç, S.A.; Kıvanç, M. Antibiofilm effect of probiotic lactic acid bacteria against Bacillus spp obtained from the ocular surface. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7799–7805. [Google Scholar] [CrossRef] [PubMed]
- Ruíz, F.O.; Pascual, L.; Giordano, W.; Barberis, L. Bacteriocins and other bioactive substances of probiotic lactobacilli as biological weapons against Neisseria gonorrhoeae. Pathog. Dis. 2015, 73, ftv013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousavi, E.; Makvandi, M.; Teimoori, A.; Ataei, A.; Ghafari, S.; Samarbaf-Zadeh, A. Antiviral effects of Lactobacillus crispatus against HSV-2 in mammalian cell lines. J. Chin. Med. Assoc. 2018, 81, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Basualdo, J.; Sparo, M.; Chiodo, P.; Ciarmela, M.; Minvielle, M. Oral treatment with a potential probiotic (Enterococcus faecalis CECT 7121) appears to reduce the parasite burden of mice infected with Toxocara canis. Ann. Trop. Med. Parasitol. 2007, 101, 559–562. [Google Scholar] [CrossRef]
- Iovieno, A.; Lambiase, A.; Sacchetti, M.; Stampachiacchiere, B.; Micera, A.; Bonini, S. Preliminary evidence of the efficacy of probiotic eye-drop treatment in patients with vernal keratoconjunctivitis. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 435–441. [Google Scholar] [CrossRef]
- Erridge, C.; Bennett-Guerrero, E.; Poxton, I.R. Structure and function of lipopolysaccharides. Microbes Infect. 2002, 4, 837–851. [Google Scholar] [CrossRef]
- De Castro, C.; Parrilli, M.; Holst, O.; Molinaro, A. Chapter Five—Microbe-Associated Molecular Patterns in Innate Immunity: Extraction and Chemical Analysis of Gram-Negative Bacterial Lipopolysaccharides. In Methods in Enzymology; Fukuda, M., Ed.; Academic Press: Cambridge, MA, USA, 2010; Volume 480, pp. 89–115. [Google Scholar]
- Ray, A.; Cot, M.; Puzo, G.; Gilleron, M.; Nigou, J. Bacterial cell wall macroamphiphiles: Pathogen-/microbe-associated molecular patterns detected by mammalian innate immune system. Biochimie 2013, 95, 33–42. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMPs and DAMPs: Signal 0s that spur autophagy and immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef]
- Moresco, E.M.Y.; LaVine, D.; Beutler, B. Toll-like receptors. Curr. Biol. 2011, 21, R488–R493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, K.; Akira, S. TLR signaling pathways. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2004; pp. 3–9. [Google Scholar]
- Bagchi, A.; Herrup, E.A.; Warren, H.S.; Trigilio, J.; Shin, H.-S.; Valentine, C.; Hellman, J. MyD88-dependent and MyD88-independent pathways in synergy, priming, and tolerance between TLR agonists. J. Immunol. 2007, 178, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Triantafilou, M.; Gamper, F.G.J.; Haston, R.M.; Mouratis, M.A.; Morath, S.; Hartung, T.; Triantafilou, K. Membrane Sorting of Toll-like Receptor (TLR)-2/6 and TLR2/1 Heterodimers at the Cell Surface Determines Heterotypic Associations with CD36 and Intracellular Targeting*. J. Biol. Chem. 2006, 281, 31002–31011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schenk, M.; Belisle, J.T.; Modlin, R.L. TLR2 looks at lipoproteins. Immunity 2009, 31, 847–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacs-Simon, A.; Titball, R.W.; Michell, S.L. Lipoproteins of bacterial pathogens. Infect. Immun. 2011, 79, 548–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Xu, K.; Ambati, B.; Fu-Shin, X.Y. Toll-like receptor 5-mediated corneal epithelial inflammatory responses to Pseudomonas aeruginosa flagellin. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4247–4254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, G.C.; Sarkar, S.N. Transcriptional signaling by double-stranded RNA: Role of TLR3. Cytokine Growth Factor Rev. 2005, 16, 1–14. [Google Scholar] [CrossRef]
- Diebold, S.S.; Kaisho, T.; Hemmi, H.; Akira, S.; Reis e Sousa, C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 2004, 303, 1529–1531. [Google Scholar] [CrossRef]
- Latz, E.; Schoenemeyer, A.; Visintin, A.; Fitzgerald, K.A.; Monks, B.G.; Knetter, C.F.; Lien, E.; Nilsen, N.J.; Espevik, T.; Golenbock, D.T. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 2004, 5, 190–198. [Google Scholar] [CrossRef]
- Ueta, M.; Nochi, T.; Jang, M.H.; Park, E.J.; Igarashi, O.; Hino, A.; Kawasaki, S.; Shikina, T.; Hiroi, T.; Kinoshita, S.; et al. Intracellularly expressed TLR2s and TLR4s contribution to an immunosilent environment at the ocular mucosal epithelium. J. Immunol. 2004, 173, 3337–3347. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Kumar, A.; Wheater, M.; Yu, F.S.X. Lack of MD-2 expression in human corneal epithelial cells is an underlying mechanism of lipopolysaccharide (LPS) unresponsiveness. Immunol. Cell Biol. 2009, 87, 141–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Gao, N.; Standiford, T.J.; Gallo, R.L.; Fu-Shin, X.Y. Topical flagellin protects the injured corneas from Pseudomonas aeruginosa infection. Microbes Infect. 2010, 12, 978–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Singh, C.N.; Glybina, I.V.; Mahmoud, T.H.; Yu, F.S. Toll-like receptor 2 ligand-induced protection against bacterial endophthalmitis. J. Infect. Dis. 2010, 201, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.K.; Lopez-Collazo, E. Endotoxin tolerance: New mechanisms, molecules and clinical significance. Trends Immunol. 2009, 30, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B. SHIP, TGF-β, and endotoxin tolerance. Immunity 2004, 21, 134–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, L.A.J.; Bowie, A.G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 2007, 7, 353–364. [Google Scholar] [CrossRef]
- Pandey, R.K.; Yu, F.-S.; Kumar, A. Targeting toll-like receptor signaling as a novel approach to prevent ocular infectious diseases. Indian J. Med. Res. 2013, 138, 609. [Google Scholar]
- Chang, J.; McCluskey, P.; Wakefield, D. Toll-like receptors in ocular immunity and the immunopathogenesis of inflammatory eye disease. Br. J. Ophthalmol. 2006, 90, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Khatri, S.; Lass, J.H.; Heinzel, F.P.; Petroll, W.M.; Gomez, J.; Diaconu, E.; Kalsow, C.M.; Pearlman, E. Regulation of Endotoxin-Induced Keratitis by PECAM-1, MIP-2, and Toll-like Receptor 4. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2278–2284. [Google Scholar]
- Petropoulos, I.K.; Vantzou, C.V.; Lamari, F.N.; Karamanos, N.K.; Anastassiou, E.D.; Pharmakakis, N.M. Expression of TNF-alpha, IL-1beta, and IFN-gamma in Staphylococcus epidermidis slime-positive experimental endophthalmitis is closely related to clinical inflammatory scores. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 1322–1328. [Google Scholar] [CrossRef]
- Sampat, K.M.; Garg, S.J. Complications of intravitreal injections. Curr. Opin. Ophthalmol. 2010, 21, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Ramalho-Santos, M.; Willenbring, H. On the Origin of the Term “Stem Cell”. Cell Stem Cell 2007, 1, 35–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Miguel, M.P.; Fuentes-Julián, S.; Blázquez-Martínez, A.; Pascual, C.Y.; Aller, M.A.; Arias, J.; Arnalich-Montiel, F. Immunosuppressive properties of mesenchymal stem cells: Advances and applications. Curr. Mol. Med. 2012, 12, 574–591. [Google Scholar] [CrossRef]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef] [Green Version]
- Francois, S.; Mouiseddine, M.; Allenet-Lepage, B.; Voswinkel, J.; Douay, L.; Benderitter, M.; Chapel, A. Human mesenchymal stem cells provide protection against radiation-induced liver injury by antioxidative process, vasculature protection, hepatocyte differentiation, and trophic effects. Biomed. Res. Int. 2013, 2013, 151679. [Google Scholar] [CrossRef] [Green Version]
- Castanheira, P.; Torquetti, L.; Nehemy, M.B.; Goes, A.M. Retinal incorporation and differentiation of mesenchymal stem cells intravitreally injected in the injured retina of rats. Arq. Bras. Oftalmol. 2008, 71, 644–650. [Google Scholar] [CrossRef] [Green Version]
- Gnecchi, M.; He, H.; Noiseux, N.; Liang, O.D.; Zhang, L.; Morello, F.; Mu, H.; Melo, L.G.; Pratt, R.E.; Ingwall, J.S.; et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006, 20, 661–669. [Google Scholar] [CrossRef]
- Kusuma, G.D.; Carthew, J.; Lim, R.; Frith, J.E. Effect of the Microenvironment on Mesenchymal Stem Cell Paracrine Signaling: Opportunities to Engineer the Therapeutic Effect. Stem Cells Dev. 2017, 26, 617–631. [Google Scholar] [CrossRef]
- Gnecchi, M.; He, H.; Liang, O.D.; Melo, L.G.; Morello, F.; Mu, H.; Noiseux, N.; Zhang, L.; Pratt, R.E.; Ingwall, J.S.; et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat. Med. 2005, 11, 367–368. [Google Scholar] [CrossRef]
- Foidart, M.; Foidart, J.-M.; Engel, W.K. Collagen localization in normal and fibrotic human skeletal muscle. Arch. Neurol. 1981, 38, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Divanyan, A.; Jourd’heuil, F.L.; Goldman, R.D.; Ridge, K.M.; Jourd’heuil, D.; Lopez-Soler, R.I. Vimentin expression is required for the development of EMT-related renal fibrosis following unilateral ureteral obstruction in mice. Am. J. Physiol.-Ren. Physiol. 2018, 315, F769–F780. [Google Scholar] [CrossRef]
- Mou, S.; Wang, Q.; Shi, B.; Gu, L.; Ni, Z. Hepatocyte growth factor suppresses transforming growth factor-beta-1 and type III collagen in human primary renal fibroblasts. Kaohsiung J. Med. Sci. 2009, 25, 577–587. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.-H.; Liu, A.-R.; Qiu, H.-B.; Yang, Y. Interaction between mesenchymal stem cells and endothelial cells restores endothelial permeability via paracrine hepatocyte growth factor in vitro. Stem Cell Res. Ther. 2015, 6, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schievenbusch, S.; Strack, I.; Scheffler, M.; Wennhold, K.; Maurer, J.; Nischt, R.; Dienes, H.P.; Odenthal, M. Profiling of anti-fibrotic signaling by hepatocyte growth factor in renal fibroblasts. Biochem. Biophys. Res. Commun. 2009, 385, 55–61. [Google Scholar] [CrossRef]
- Corbel, M.; Boichot, E.; Lagente, V. Role of gelatinases MMP-2 and MMP-9 in tissue remodeling following acute lung injury. Braz. J. Med. Biol. Res. 2000, 33, 749–754. [Google Scholar] [CrossRef] [Green Version]
- Nagaya, N.; Kangawa, K.; Itoh, T.; Iwase, T.; Murakami, S.; Miyahara, Y.; Fujii, T.; Uematsu, M.; Ohgushi, H.; Yamagishi, M.; et al. Transplantation of Mesenchymal Stem Cells Improves Cardiac Function in a Rat Model of Dilated Cardiomyopathy. Circulation 2005, 112, 1128–1135. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Chen, Y.; Wang, S.; Qin, F.; Wang, L. MSCs helped reduce scarring in the cornea after fungal infection when combined with anti-fungal treatment. BMC Ophthalmol. 2019, 19, 226. [Google Scholar] [CrossRef]
- Ueno, T.; Nakashima, A.; Doi, S.; Kawamoto, T.; Honda, K.; Yokoyama, Y.; Doi, T.; Higashi, Y.; Yorioka, N.; Kato, Y.; et al. Mesenchymal stem cells ameliorate experimental peritoneal fibrosis by suppressing inflammation and inhibiting TGF-β1 signaling. Kidney Int. 2013, 84, 297–307. [Google Scholar] [CrossRef] [Green Version]
- Krasnodembskaya, A.; Song, Y.; Fang, X.; Gupta, N.; Serikov, V.; Lee, J.-W.; Matthay, M.A. Antibacterial Effect of Human Mesenchymal Stem Cells Is Mediated in Part from Secretion of the Antimicrobial Peptide LL-37. Stem Cells 2010, 28, 2229–2238. [Google Scholar] [CrossRef] [Green Version]
- Sung, D.K.; Chang, Y.S.; Sung, S.I.; Yoo, H.S.; Ahn, S.Y.; Park, W.S. Antibacterial effect of mesenchymal stem cells against Escherichia coli is mediated by secretion of beta- defensin- 2 via toll- like receptor 4 signalling. Cell. Microbiol. 2016, 18, 424–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goetz, D.H.; Holmes, M.A.; Borregaard, N.; Bluhm, M.E.; Raymond, K.N.; Strong, R.K. The Neutrophil Lipocalin NGAL Is a Bacteriostatic Agent that Interferes with Siderophore-Mediated Iron Acquisition. Mol. Cell 2002, 10, 1033–1043. [Google Scholar] [CrossRef]
- Borregaard, N.; Cowland, J.B. Neutrophil gelatinase-associated lipocalin, a siderophore-binding eukaryotic protein. Biometals 2006, 19, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Maisetta, G.; Petruzzelli, R.; Brancatisano, F.L.; Esin, S.; Vitali, A.; Campa, M.; Batoni, G. Antimicrobial activity of human hepcidin 20 and 25 against clinically relevant bacterial strains: Effect of copper and acidic pH. Peptides 2010, 31, 1995–2002. [Google Scholar] [CrossRef]
- Alcayaga-Miranda, F.; Cuenca, J.; Martin, A.; Contreras, L.; Figueroa, F.E.; Khoury, M. Combination therapy of menstrual derived mesenchymal stem cells and antibiotics ameliorates survival in sepsis. Stem Cell Res. Ther. 2015, 6, 199. [Google Scholar] [CrossRef] [Green Version]
- Meisel, R.; Brockers, S.; Heseler, K.; Degistirici, Ö.; Bülle, H.; Woite, C.; Stuhlsatz, S.; Schwippert, W.; Jäger, M.; Sorg, R.; et al. Human but not murine multipotent mesenchymal stromal cells exhibit broad-spectrum antimicrobial effector function mediated by indoleamine 2,3-dioxygenase. Leukemia 2011, 25, 648–654. [Google Scholar] [CrossRef]
- Holland, E.J.; Fingeret, M.; Mah, F.S. Use of Topical Steroids in Conjunctivitis: A Review of the Evidence. Cornea 2019, 38, 1062–1067. [Google Scholar] [CrossRef]
- Talamo, J.H.; Gollamudi, S.; Green, W.R.; De La Cruz, Z.; Filatov, V.; Stark, W.J. Modulation of Corneal Wound Healing After Excimer Laser Keratomileusis Using Topical Mitomycin C and Steroids. Arch. Ophthalmol. 1991, 109, 1141–1146. [Google Scholar] [CrossRef]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef]
- Asari, S.; Itakura, S.; Ferreri, K.; Liu, C.P.; Kuroda, Y.; Kandeel, F.; Mullen, Y. Mesenchymal stem cells suppress B-cell terminal differentiation. Exp. Hematol. 2009, 37, 604–615. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Liu, J.; Liu, Y.; Qin, Y.; Luo, Q.; Wang, Q.; Duan, H. TLR4 plays a crucial role in MSC-induced inhibition of NK cell function. Biochem. Biophys. Res. Commun. 2015, 464, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Bouffi, C.; Bony, C.; Courties, G.; Jorgensen, C.; Noël, D. IL-6-dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS ONE 2010, 5, e14247. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lee, R.H.; Bazhanov, N.; Oh, J.Y.; Prockop, D.J. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood J. Am. Soc. Hematol. 2011, 118, 330–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Ren, G.; Huang, Y.; Su, J.; Han, Y.; Li, J.; Chen, X.; Cao, K.; Chen, Q.; Shou, P. Mesenchymal stem cells: A double-edged sword in regulating immune responses. Cell Death Differ. 2012, 19, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- François, M.; Romieu-Mourez, R.; Li, M.; Galipeau, J. Human MSC Suppression Correlates With Cytokine Induction of Indoleamine 2,3-Dioxygenase and Bystander M2 Macrophage Differentiation. Mol. Ther. 2012, 20, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Galleu, A.; Riffo-Vasquez, Y.; Trento, C.; Lomas, C.; Dolcetti, L.; Cheung, T.S.; von Bonin, M.; Barbieri, L.; Halai, K.; Ward, S.; et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 2017, 9, eaam7828. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.; Ha, S.-J. Generation of tolerogenic dendritic cells and their therapeutic applications. Immune Netw. 2016, 16, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Shahir, M.; Mahmoud Hashemi, S.; Asadirad, A.; Varahram, M.; Kazempour-Dizaji, M.; Folkerts, G.; Garssen, J.; Adcock, I.; Mortaz, E. Effect of mesenchymal stem cell-derived exosomes on the induction of mouse tolerogenic dendritic cells. J. Cell. Physiol. 2020, 235, 7043–7055. [Google Scholar] [CrossRef] [Green Version]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef]
- Yu, B.; Shao, H.; Su, C.; Jiang, Y.; Chen, X.; Bai, L.; Zhang, Y.; Li, Q.; Zhang, X.; Li, X. Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci. Rep. 2016, 6, 34562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermankova, B.; Kossl, J.; Bohacova, P.; Javorkova, E.; Hajkova, M.; Krulova, M.; Zajicova, A.; Holan, V. The Immunomodulatory Potential of Mesenchymal Stem Cells in a Retinal Inflammatory Environment. Stem Cell Rev. Rep. 2019, 15, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Frisch, S.M.; Screaton, R.A. Anoikis mechanisms. Curr. Opin. Cell Biol. 2001, 13, 555–562. [Google Scholar] [CrossRef]
- Tsuji, K.; Ojima, M.; Otabe, K.; Horie, M.; Koga, H.; Sekiya, I.; Muneta, T. Effects of different cell-detaching methods on the viability and cell surface antigen expression of synovial mesenchymal stem cells. Cell Transplant. 2017, 26, 1089–1102. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Choi, E.; Cha, M.J.; Hwang, K.C. Cell adhesion and long-term survival of transplanted mesenchymal stem cells: A prerequisite for cell therapy. Oxid. Med. Cell Longev. 2015, 2015, 632902. [Google Scholar] [CrossRef] [Green Version]
- Robey, T.E.; Saiget, M.K.; Reinecke, H.; Murry, C.E. Systems approaches to preventing transplanted cell death in cardiac repair. J. Mol. Cell. Cardiol. 2008, 45, 567–581. [Google Scholar] [CrossRef] [Green Version]
- Bollini, S.; Vieira, J.M.; Howard, S.; Dubè, K.N.; Balmer, G.M.; Smart, N.; Riley, P.R. Re-activated adult epicardial progenitor cells are a heterogeneous population molecularly distinct from their embryonic counterparts. Stem Cells Dev. 2014, 23, 1719–1730. [Google Scholar] [CrossRef]
- Yang, Y.J.; Qian, H.Y.; Huang, J.; Geng, Y.J.; Gao, R.L.; Dou, K.F.; Yang, G.S.; Li, J.J.; Shen, R.; He, Z.X.; et al. Atorvastatin treatment improves survival and effects of implanted mesenchymal stem cells in post-infarct swine hearts. Eur. Heart J. 2008, 29, 1578–1590. [Google Scholar] [CrossRef] [Green Version]
- Mias, C.; Trouche, E.; Seguelas, M.H.; Calcagno, F.; Dignat-George, F.; Sabatier, F.; Piercecchi-Marti, M.D.; Daniel, L.; Bianchi, P.; Calise, D.; et al. Ex vivo pretreatment with melatonin improves survival, proangiogenic/mitogenic activity, and efficiency of mesenchymal stem cells injected into ischemic kidney. Stem Cells 2008, 26, 1749–1757. [Google Scholar] [CrossRef]
- Yu, X.; Cohen, D.M.; Chen, C.S. miR-125b Is an adhesion-regulated microRNA that protects mesenchymal stem cells from anoikis. Stem Cells 2012, 30, 956–964. [Google Scholar] [CrossRef] [Green Version]
- White, M.L.; Chodosh, J. Herpes simplex virus keratitis: A treatment guideline. In Hoskins Center for Quality Eye Care and American Academy of Ophthalmology Website; American Academy of Opthalmology: San Francisco, CA, USA, 2014. [Google Scholar]
- Krieg, A.M.; Yi, A.-K.; Matson, S.; Waldschmidt, T.J.; Bishop, G.A.; Teasdale, R.; Koretzky, G.A.; Klinman, D.M. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 1995, 374, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Krieg, A.M. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 2002, 20, 709–760. [Google Scholar] [CrossRef] [PubMed]
- Schnare, M.; Holt, A.C.; Takeda, K.; Akira, S.; Medzhitov, R. Recognition of CpG DNA is mediated by signaling pathways dependent on the adaptor protein MyD88. Curr. Biol. 2000, 10, 1139–1142. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Klinman, D.M.; Gierynska, M.; Rouse, B.T. DNA containing CpG motifs induces angiogenesis. Proc. Natl. Acad. Sci. USA 2002, 99, 8944–8949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chernikov, I.V.; Vlassov, V.V.; Chernolovskaya, E.L. Current Development of siRNA Bioconjugates: From Research to the Clinic. Front. Pharmacol. 2019, 10, 444. [Google Scholar] [CrossRef] [Green Version]
- Filipowicz, W.; Jaskiewicz, L.; Kolb, F.A.; Pillai, R.S. Post-transcriptional gene silencing by siRNAs and miRNAs. Curr. Opin. Struct. Biol. 2005, 15, 331–341. [Google Scholar] [CrossRef]
- Kim, B.; Tang, Q.; Biswas, P.S.; Xu, J.; Schiffelers, R.M.; Xie, F.Y.; Ansari, A.M.; Scaria, P.V.; Woodle, M.C.; Lu, P.; et al. Inhibition of Ocular Angiogenesis by siRNA Targeting Vascular Endothelial Growth Factor Pathway Genes: Therapeutic Strategy for Herpetic Stromal Keratitis. Am. J. Pathol. 2004, 165, 2177–2185. [Google Scholar] [CrossRef]
- Pratt, A.J.; MacRae, I.J. The RNA-induced Silencing Complex: A Versatile Gene-silencing Machine *. J. Biol. Chem. 2009, 284, 17897–17901. [Google Scholar] [CrossRef] [Green Version]
- Siomi, H.; Siomi, M.C. On the road to reading the RNA-interference code. Nature 2009, 457, 396–404. [Google Scholar] [CrossRef]
- Preall, J.B.; He, Z.; Gorra, J.M.; Sontheimer, E.J. Short interfering RNA strand selection is independent of dsRNA processing polarity during RNAi in Drosophila. Curr. Biol. 2006, 16, 530–535. [Google Scholar] [CrossRef] [Green Version]
- Gregory, R.I.; Chendrimada, T.P.; Cooch, N.; Shiekhattar, R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell 2005, 123, 631–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking down barriers: Advances in siRNA delivery. Nat. Rev. Drug Discov. 2009, 8, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.S.; Jain, A.; Zhao, Z.; Cheng, K. Intracellular trafficking and exocytosis of a multi-component siRNA nanocomplex. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1323–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, A.L.; Linsley, P.S. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat. Rev. Drug Discov. 2010, 9, 57–67. [Google Scholar] [CrossRef]
- Fedorov, Y.; Anderson, E.M.; Birmingham, A.; Reynolds, A.; Karpilow, J.; Robinson, K.; Leake, D.; Marshall, W.S.; Khvorova, A. Off-target effects by siRNA can induce toxic phenotype. RNA 2006, 12, 1188–1196. [Google Scholar] [CrossRef] [Green Version]
- Deleavey, G.F.; Watts, J.K.; Damha, M.J. Chemical Modification of siRNA. Curr. Protoc. Nucleic Acid Chem. 2009, 39, nc1603s39. [Google Scholar] [CrossRef]
- Davis, M.E. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: From concept to clinic. Mol. Pharm. 2009, 6, 659–668. [Google Scholar] [CrossRef]
- Akinc, A.; Goldberg, M.; Qin, J.; Dorkin, J.R.; Gamba-Vitalo, C.; Maier, M.; Jayaprakash, K.N.; Jayaraman, M.; Rajeev, K.G.; Manoharan, M. Development of lipidoid–siRNA formulations for systemic delivery to the liver. Mol. Ther. 2009, 17, 872–879. [Google Scholar] [CrossRef]
- Bolaños-Jiménez, R.; Navas, A.; López-Lizárraga, E.P.; De Ribot, F.M.; Peña, A.; Graue-Hernández, E.O.; Garfias, Y. Ocular surface as barrier of innate immunity. Open Ophthalmol. J. 2015, 9, 49. [Google Scholar] [CrossRef] [Green Version]
- Ni, M.; Evans, D.J.; Hawgood, S.; Anders, E.M.; Sack, R.A.; Fleiszig, S.M. Surfactant protein D is present in human tear fluid and the cornea and inhibits epithelial cell invasion by Pseudomonas aeruginosa. Infect. Immun. 2005, 73, 2147–2156. [Google Scholar] [CrossRef] [Green Version]
- Hou, X.; Zhang, X.; Zhang, Z. Role of surfactant protein-D in ocular bacterial infection. Int. Ophthalmol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Hoge, R.; Pelzer, A.; Rosenau, F.; Wilhelm, S. Weapons of a pathogen: Proteases and their role in virulence of Pseudomonas aeruginosa. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 2, 383–395. [Google Scholar]
- Zhang, Z.; Abdel-Razek, O.; Hawgood, S.; Wang, G. Protective Role of Surfactant Protein D in Ocular Staphylococcus aureus Infection. PLoS ONE 2015, 10, e0138597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sieg, J.W.; Robinson, J.R. Mechanistic studies on transcorneal permeation of pilocarpine. J. Pharm. Sci. 1976, 65, 1816–1822. [Google Scholar] [CrossRef]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47. [Google Scholar] [CrossRef]
- Topical Ocular Drug Delivery: Recent Developments and Future Challenges. J. Ocul. Pharmacol. Ther. 1986, 2, 67–108. [CrossRef]
- Mythili, L.; Ganesh, G.; Monisha, C.; Kayalvizhi, R. Ocular Drug Delivery System—An Update Review. Res. J. Pharm. Technol. 2019, 12, 2527–2538. [Google Scholar] [CrossRef]
- Nirmal, H.B.; Bakliwal, S.; Pawar, S. In-situ gel: New trends in controlled and sustained drug delivery system. Int. J. PharmTech Res. 2010, 2, 1398–1408. [Google Scholar]
- Makwana, S.B.; Patel, V.A.; Parmar, S.J. Development and characterization of in-situ gel for ophthalmic formulation containing ciprofloxacin hydrochloride. Results Pharma Sci. 2016, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Rajput, R.; Singh, S. The use of in situ hydrogel in ocular drug delivery. IJPPR 2016, 7, 1320–1325. [Google Scholar]
- Wu, Y.; Liu, Y.; Li, X.; Kebebe, D.; Zhang, B.; Ren, J.; Lu, J.; Li, J.; Du, S.; Liu, Z. Research progress of in-situ gelling ophthalmic drug delivery system. Asian J. Pharm. Sci. 2019, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, H.; Okeke, C.I.; Li, L.; Liu, Z.; Yin, Z.; Zhuang, P.; Sun, J.; Wu, T.; Wang, M.; et al. Comparison of systemic absorption between ofloxacin ophthalmic in situ gels and ofloxacin conventional ophthalmic solutions administration to rabbit eyes by HPLC–MS/MS. Int. J. Pharm. 2013, 450, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Irimia, T.; Dinu-Pîrvu, C.-E.; Ghica, M.V.; Lupuleasa, D.; Muntean, D.-L.; Udeanu, D.I.; Popa, L. Chitosan-Based In Situ Gels for Ocular Delivery of Therapeutics: A State-of-the-Art Review. Mar. Drugs 2018, 16, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016, 10, 483. [Google Scholar] [CrossRef] [Green Version]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Vyas, S.P. Carbopol/Chitosan Based pH Triggered In Situ Gelling System for Ocular Delivery of Timolol Maleate. Sci. Pharm. 2010, 78, 959–976. [Google Scholar] [CrossRef] [Green Version]
- Burka, J.M.; Bower, K.S.; Vanroekel, R.C.; Stutzman, R.D.; Kuzmowych, C.P.; Howard, R.S. The effect of fourth-generation fluoroquinolones gatifloxacin and moxifloxacin on epithelial healing following photorefractive keratectomy. Am. J. Ophthalmol. 2005, 140, 83–87. [Google Scholar] [CrossRef] [Green Version]
- Abdul Malik, P.H.; Satyananda, S. pH-induced in situ gelling system of an anti-infective drug for sustained ocular delivery. J. Appl. Pharm. Sci. 2014, 4, 101–104. [Google Scholar]
- Gong, C.; Qi, T.; Wei, X.; Qu, Y.; Wu, Q.; Luo, F.; Qian, Z. Thermosensitive polymeric hydrogels as drug delivery systems. Curr. Med. Chem. 2013, 20, 79–94. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Tsai, T.-H.; Jhan, Y.-Y.; Chiu, A.W.-H.; Tsai, K.-L.; Chien, C.-S.; Chiou, S.-H.; Liu, C.J.-L. Thermosensitive chitosan-based hydrogel as a topical ocular drug delivery system of latanoprost for glaucoma treatment. Carbohydr. Polym. 2016, 144, 390–399. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Woung, L.-C.; Yen, J.-C.; Tseng, P.-C.; Chiou, S.-H.; Sung, Y.-J.; Liu, K.-T.; Cheng, Y.-H. Thermosensitive chitosan-based hydrogels for sustained release of ferulic acid on corneal wound healing. Carbohydr. Polym. 2016, 135, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Nagai, N.; Saijo, S.; Kaji, H.; Nishizawa, M.; Abe, T. In situ formation of injectable chitosan-gelatin hydrogels through double crosslinking for sustained intraocular drug delivery. Mater. Sci. Eng. C 2018, 88, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zuo, Z.; Cheung, C.K.C.; Leung, S.S.Y. Updates on thermosensitive hydrogel for nasal, ocular and cutaneous delivery. Int. J. Pharm. 2019, 559, 86–101. [Google Scholar] [CrossRef]
- Cho, I.S.; Park, C.G.; Huh, B.K.; Cho, M.O.; Khatun, Z.; Li, Z.; Kang, S.-W.; Choy, Y.B.; Huh, K.M. Thermosensitive hexanoyl glycol chitosan-based ocular delivery system for glaucoma therapy. Acta Biomater. 2016, 39, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Xu, W.; Zhang, C.; Kong, W. Chitosan temperature-sensitive gel loaded with drug microspheres has excellent effectiveness, biocompatibility and safety as an ophthalmic drug delivery system. Exp. Ther. Med. 2018, 15, 1442–1448. [Google Scholar] [CrossRef] [Green Version]
- Fabiano, A.; Bizzarri, R.; Zambito, Y. Thermosensitive hydrogel based on chitosan and its derivatives containing medicated nanoparticles for transcorneal administration of 5-fluorouracil. Int. J. Nanomed. 2017, 12, 633. [Google Scholar] [CrossRef] [Green Version]
- Abbas, M.N.; Khan, S.A.; Sadozai, S.K.; Khalil, I.A.; Anter, A.; Fouly, M.E.; Osman, A.H.; Kazi, M. Nanoparticles Loaded Thermoresponsive In Situ Gel for Ocular Antibiotic Delivery against Bacterial Keratitis. Polymers 2022, 14, 1135. [Google Scholar] [CrossRef]
- Rupenthal, I.D.; Green, C.R.; Alany, R.G. Comparison of ion-activated in situ gelling systems for ocular drug delivery. Part 2: Precorneal retention and in vivo pharmacodynamic study. Int. J. Pharm. 2011, 411, 78–85. [Google Scholar] [CrossRef]
- Milas, M.; Shi, X.; Rinaudo, M. On the physicochemical properties of gellan gum. Biopolymers 1990, 30, 451–464. [Google Scholar] [CrossRef]
- Das, M.; Giri, T.K. Hydrogels based on gellan gum in cell delivery and drug delivery. J. Drug Deliv. Sci. Technol. 2020, 56, 101586. [Google Scholar] [CrossRef]
- Maiti, S.; Ranjit, S.; Mondol, R.; Ray, S.; Sa, B. Al+ 3 ion cross-linked and acetalated gellan hydrogel network beads for prolonged release of glipizide. Carbohydr. Polym. 2011, 85, 164–172. [Google Scholar] [CrossRef]
- Osmałek, T.; Froelich, A.; Tasarek, S. Application of gellan gum in pharmacy and medicine. Int. J. Pharm. 2014, 466, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.R.; Nishinari, K.; Rinaudo, M. Gelation of gellan—A review. Food Hydrocoll. 2012, 28, 373–411. [Google Scholar] [CrossRef]
- Geethalakshmi, A.; Karki, R.; Jha, S.K.; Venkatesh, D.P.; Nikunj, B. Sustained ocular delivery of brimonidine tartrate using ion activated in situ gelling system. Curr. Drug Deliv. 2012, 9, 197–204. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, Z. A novel ocular delivery of brinzolamide based on gellan gum: In vitro and in vivo evaluation. Drug Des. Dev. Ther. 2018, 12, 383–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Ferreiro, A.; Bargiela, N.F.; Varela, M.S.; Martínez, M.G.; Pardo, M.; Ces, A.P.; Méndez, J.B.; Barcia, M.G.; Lamas, M.J.; Otero-Espinar, F.J. Cyclodextrin–polysaccharide-based, in situ-gelled system for ocular antifungal delivery. Beilstein J. Org. Chem. 2014, 10, 2903–2911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tayel, S.A.; El-Nabarawi, M.A.; Tadros, M.I.; Abd-Elsalam, W.H. Promising ion-sensitive in situ ocular nanoemulsion gels of terbinafine hydrochloride: Design, in vitro characterization and in vivo estimation of the ocular irritation and drug pharmacokinetics in the aqueous humor of rabbits. Int. J. Pharm. 2013, 443, 293–305. [Google Scholar] [CrossRef]
- Hecht, H.; Srebnik, S. Structural Characterization of Sodium Alginate and Calcium Alginate. Biomacromolecules 2016, 17, 2160–2167. [Google Scholar] [CrossRef]
- Pahuja, P.; Arora, S.; Pawar, P. Ocular drug delivery system: A reference to natural polymers. Expert Opin. Drug Deliv. 2012, 9, 837–861. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Nie, S.; Liu, H.; Ding, P.; Pan, W. Study of an alginate/HPMC-based in situ gelling ophthalmic delivery system for gatifloxacin. Int. J. Pharm. 2006, 315, 12–17. [Google Scholar] [CrossRef]
- Chang, M.-C.; Kuo, Y.-J.; Hung, K.-H.; Peng, C.-L.; Chen, K.-Y.; Yeh, L.-K. Liposomal dexamethasone–moxifloxacin nanoparticle combinations with collagen/gelatin/alginate hydrogel for corneal infection treatment and wound healing. Biomed. Mater. 2020, 15, 055022. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Silva, N.; Sarmento, B.; Pintado, M. Potential chitosan-coated alginate nanoparticles for ocular delivery of daptomycin. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- MohammadSadeghi, A.; Farjadian, F.; Alipour, S. Sustained release of linezolid in ocular insert based on lipophilic modified structure of sodium alginate. Iran. J. Basic Med. Sci. 2021, 24, 331. [Google Scholar] [PubMed]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in biomedical applications of pectin gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Vijaya, C.; Goud, K.S. Ion-activated in situ gelling ophthalmic delivery systems of azithromycin. Indian J. Pharm. Sci. 2011, 73, 615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, A.; Sharma, P.K.; Garg, V.K.; Garg, G. Ocular inserts—Advancement in therapy of eye diseases. J. Adv. Pharm. Technol. Res. 2010, 1, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Gurtler, F.; Gurny, R. Patent literature review of ophthalmic inserts. Drug Dev. Ind. Pharm. 1995, 21, 1–18. [Google Scholar] [CrossRef]
- Devhadrao, N.; Siddhaia, M. Review on ocular insert drug delivery system. J. Drug Deliv. Ther. 2018, 8, 115–121. [Google Scholar] [CrossRef]
- Kaul, S.; Kumar, G.; Kothiyal, P. An insight into ocular insert. Int. J. Pharm. Sci. Res. 2012, 3, 1905. [Google Scholar]
- Attia, M.A.; Al-Azizi, M.; Hashish, M.S. Design and evaluation of ciprofloxacin hydrochloride ocular inserts. Int. J. PharmTech Res. 2011, 3, 1750–1763. [Google Scholar]
- Rao, P.C.M.; Nappinnai, M.; Raju, S.; Rao, U.M.V.; Reddy, V.B. Fluconazole Ocular Inserts: Formulation and In-Vitro Evaluation. J. Pharm. Sci. Res. 2010, 2, 344–350. [Google Scholar]
- Taghe, S.; Mirzaeei, S.; Alany, R.G.; Nokhodchi, A. Polymeric inserts containing Eudragit® L100 nanoparticle for improved ocular delivery of azithromycin. Biomedicines 2020, 8, 466. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Panwar, A.S.; Dwivedi, P.; Jain, P.; Agrawal, A.; Jain, D. Design and evaluation of Ocular Inserts for controlled drug delivery of Acyclovir. Int. J. Pharm. Biol. Arch. 2011, 2, 1106–1110. [Google Scholar]
- Friedrich, S.W.; Saville, B.A.; Cheng, Y.-L.; Rootman, D.S. Pharmacokinetic differences between ocular inserts and eyedrops. J. Ocul. Pharmacol. Ther. 1996, 12, 5–18. [Google Scholar] [CrossRef]
- Deepak, A.; Goyal, A.K.; Rath, G. Nanofiber in transmucosal drug delivery. J. Drug Deliv. Sci. Technol. 2018, 43, 379–387. [Google Scholar] [CrossRef]
- Tawfik, E.A.; Alshamsan, A.; Kalam, M.A.; Raish, M.; Alkholief, M.; Stapleton, P.; Harvey, K.; Craig, D.Q.; Barker, S.A. In vitro and in vivo biological assessment of dual drug-loaded coaxial nanofibers for the treatment of corneal abrasion. Int. J. Pharm. 2021, 604, 120732. [Google Scholar] [CrossRef] [PubMed]
- Mirzaeei, S.; Barfar, D. Design and development of antibacterial/anti-inflammatory dual drug-loaded nanofibrous inserts for ophthalmic sustained delivery of gentamicin and methylprednisolone: In vitro bioassay, solvent, and method effects’ evaluation. Adv. Pharm. Bull. 2021, 12, 531–540. [Google Scholar] [CrossRef]
- Mirzaeei, S.; Taghe, S.; Asare-Addo, K.; Nokhodchi, A. Polyvinyl alcohol/chitosan single-layered and polyvinyl alcohol/chitosan/eudragit rl100 multi-layered electrospun nanofibers as an ocular matrix for the controlled release of ofloxacin: An in vitro and in vivo evaluation. AAPS PharmSciTech 2021, 22, 170. [Google Scholar] [CrossRef]
- Hui, A.; Willcox, M.; Jones, L. In vitro and in vivo evaluation of novel ciprofloxacin-releasing silicone hydrogel contact lenses. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4896–4904. [Google Scholar] [CrossRef]
- Gade, S.K.; Nirmal, J.; Garg, P.; Venuganti, V.V.K. Corneal delivery of moxifloxacin and dexamethasone combination using drug-eluting mucoadhesive contact lens to treat ocular infections. Int. J. Pharm. 2020, 591, 120023. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, M.-C.; Chern, E. More than Antibiotics: Latest Therapeutics in the Treatment and Prevention of Ocular Surface Infections. J. Clin. Med. 2022, 11, 4195. https://doi.org/10.3390/jcm11144195
Chiang M-C, Chern E. More than Antibiotics: Latest Therapeutics in the Treatment and Prevention of Ocular Surface Infections. Journal of Clinical Medicine. 2022; 11(14):4195. https://doi.org/10.3390/jcm11144195
Chicago/Turabian StyleChiang, Ming-Cheng, and Edward Chern. 2022. "More than Antibiotics: Latest Therapeutics in the Treatment and Prevention of Ocular Surface Infections" Journal of Clinical Medicine 11, no. 14: 4195. https://doi.org/10.3390/jcm11144195
APA StyleChiang, M.-C., & Chern, E. (2022). More than Antibiotics: Latest Therapeutics in the Treatment and Prevention of Ocular Surface Infections. Journal of Clinical Medicine, 11(14), 4195. https://doi.org/10.3390/jcm11144195





