Prognostic Implications of the Novel Pulmonary Hypertension Definition in Patients with Aortic Stenosis after Transcatheter Valve Replacement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Invasive Hemodynamics
2.3. Baseline Echocardiographic Assessment
2.4. Procedure Characteristics
2.5. Follow-Up
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
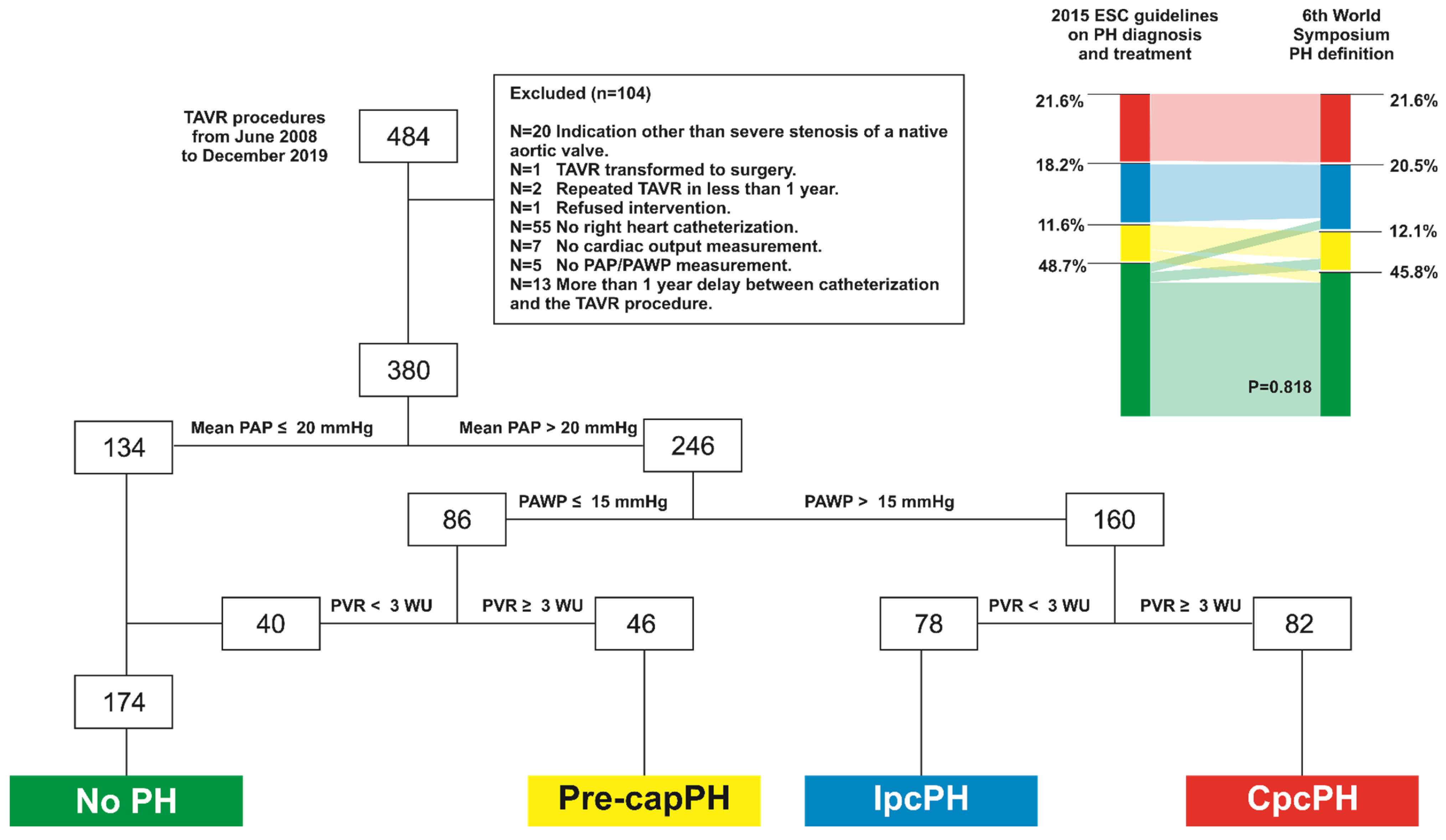
3.2. 2015. ESC Guidelines vs. 6th World Symposium PH Definition Groups
3.3. Echocardiographic and Heart Catheterization Parameters
3.4. TAVR Intervention
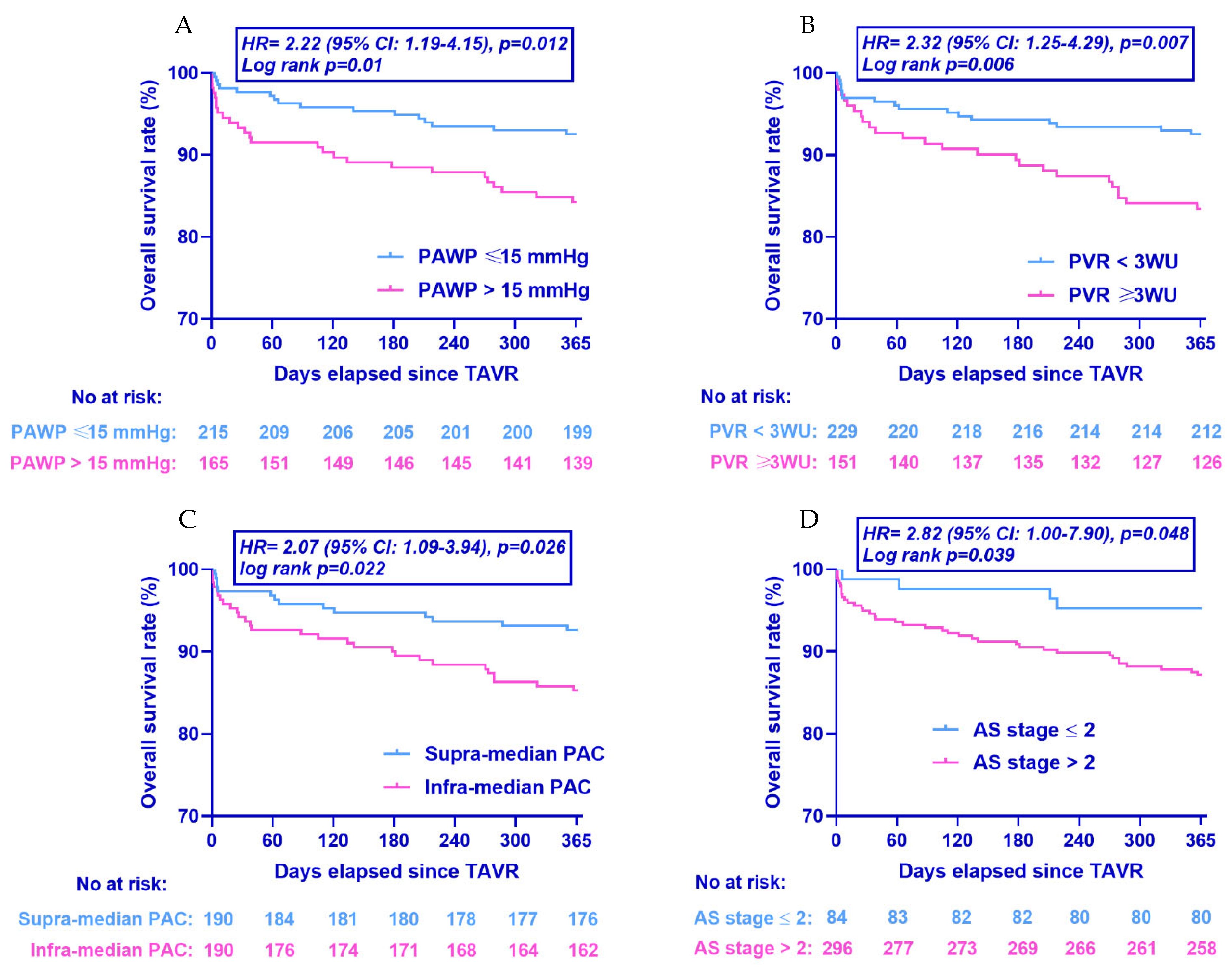
3.5. Clinical Outcomes
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Genereux, P.; Pibarot, P.; Redfors, B.; Mack, M.J.; Makkar, R.R.; Jaber, W.A.; Svensson, L.G.; Kapadia, S.; Tuzcu, E.M.; Thourani, V.H.; et al. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur. Heart J. 2017, 38, 3351–3358. [Google Scholar] [CrossRef] [Green Version]
- Alushi, B.; Beckhoff, F.; Leistner, D.; Franz, M.; Reinthaler, M.; Stahli, B.E.; Morguet, A.; Figulla, H.R.; Doenst, T.; Maisano, F.; et al. Pulmonary Hypertension in Patients with Severe Aortic Stenosis: Prognostic Impact After Transcatheter Aortic Valve Replacement: Pulmonary Hypertension in Patients Undergoing TAVR. JACC Cardiovasc. Imaging 2019, 12, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Lucon, A.; Oger, E.; Bedossa, M.; Boulmier, D.; Verhoye, J.P.; Eltchaninoff, H.; Iung, B.; Leguerrier, A.; Laskar, M.; Leprince, P.; et al. Prognostic implications of pulmonary hypertension in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation: Study from the FRANCE 2 Registry. Circ. Cardiovasc. Interv. 2014, 7, 240–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Sullivan, C.J.; Wenaweser, P.; Ceylan, O.; Rat-Wirtzler, J.; Stortecky, S.; Heg, D.; Spitzer, E.; Zanchin, T.; Praz, F.; Tuller, D.; et al. Effect of Pulmonary Hypertension Hemodynamic Presentation on Clinical Outcomes in Patients with Severe Symptomatic Aortic Valve Stenosis Undergoing Transcatheter Aortic Valve Implantation: Insights From the New Proposed Pulmonary Hypertension Classification. Circ. Cardiovasc. Interv. 2015, 8, e002358. [Google Scholar] [PubMed] [Green Version]
- Cavalcante, J.L.; Simon, M.A.; Chan, S.Y. Comprehensive Right-Sided Assessment for Transcatheter Aortic Valve Replacement Risk Stratification: Time for a Change. J. Am. Soc. Echocardiogr. 2017, 30, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Hatano, S.; Strasser, T. Primary Pulmonary-Hypertension-Report on a WHO Meeting. WHO Chron. 1975, 29, 371. [Google Scholar]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Rev. Esp. Cardiol. 2016, 69, 177. [Google Scholar] [CrossRef]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Kovacs, G.; Avian, A.; Olschewski, A.; Olschewski, H. Zero reference level for right heart catheterisation. Eur. Respir. J. 2013, 42, 1586–1594. [Google Scholar] [CrossRef]
- Gorlin, R.; Gorlin, S.G. Hydraulic formula for calculation of the area of the stenotic mitral valve, other cardiac valves, and central circulatory shunts. I. Am. Heart J. 1951, 41, 1–29. [Google Scholar] [CrossRef]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Evangelista, A.; Griffin, B.P.; Iung, B.; Otto, C.M.; Pellikka, P.A.; Quinones, M.; et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur. J. Echocardiogr. 2009, 10, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Kappetein, A.P.; Head, S.J.; Genereux, P.; Piazza, N.; van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; van Es, G.A.; et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. J. Am. Coll. Cardiol. 2012, 60, 1438–1454. [Google Scholar] [CrossRef] [Green Version]
- Kokkinidis, D.G.; Papanastasiou, C.A.; Jonnalagadda, A.K.; Oikonomou, E.K.; Theochari, C.A.; Palaiodimos, L.; Karvounis, H.I.; Armstrong, E.J.; Faillace, R.T.; Giannakoulas, G. The predictive value of baseline pulmonary hypertension in early and long term cardiac and all-cause mortality after transcatheter aortic valve implantation for patients with severe aortic valve stenosis: A systematic review and meta-analysis. Cardiovasc. Revasc. Med. 2018, 19, 859–867. [Google Scholar] [CrossRef]
- Sultan, I.; Fukui, M.; Bianco, V.; Brown, J.A.; Kliner, D.E.; Hickey, G.; Thoma, F.W.; Lee, J.S.; Schindler, J.T.; Kilic, A.; et al. Impact of Combined Pre and Postcapillary Pulmonary Hypertension on Survival after Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2020, 131, 60–66. [Google Scholar] [CrossRef]
- Schewel, J.; Schmidt, T.; Kuck, K.H.; Frerker, C.; Schewel, D. Impact of Pulmonary Hypertension Hemodynamic Status on Long-Term Outcome after Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 2155–2168. [Google Scholar] [CrossRef]
- Maeder, M.T.; Weber, L.; Weilenmann, D.; Chronis, J.; Joerg, L.; Pohle, S.; Haager, P.K.; Brutsche, M.; Neumann, T.; Schoch, O.D.; et al. Impact of the new pulmonary hypertension definition on long-term mortality in patients with severe aortic stenosis undergoing valve replacement. Clin. Cardiol. 2021, 44, 1276–1285. [Google Scholar] [CrossRef]
- Douschan, P.; Kovacs, G.; Avian, A.; Foris, V.; Gruber, F.; Olschewski, A.; Olschewski, H. Mild Elevation of Pulmonary Arterial Pressure as a Predictor of Mortality. Am. J. Respir. Crit. Care Med. 2018, 197, 509–516. [Google Scholar] [CrossRef]
- Valerio, C.J.; Schreiber, B.E.; Handler, C.E.; Denton, C.P.; Coghlan, J.G. Borderline mean pulmonary artery pressure in patients with systemic sclerosis: Transpulmonary gradient predicts risk of developing pulmonary hypertension. Arthritis Rheum. 2013, 65, 1074–1084. [Google Scholar] [CrossRef]
- Maron, B.A.; Hess, E.; Maddox, T.M.; Opotowsky, A.R.; Tedford, R.J.; Lahm, T.; Joynt, K.E.; Kass, D.J.; Stephens, T.; Stanislawski, M.A.; et al. Association of Borderline Pulmonary Hypertension with Mortality and Hospitalization in a Large Patient Cohort: Insights from the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. Circulation 2016, 133, 1240–1248. [Google Scholar] [CrossRef] [Green Version]
- Assad, T.R.; Maron, B.A.; Robbins, I.M.; Xu, M.; Huang, S.; Harrell, F.E.; Farber-Eger, E.H.; Wells, Q.S.; Choudhary, G.; Hemnes, A.R.; et al. Prognostic Effect and Longitudinal Hemodynamic Assessment of Borderline Pulmonary Hypertension. JAMA Cardiol. 2017, 2, 1361–1368. [Google Scholar] [CrossRef]
- Lau, E.M.; Godinas, L.; Sitbon, O.; Montani, D.; Savale, L.; Jais, X.; Lador, F.; Gunther, S.; Celermajer, D.S.; Simonneau, G.; et al. Resting pulmonary artery pressure of 21–24 mmHg predicts abnormal exercise haemodynamics. Eur. Respir. J. 2016, 47, 1436–1444. [Google Scholar] [CrossRef] [Green Version]
- Kaple, R.K.; Wilson, S.; Kampaktsis, P.; Baduashvili, A.; Zemedkun, M.; Kyaw, H.; Bergman, G.; Minutello, R.; Devereux, R.; Salemi, A.; et al. Impact of Etiology of Pulmonary Hypertension on Post-Procedural Management and Outcomes in Patients Undergoing Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2013, 61, E1954. [Google Scholar] [CrossRef] [Green Version]
- Hunt, J.M.; Bethea, B.; Liu, X.; Gandjeva, A.; Mammen, P.P.; Stacher, E.; Gandjeva, M.R.; Parish, E.; Perez, M.; Smith, L.; et al. Pulmonary veins in the normal lung and pulmonary hypertension due to left heart disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L725–L736. [Google Scholar] [CrossRef]

| Definition | 6th World Symposium Criteria | 2015 ESC Guidelines For PH Diagnosis | Pathophysiological Mechanisms |
|---|---|---|---|
| No PH | Mean PAP ≤ 20 mmHg * | Mean PAP < 25 mmHg | |
| Pre-capillary PH (Pre-capPH) | Mean PAP > 20 mmHg and PAWP ≤ 15 mmHg and PVR ≥ 3 WU | Mean PAP ≥ 25 mmHg and PAWP ≤ 15 mmHg |
1. Pulmonary arterial hypertension 3. PH due to lung diseases or hypoxia 4. PH due to pulmonary artery obstructions 5. PH with unclear and/or multifactorial mechanisms |
| Isolated post-capillary PH (IpcPH) | Mean PAP > 20 mmHg and PAWP > 15 mmHg and PVR < 3 WU | Mean PAP ≥ 25 mmHg and PAWP > 15 mmHg and DPG < 7 mmHg and/or PVR ≤ 3WU † | 2.
PH due to left heart disease - Heart failure with preserved ejection fraction - Heart failure with reduced ejection fraction - Valvular heart disease - Congenital/acquired conditions leading to post-capillary PH 5. PH with unclear and/or multifactorial mechanisms |
| Combined pre-and post-capillary PH (CpcPH) | Mean PAP > 20 mmHg and PAWP > 15 mmHg and PVR ≥ 3 WU | Mean PAP ≥ 25 mmHg and PAWP > 15 mmHg and DPG ≥ 7 mmHg and/or PVR > 3 WU † |
| PH | |||||
|---|---|---|---|---|---|
| NoPH | Pre-capPH | IpcPH | CpcPH | p | |
| n = 174 | n = 46 | n = 78 | n = 82 | Value | |
| Demographics | |||||
| Age (years) | 84 ± 6 | 84 ± 6 | 82 ± 7 | 84 ± 6 | 0.165 |
| Height (cm) | 165 ± 9 | 163 ± 9 | 167 ± 9 | 163 ± 9 | 0.014 |
| Weight (kg) | 72 ± 15 | 68 ± 11 | 74 ± 15 | 69 ± 15 | 0.058 |
| BMI (kg/m2) | 26.3 ± 4.8 | 25.7 ± 4.2 | 26.5 ± 4.6 | 25.9 ± 5.7 | 0.767 |
| BSA (m2) | 1.81 ± 0.21 | 1.75 ± 0.18 | 1.84 ± 0.22 | 1.75 ± 0.21 | 0.021 |
| Gender (males, n, %) | 84 (48) | 15 (33) | 41 (53) ‡ | 27 (33) ‡ | 0.018 |
| Pre-intervention risk scores | |||||
| EuroSCORE (%, n = 372) | 12.1 [8.4–16.7] | 13.1 [9.7–20.1] | 14.9 [11.4–23.5] ‡ | 24.6 [16.1–39.9] ‡ | <0.001 |
| STS Score (%, n = 372) | 4.1 [2.9–6.4] | 5.3 [3.7–8.0] ‡ | 5.0 [3.6–9.1] ‡ | 6.7 [3.9–9.8] ‡ | <0.001 |
| Comorbidities and risk factors | |||||
| Diabetes (n, %) | 41 (24) | 13 (28) | 33 (42) ‡ | 22 (27) | 0.024 |
| Dyslipidaemia (n, %) | 119 (68) | 32 (70) | 55 (71) | 57 (70) | 0.989 |
| Arterial hypertension (n, %) | 133 (76) | 36 (78) | 72 (92) ‡ | 65 (79) | 0.029 |
| Smokers (n, %) | 9 (5) | 4 (9) | 8 (10) | 7 (9) | 0.482 |
| CAD (n, %) | 93 (53) | 21 (46) | 45 (58) | 48 (59) | 0.496 |
| Previous MI (n, %) | 22 (13) | 5 (11) | 9 (12) | 14 (17) | 0.675 |
| PAD (n, %) | 16 (9) | 8 (17) | 14 (18) | 17 (21) | 0.055 |
| COPD (n, %) | 20 (12) | 13 (28) ‡ | 16 (21) | 19 (23) ‡ | 0.017 |
| Renal failure (n, %) | 83 (48) | 20 (43) | 45 (58) | 45 (55) | 0.298 |
| Cancer (n, %) | 34 (20) | 12 (26) | 15 (19) | 17 (21) | 0.787 |
| Atrial fibrillation/flutter (n, %) | 35 (20) | 19 (41) ‡ | 34 (44) ‡ | 39 (48) ‡ | <0.001 |
| Presence of symptoms | |||||
| NYHA III or IV (n, %) | 116 (67) | 37 (80) | 57 (73) | 69 (84) ‡ | 0.018 |
| Syncope (n, %, n = 368) | 29 (17) | 3 (7) | 5 (7) | 7 (9) | 0.051 |
| Angina (n, %, n = 368) | 33 (19) | 8 (18) | 14 (19) | 18 (23) | 0.894 |
| Baseline medications | |||||
| Aspirin (n, %) | 98 (56) | 25 (54) | 43 (55) | 43 (52) | 0.951 |
| Oral anticoagulation (n, %) | 34 (20) | 15 (33) | 30 (39) ‡ | 40 (49) ‡ | <0.001 |
| Beta-blockers (n, %) | 53 (31) | 20 (44) | 35 (45) ‡ | 45 (55) ‡ | 0.002 |
| ACE inhibitors (n, %) | 39 (22) | 11 (24) | 20 (26) | 17 (21) | 0.896 |
| ARBs (n, %) | 60 (35) | 13 (28) | 34 (44) | 25 (31) | 0.240 |
| Ca channel blockers (n, %) | 28 (16) | 9 (20) | 22 (28) | 19 (23) | 0.151 |
| Statin (n, %) | 101 (58) | 26 (57) | 44 (56) | 42 (51) | 0.785 |
| PH | |||||
|---|---|---|---|---|---|
| NoPH | Pre-capPH | IpcPH | CpcPH | p | |
| n = 174 | n = 46 | n = 78 | n = 82 | Value | |
| Aortic valve stenosis severity | |||||
| Transvalvular mean pressure gradient (mmHg, n = 378) | 43 ± 13 | 42 ± 17 | 40 ± 14 | 41 ± 14 | 0.051 |
| Transvalvular max pressure gradient (mmHg, n = 378) | 73 ± 21 | 70 ± 25 | 68 ± 22 | 65 ± 20 ‡ | 0.044 |
| Transvalvular max velocity (cm/s, n = 378) | 422 ± 62 | 413 ± 75 | 408 ± 66 | 398 ± 61 ‡ | 0.046 |
| AVA (cm2, n = 378) | 0.77 ± 0.2 | 0.69 ± 0.19 | 0.73 ± 0.21 | 0.65 ± 0.2 ‡ | <0.001 |
| AVA indexed for BSA (cm2/m2, n = 378) | 0.43 ± 0.12 | 0.40 ± 0.11 | 0.40 ± 0.12 | 0.37 ± 0.11 ‡ | 0.002 |
| LV geometry | |||||
| LV End-diastolic diameter (cm, n = 377) | 4.4 ± 0.7 | 4.5 ± 0.7 | 4.8 ± 0.9 ‡ | 4.8 ± 0.7 ‡ | <0.001 |
| LV mass (g, n = 375) | 199 ± 69 | 201 ± 73 ‡ | 228 ± 68 ‡ | 208 ± 62 | 0.019 |
| LV mass indexed for BSA (g/m2, n = 375) | 110 ± 33 | 115 ± 41 | 120 ± 35 ‡ | 116 ± 36 ‡ | 0.009 |
| RWT (n = 374) | 0.48 ± 0.13 | 0.47 ± 0.11 | 0.45 ± 0.14 | 0.44 ± 0.13 | 0.082 |
| LV systolic function | |||||
| Ejection fraction (%, n = 378) | 63 [59–65] | 63 [55–66] | 58 [43–65] ‡ | 55 [41–63] ‡ | <0.001 |
| LVOT flow max (mL/s, n = 351) | 254 ± 80 | 201 ± 60 ‡ | 268 ± 90 | 197 ± 66 ‡ | <0.001 |
| LV diastolic function | |||||
| Mitral E wave maximal velocity (cm/s, n = 373) | 84 ± 31 | 69 ± 31 | 109 ± 34 ‡ | 116 ± 37 ‡ | <0.001 |
| Mitral A wave maximal velocity (cm/s, n = 288) | 110 ± 33 | 116 ± 33 | 92 ± 32 ‡ | 83 ± 37 ‡ | <0.001 |
| e’ mean (m/s, n = 370) | 5.4 ± 1.7 | 5.5 ± 1.7 | 5.8 ± 2.2 | 5.5 ± 1.8 | 0.450 |
| Left atrial volume (mL, n = 375) | 71 [58–85] | 76 [65–87] | 87 [70–108] ‡ | 83 [74–107] ‡ | <0.001 |
| Left atrial volume indexed BSA (mL/m2, n = 375) | 40 [32–48] | 43 [34–51] | 46 [36–62] ‡ | 49 [41–60] ‡ | <0.001 |
| RV longitudinal function | |||||
| TAPSE (mm, n = 372) | 21 ± 4.5 | 20 ± 4.5 | 20 ± 5.0 | 17 ± 5.1 ‡ | <0.001 |
| DTI (cm/s, n = 370) | 11.9 ± 2.7 | 11.6 ± 2.6 | 11.3 ± 3.0 | 10.2 ± 2.9 ‡ | <0.001 |
| Aortic regurgitation | 0.866 | ||||
| None (%) | 42 (24) | 46 (17) | 78 (31) | 82 (28) | |
| Discrete (%) | 117 (67) | 32 (69) | 48 (61) | 52 (63) | |
| Discrete to moderate (%) | 7 (4) | 3 (7) | 4 (5) | 3 (4) | |
| Moderate (%) | 8 (5) | 3 (7) | 2 (3) | 4 (5) | |
| Mitral regurgitation | ‡ | ‡ | <0.001 | ||
| None (%) | 87 (50) | 18 (39) | 25 (32) | 24 (29) | |
| Discrete (%) | 75 (43) | 21 (46) | 41 (53) | 35 (43) | |
| Discrete to moderate (%) | 9 (5) | 6 (13) | 7 (9) | 11 (13) | |
| Moderate (%) | 3 (2) | 1 (2) | 5 (6) | 12 (15) | |
| Tricuspid regurgitation | ‡ | ‡ | ‡ | <0.001 | |
| None (%) | 127 (73) | 23 (50) ‡ | 43 (55) | 27 (33) | |
| Discrete (%) | 41 (23) | 15 (33) | 23 (30) | 34 (42) | |
| Discrete to moderate (%) | 3 (2) | 5 (11) ‡ | 4 (5) | 11 (13) | |
| Moderate (%) | 3 (2) | 2 (4) | 7 (9) | 7 (9) | |
| Moderate to severe (%) | 0 (0) | 1 (2) | 1 (1) | 3 (4) | |
| PH | |||||
|---|---|---|---|---|---|
| No PH | Pre-capPH | IpcPH | CpcPH | p | |
| n = 174 | n = 46 | n = 78 | n = 82 | Value | |
| Aortic valve stenosis severity | |||||
| Transvalvular mean pressure gradient (mmHg, n = 343) | 33 ± 13 | 36 ± 18 | 32 ± 16 | 31 ± 14 | 0.470 |
| Transvalvular peak to peak pressure gradient (mmHg, n = 366) | 44 ± 18 | 49 ± 24 | 42 ± 23 | 41 ± 23 | 0.312 |
| Transvalvular pressure gradient AUC (mmHg·s, n = 343) | 14 ± 6 | 15 ± 9 | 13 ± 6 | 13 ± 6 | 0.126 |
| AVA (Gorlin, cm2, n = 341) | 0.60 ± 0.28 | 0.51 ± 0.23 | 0.66 ± 0.25 | 0.46 ± 0.14 ‡ | <0.001 |
| AVA indexed for BSA (Gorlin, cm2/m2, n = 341) | 0.33 ± 0.14 | 0.29 ± 0.13 | 0.36 ± 0.14 | 0.26 ± 0.08 ‡ | <0.001 |
| Aortic valve stenosis staging | ‡ | ‡ | ‡ | <0.001 | |
| Stage 0 | 10 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Stage 1 | 10 (6) | 2 (4) | 1 (1) | 0 (0) | |
| Stage 2 | 55 (34) | 1 (2) | 5 (6) | 0 (0) | |
| Stage 3 | 4 (2) | 4 (9) | 14 (18) | 1 (1) | |
| Stage 4 | 95 (55) | 39 (85) | 58 (74) | 81 (99) | |
| Systemic afterload | |||||
| Aortic systolic blood pressure (mmHg) | 128 ± 25 | 120 ± 28 | 119 ± 27 | 126 ± 27 | 0.070 |
| Aortic diastolic blood pressure (mmHg) | 54 ± 11 | 52 ± 13 | 53 ± 12 | 56 ± 15 | 0.192 |
| Aortic pulse pressure (mmHg) | 74 ± 20 | 68 ± 24 | 66 ± 24 ‡ | 69 ± 24 | 0.051 |
| Aortic mean pressure (mmHg) | 82 ± 15 | 78 ± 17 | 79 ± 16 | 83 ± 17 | 0.112 |
| Total arterial compliance (mL/mmHg, n = 351) | 0.58 ± 0.27 | 0.51 ± 0.26 | 0.67 ± 0.33 | 0.46 ± 0.2 ‡ | <0.001 |
| Total vascular resistance (mmHg·min/mL, n = 351) | 1.38 ± 0.45 | 1.54 ± 0.47 | 1.27 ± 0.45 | 1.82 ± 0.73 ‡ | <0.001 |
| Zva (mmHg/mL/m2, n = 345) | 5.49 ± 1.6 | 6.46 ± 1.68 ‡ | 5.24 ± 1.60 | 7.23 ± 2.46 ‡ | <0.001 |
| LV systolic function | |||||
| Stroke volume (mL) | 60 ± 17 | 48 ± 15 ‡ | 59 ± 17 | 43 ± 15 ‡ | <0.001 |
| Stroke volume index (mL/m2) | 33 ± 7 | 27 ± 7 ‡ | 32 ± 8 | 25 ± 8 ‡ | <0.001 |
| Cardiac output (L/min) | 4.2 ± 1.1 | 3.7 ± 0.9 ‡ | 4.4 ± 1.1 | 3.3 ± 0.8 ‡ | <0.001 |
| Cardiac Index (L/min/m2) | 2.3 ± 0.5 | 2.1 ± 0.4 ‡ | 2.4 ± 0.5 | 1.9 ± 0.4 ‡ | <0.001 |
| Heart rate (bpm) | 65 ± 10 | 69 ± 14 | 68 ± 14 | 71 ± 17 ‡ | 0.002 |
| LV systolic pressure (mmHg, n = 366) | 172 ± 28 | 169 ± 37 | 161 ± 29 | 165 ± 31 | 0.055 |
| LV end-diastolic pressure (mmHg, n = 366) | 15 ± 7 | 18 ± 10 | 18 ± 7 ‡ | 20 ± 8 ‡ | <0.001 |
| Right heart hemodynamics | |||||
| Pulmonary arterial wedge pressure (mmHg) | 9 ± 4 | 12 ± 3 ‡ | 23 ± 6 ‡ | 23 ± 5 ‡ | <0.001 |
| Pulmonary arterial systolic pressure (mmHg) | 33 ± 7 | 49 ± 12 ‡ | 49 ± 10 ‡ | 64 ± 13 ‡ | <0.001 |
| Pulmonary arterial diastolic pressure (mmHg) | 10 ± 4 | 15 ± 5 ‡ | 19 ± 6 ‡ | 24 ± 7 ‡ | <0.001 |
| Pulmonary arterial mean pressure (mmHg) | 18 ± 4 | 28 ± 5 ‡ | 30 ± 5 ‡ | 39 ± 8 ‡ | <0.001 |
| Pulmonary arterial compliance (mL/mmHg) | 2.8 ± 1.1 | 1.5 ± 0.5 ‡ | 2.0 ± 0.7 ‡ | 1.2 ± 0.5 ‡ | <0.001 |
| Transpulmonic gradient (mmHg) | 9 ± 3 | 16 ± 5 ‡ | 8 ± 3 | 17 ± 6 ‡ | <0.001 |
| Pulmonary diastolic pressure gradient (mmHg) | 1 ± 4 | 3 ± 4 ‡ | −4 ± 5 ‡ | 1 ± 6 | <0.001 |
| Pulmonary vascular resistance (mmHg·min/mL) | 2.1 ± 0.8 | 4.4 ± 1.5 ‡ | 1.8 ± 0.7 | 5.3 ± 2.5 ‡ | <0.001 |
| Effective pulmonary arterial elastance (mmHg/mL) | 0.38 ± 0.19 | 1.1 ± 0.38 ‡ | 0.91 ± 0.36 ‡ | 1.61 ± 0.65 ‡ | <0.001 |
| PH | |||||
|---|---|---|---|---|---|
| No PH | Pre-capPH | IpcPH | CpcPH | p | |
| n = 174 | n = 46 | n = 78 | n = 82 | Value | |
| Access site | 0.086 | ||||
| Femoral (n, %) | 170 (98) | 42 (91) | 75 (96) | 74 (90) | |
| Apical (n, %) | 1 (1) | 2 (5) | 1 (1) | 4 (4) | |
| Sub-clavian (n, %) | 3 (1) | 1 (2) | 1 (1) | 2 (2) | |
| Other (n, %) | 0 (0) | 1 (2) | 1 (1) | 3 (4) | |
| Prosthetic valve type | 0.962 | ||||
| Medtronic CoreValve (n, %) | 156 (90) | 43 (94) | 68 (87) | 75 (92) | |
| Edwards Sapien (n, %) | 15 (8) | 3 (7) | 9 (12) | 6 (7) | |
| Boston Acurate (n, %) | 3 (2) | 0 (0) | 1 (1) | 1 (1) | |
| Procedural specifications | |||||
| Concomitant procedure (n, %) | 19 (11) | 6 (13) | 7 (9) | 15 (18) | 0.279 |
| Device success (n, %) | 162 (93) | 41 (89) | 73 (94) | 70 (85) | 0.179 |
| PH | PH vs. No PH | Pre-capPH vs. No PH | IpcPH vs. No PH | CpcPH vs. No PH | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No PH | Pre-capPH | IpcPH | CpcPH | |||||||||
| n = 174 | n = 46 | n = 78 | n = 82 | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Unadjusted | ||||||||||||
| All-cause death (n, %) | 10 (5.7) | 7 (15.2) | 8 (10.3) | 17 (20.7) | 2.8 (1.4–5.8) | 0.004 | 2.7 (1.0–7.2) | 0.041 | 1.8 (0.7–4.6) | 0.202 | 3.9 (1.8–8.5) | 0.001 |
| Adjusted Model A | ||||||||||||
| All-cause death (n, %) | 2.5 (1.2–5.3) | 0.013 | 2.8 (1.1–7.4) | 0.037 | 1.6 (0.6–8.6) | 0.361 | 3.7 (1.6–8.6) | 0.003 | ||||
| Adjusted Model B | ||||||||||||
| All-cause death (n, %) | 2.7 (1.3–5.7) | 0.011 | 2.7 (1.0–7.4) | 0.049 | 1.8 (0.7–4.8) | 0.248 | 3.9 (1.7–9.1) | 0.001 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamopoulos, D.; Pagoulatou, S.; Rovas, G.; Bikia, V.; Müller, H.; Giannakopoulos, G.; Mauler-Wittwer, S.; Licker, M.-J.; Stergiopulos, N.; Lador, F.; et al. Prognostic Implications of the Novel Pulmonary Hypertension Definition in Patients with Aortic Stenosis after Transcatheter Valve Replacement. J. Clin. Med. 2022, 11, 4279. https://doi.org/10.3390/jcm11154279
Adamopoulos D, Pagoulatou S, Rovas G, Bikia V, Müller H, Giannakopoulos G, Mauler-Wittwer S, Licker M-J, Stergiopulos N, Lador F, et al. Prognostic Implications of the Novel Pulmonary Hypertension Definition in Patients with Aortic Stenosis after Transcatheter Valve Replacement. Journal of Clinical Medicine. 2022; 11(15):4279. https://doi.org/10.3390/jcm11154279
Chicago/Turabian StyleAdamopoulos, Dionysios, Stamatia Pagoulatou, Georgios Rovas, Vasiliki Bikia, Hajo Müller, Georgios Giannakopoulos, Sarah Mauler-Wittwer, Marc-Joseph Licker, Nikolaos Stergiopulos, Frédéric Lador, and et al. 2022. "Prognostic Implications of the Novel Pulmonary Hypertension Definition in Patients with Aortic Stenosis after Transcatheter Valve Replacement" Journal of Clinical Medicine 11, no. 15: 4279. https://doi.org/10.3390/jcm11154279
APA StyleAdamopoulos, D., Pagoulatou, S., Rovas, G., Bikia, V., Müller, H., Giannakopoulos, G., Mauler-Wittwer, S., Licker, M.-J., Stergiopulos, N., Lador, F., & Noble, S. (2022). Prognostic Implications of the Novel Pulmonary Hypertension Definition in Patients with Aortic Stenosis after Transcatheter Valve Replacement. Journal of Clinical Medicine, 11(15), 4279. https://doi.org/10.3390/jcm11154279







