European Kidney Function Consortium Equation vs. Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Refit Equations for Estimating Glomerular Filtration Rate: Comparison with CKD-EPI Equations in the Korean Population
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Estimation of GFR
2.3. Statistical Analysis
3. Results
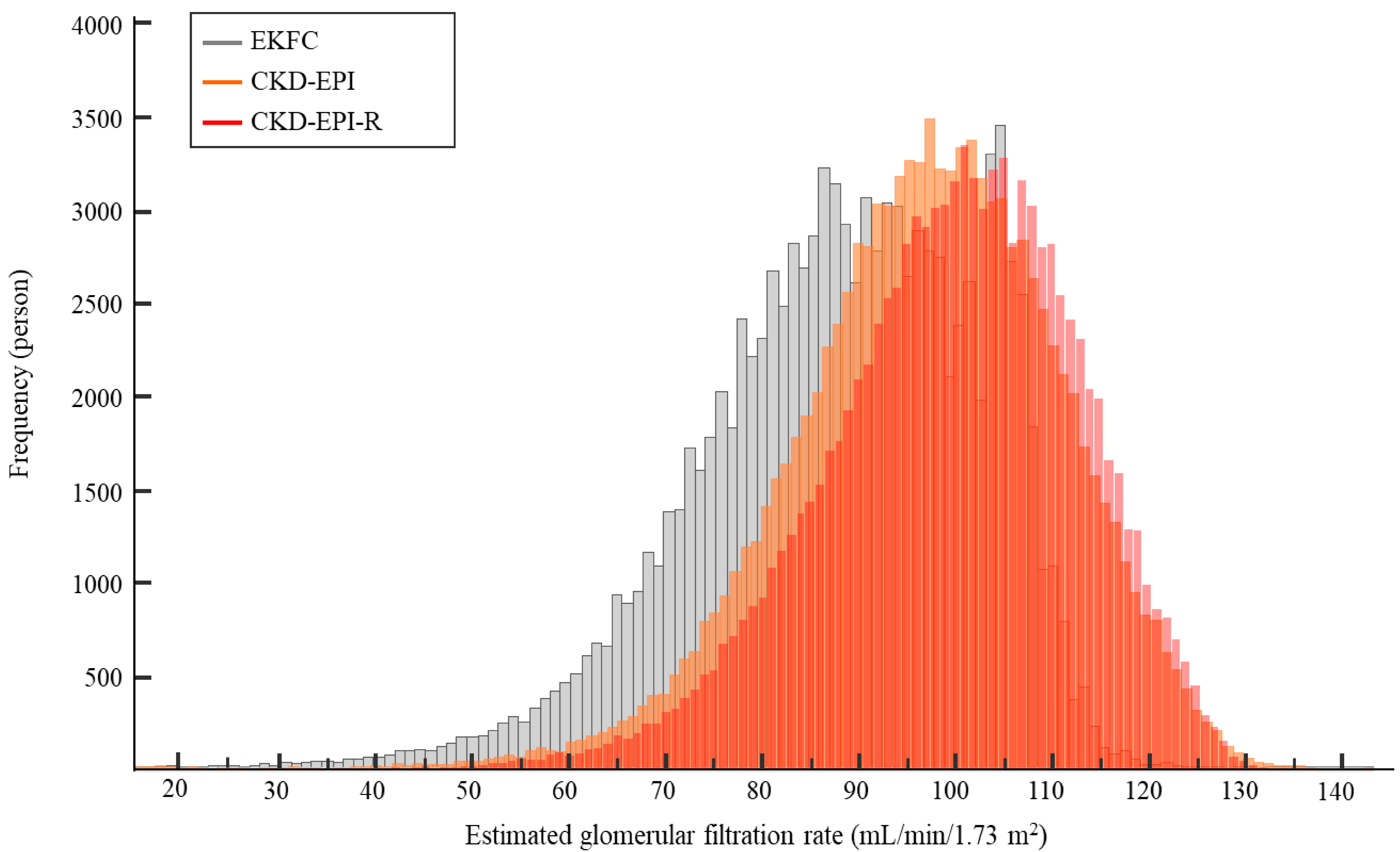
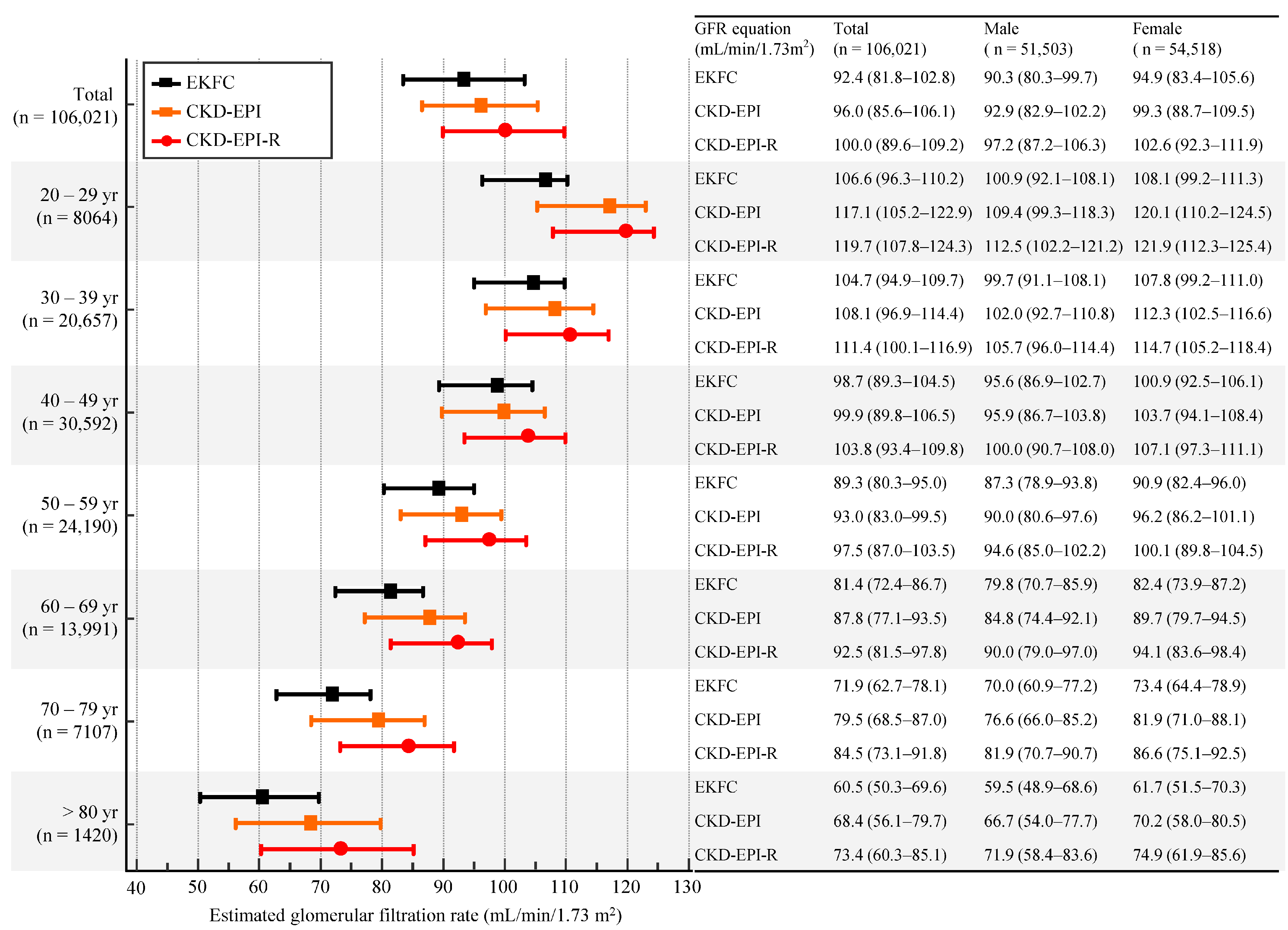
3.1. eGFR Based on sCr Level
| Age (yr) | CKD−EPI vs. EKFC | CKD−EPI vs. CKD−EPI−R | CKD−EPI−R vs. EKFC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Equation | r (95% CI) | Mean Difference (95% CI) | Equation | r (95% CI) | Mean Difference (95% CI) | Equation | r (95% CI) | Mean Difference (95% CI) | |
| 20–29 (n = 8064) | y = 0.81x + 11.59 | 0.97 (0.97–0.97) | 10.6 (4.6–16.6) | y = 0.93x + 9.38 | 1.00 (1.00–1.00) | −1.9 (−4.1–0.4) | y = 0.86x + 4.37 | 0.97 (0.97–0.97) | 12.5 (7.6–17.4) |
| 30–39 (n = 20,657) | y = 0.86x + 10.98 | 0.97 (0.97–0.97) | 3.5 (−1.7–8.6) | y = 0.95x + 7.81 | 1.00 (1.00–1.00) | −2.7 (−4.7–−0.7) | y = 0.91x + 3.93 | 0.97 (0.97–0.97) | 6.1 (1.8–10.4) |
| 40–49 (n = 30,592) | y = 0.93x + 5.65 | 0.99 (0.99–0.99) | 1.1 (−1.8–4.1) | y = 0.97x + 6.26 | 1.00 (1.00–1.00) | −3.4 (−5.1–−1.7) | y = 0.96x–0.92 | 0.99 (0.99–0.99) | 4.5 (1.8–7.2) |
| 50–59 (n = 24,190) | y = 0.90x + 5.13 | 0.99 (0.99–0.99) | 3.6 (0.4–6.7) | y = 0.99x + 5.22 | 1.00 (1.00–1.00) | −3.9 (−5.4–−2.3) | y = 0.92x–0.01 | 1.00 (0.99–1.00) | 7.4 (4.4–10.5) |
| 60–69 (n = 13,991) | y = 0.88x + 4.21 | 0.99 (0.99–0.99) | 5.8 (2.3–9.3) | y = 0.99x + 4.82 | 1.00 (1.00–1.00) | −4.2 (−5.6–−2.7) | y = 0.89x–0.30 | 1.00 (1.00–1.00) | 10.0 (6.4–13.6) |
| 70–79 (n = 7107) | y = 0.86x + 3.78 | 1.00 (1.00–1.00) | 7.2 (3.0–11.4) | y = 1.01x + 3.42 | 1.00 (1.00–1.00) | −4.4 (−6.0–−2.9) | y = 0.85x + 0.81 | 1.00 (1.00–1.00) | 11.6 (6.9–16.4) |
| >80 (n = 1420) | y = 0.84x + 3.17 | 1.00 (1.00–1.00) | 7.6 (2.5–12.6) | y = 1.04x + 1.70 | 1.00 (1.00–1.00) | −4.5 (−6.3–−2.8) | y = 0.81x + 1.57 | 1.00 (1.00–1.00) | 12.1 (5.8–18.4) |
| Total (n = 106,021) | y = 0.94x + 2.12 | 0.98 (0.98–0.98) | 4.0 (−2.6–10.5) | y = 0.96x + 7.10 | 1.00 (1.00–1.00) | −3.4 (−5.6–−1.2) | y = 0.97x–4.57 | 0.98 (0.97–0.99) | 7.4 (1.0–13.8) |
| GFR Category | CKD-EPI | Categorical Agreement | Agreement of Estimated CKD Prevalence | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3a | G3b | G4 | G5 | Total | % | κ (95% CI) | κ (95% CI) | Estimated CKD Prevalence (%) | Difference and Rate in Prevalence (%, 95% CI) | p | ||
| EKFC | G1 | 59,439 | 291 | 0 | 0 | 0 | 0 | 59,730 | 89.1 | 0.80 (0.80–0.81) | 0.80 (0.79–0.81) | 3.0 | 1.0 (0.8–1.1) 1.5 (1.4–1.5) | < 0.001 |
| G2 | 9954 | 33,104 | 12 | 0 | 0 | 0 | 43,070 | |||||||
| G3a | 0 | 1031 | 1461 | 1 | 0 | 0 | 2493 | |||||||
| G3b | 0 | 0 | 194 | 349 | 4 | 0 | 547 | |||||||
| G4 | 0 | 0 | 0 | 13 | 80 | 4 | 97 | |||||||
| G5 | 0 | 0 | 0 | 0 | 0 | 84 | 84 | |||||||
| CKD-EPI-R | G1 | 69,393 | 9346 | 0 | 0 | 0 | 0 | 78,739 | 90.4 | 0.80 (0.79–0.80) | 0.82 (0.81–0.83) | 1.5 | −0.6 (−0.7–−0.5) 0.7 (0.7–0.7) | < 0.001 |
| G2 | 0 | 25,080 | 662 | 0 | 0 | 0 | 25,742 | |||||||
| G3a | 0 | 0 | 1005 | 120 | 0 | 0 | 1125 | |||||||
| G3b | 0 | 0 | 0 | 243 | 20 | 0 | 263 | |||||||
| G4 | 0 | 0 | 0 | 0 | 64 | 4 | 68 | |||||||
| G5 | 0 | 0 | 0 | 0 | 0 | 84 | 84 | |||||||
| Total | 69,393 | 34,426 | 1667 | 363 | 84 | 88 | 106,021 | |||||||

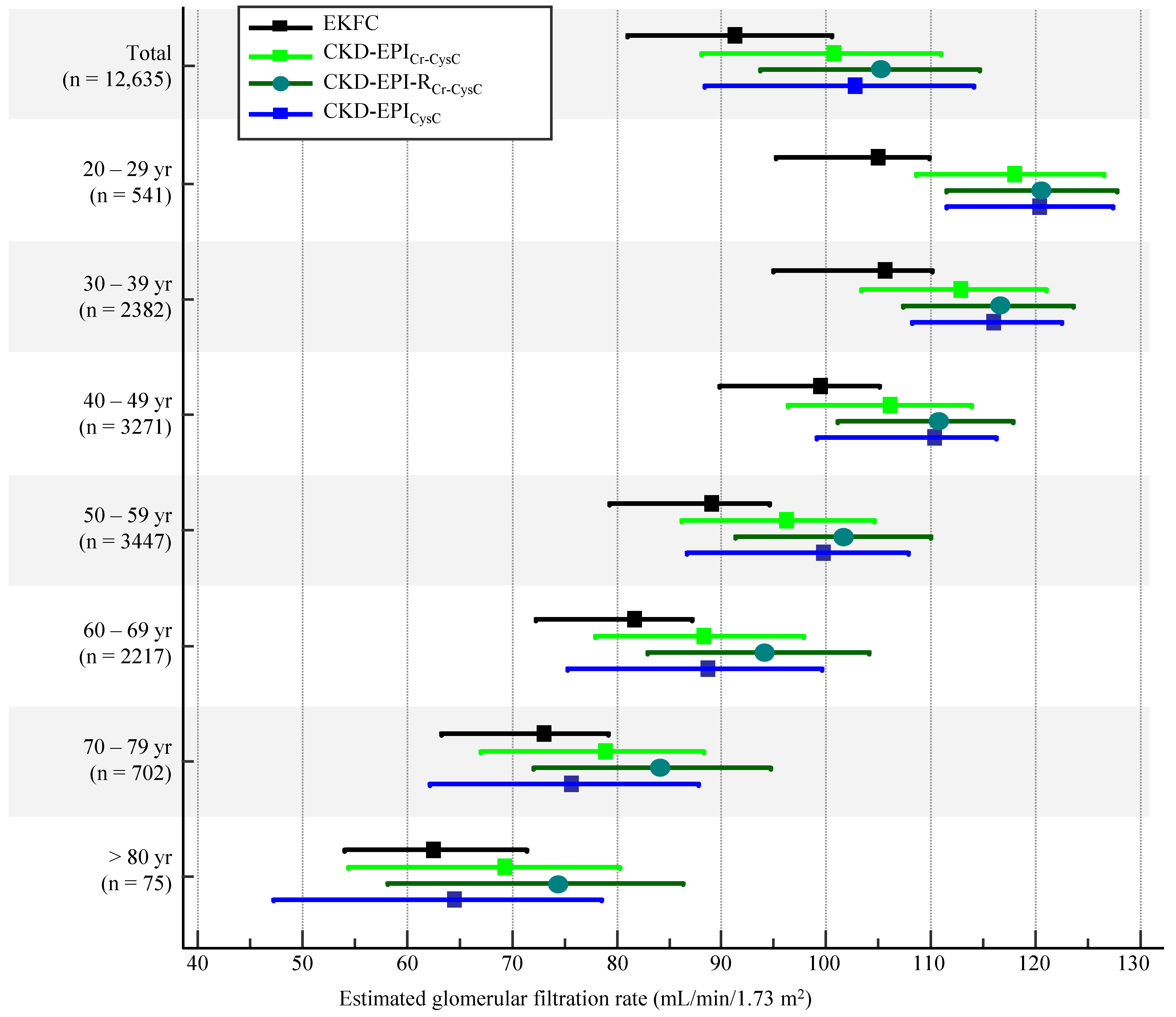
| 20–29 yr (n = 541) | 30–39 yr (n = 2382) | 40–49 yr (n = 3271) | 50–59 yr (n = 3447) | 60–69 yr (n = 2217) | 70–79 yr (n = 702) | >80 yr (n = 75) | Total (n = 12,635) | |
|---|---|---|---|---|---|---|---|---|
| CKD-EPICr-CysC | 106.7 (96.3–115.7) | 112.9 (103.3–121.0) | 106.0 (96.4–113.9) | 96.2 (86.2–104.5) | 88.3 (77.8–97.8) | 78.9 (67.1–88.3) | 69.3 (54.5–80.3) | 100.4 (88.0–111.0) |
| EKFC | 105.0 (95.2–110.0) | 105.6 (94.9–110.1) | 99.4 (89.8–105.0) | 89.1 (79.3–94.6) | 81.7 (72.3–87.1) | 73.0 (63.3–79.1) | 62.5 (54.0–71.4) | 91.2 (81.2–101.8) |
| CKD-EPI-RCr-CysC | 120.6 (111.5–127.7) | 116.5 (107.4–123.7) | 110.8 (101.0–117.9) | 101.7 (91.3–110.0) | 94.1 (82.9–104.1) | 84.2 (72.1–94.7) | 74.5 (58.2–86.3) | 105.5 (92.9–115.3) |
| CKD-EPICysC | 120.4 (111.4–127.4) | 115.9 (108.2–112.5) | 110.4 (99.0–116.2) | 99.7 (86.7–107.8) | 88.7 (75.3–99.6) | 75.6 (62.1–87.8) | 64.5 (47.3–78.6) | 103.7 (88.3–114.2) |
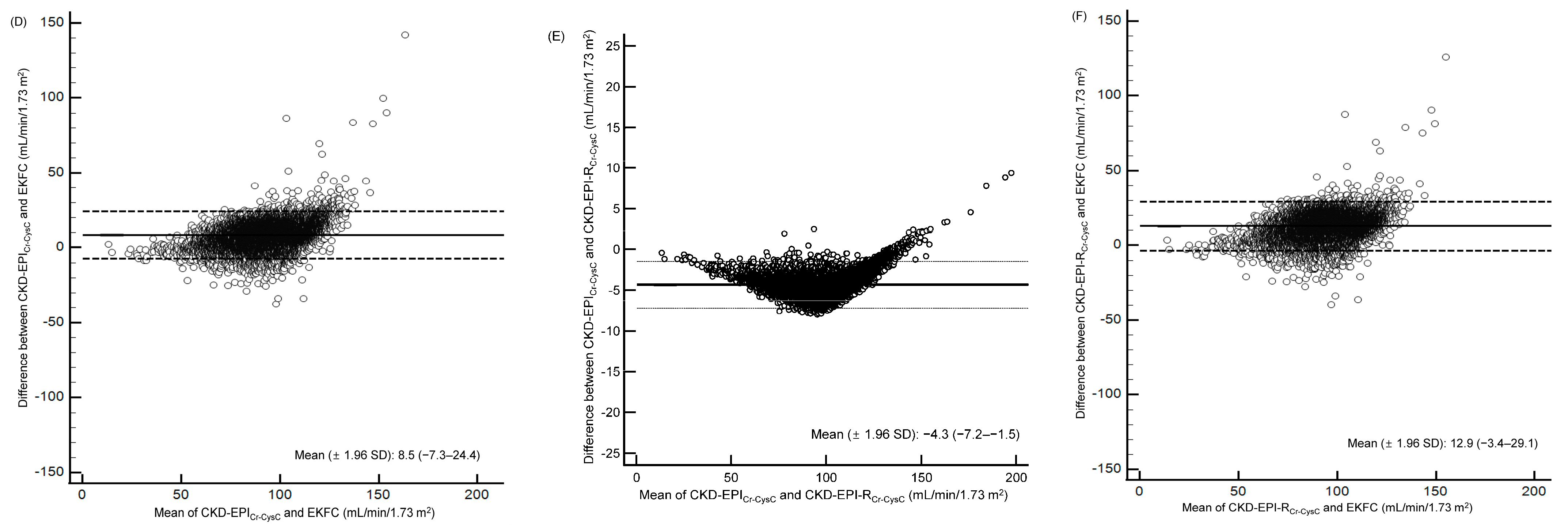
3.2. eGFR Based on sCr and sCysC Levels
| Age (yr) | CKD−EPICr−CysC vs. EKFC | CKD−EPICr−CysC vs. CKD−EPI−RCr−CysC | CKD−EPI−RCr−CysC vs. EKFC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Equation | r (95% CI) | Mean Difference (95% CI) | Equation | r (95% CI) | Mean Difference (95% CI) | Equation | r (95% CI) | Mean Difference (95% CI) | |
| 20–29 (n = 541) | y = 0.70x + 20.09 | 0.79 (0.75–0.82) | 14.9 (−2.6–32.4) | y = 0.92x + 11.40 | 1.00 (1.00–1.00) | −2.0 (−4.6–0.6) | y = 0.76x + 11.10 | 0.78 (0.74–0.81) | 16.9 (0.3–33.5) |
| 30–39 (n = 2382) | y = 0.76x + 17.19 | 0.81 (0.79–0.82) | 9.6 (−6.7–25.9) | y = 0.92x + 11.89 | 1.00 (1.00–1.00) | −3.1 (−5.6–−0.6) | y = 0.82x + 7.72 | 0.80 (0.78–0.81) | 12.7 (−3.0–28.5) |
| 40–49 (n = 3271) | y = 0.80x + 13.07 | 0.81 (0.80–0.82) | 7.6 (−8.2–23.5) | y = 0.96x + 8.54 | 1.00 (1.00–1.00) | −4.1 (−6.3–−2.0) | y = 0.84x + 5.68 | 0.81 (0.80–0.82) | 11.8 (−4.1–27.6) |
| 50–59 (n = 3447) | y = 0.76x + 14.12 | 0.81 (0.79–0.82) | 8.3 (−6.6–23.2) | y = 1.01x + 3.73 | 1.00 (1.00–1.00) | −5.0 (−6.8–−3.1) | y = 0.75x + 11.35 | 0.78 (0.77–0.80) | 13.2 (−2.7–29.1) |
| 60–69 (n = 2217) | y = 0.69x + 18.66 | 0.83 (0.82–0.84) | 8.1 (−7.1–23.3) | y = 1.05x + 1.00 | 1.00 (1.00–1.00) | −5.3 (−7.4–−3.2) | y = 0.65x + 18.33 | 0.81 (0.79–0.82) | 13.4 (−3.4–30.1) |
| 70–79 (n = 702) | y = 0.69x + 17.28 | 0.86 (0.84–0.88) | 6.8 (−7.7–21.4) | y = 1.07x − 0.37 | 1.00 (1.00–1.00) | −5.3 (−7.8–−2.8) | y = 0.63x + 17.89 | 0.84 (0.82–0.86) | 12.2 (−4.6–29.0) |
| >80 (n = 75) | y = 0.65x + 17.55 | 0.92 (0.88–0.95) | 6.0 (−9.4–21.4) | y = 1.09x–0.78 | 1.00 (1.00–1.00) | −5.2 (−8.4–−1.9) | y = 0.59x + 17.99 | 0.99 (0.99–0.99) | 11.2 (−7.3–29.6) |
| Total (n = 12,635) | y = 0.84x + 7.41 | 0.88 (0.88–0.89) | 8.5 (−7.3–24.4) | y = 0.96x + 8.27 | 1.00 (1.00–1.00) | −4.3 (−7.2–−1.5) | y = 0.87x + 0.20 | 0.87 (0.86–0.87) | 12.9 (−3.4–29.1) |
| GFR Category | CKD-EPICr-CysC | Categorical Agreement | Agreement of Estimated CKD Prevalence | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3a | G3b | G4 | G5 | Total | % | κ (95% CI) | κ (95% CI) | Estimated CKD Prevalence (%) | Difference and Rate in Prevalence (%, 95% CI) | p | ||
| EKFC | G1 | 6531 | 230 | 0 | 0 | 0 | 0 | 6761 | 76.9 | 0.57 (0.56–0.58) | 0.69 (0.64–0.73) | 2.5 | 0.6 (0.2–0.9) 1.3 (1.1–1.5) | 0.002 |
| G2 | 2500 | 3015 | 48 | 1 | 0 | 0 | 5564 | |||||||
| G3a | 0 | 120 | 132 | 8 | 0 | 0 | 260 | |||||||
| G3b | 0 | 0 | 7 | 32 | 5 | 0 | 44 | |||||||
| G4 | 0 | 0 | 0 | 0 | 4 | 0 | 5 | |||||||
| G5 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | |||||||
| CKD-EPI-RCr-CysC | G1 | 9031 | 1061 | 0 | 0 | 0 | 0 | 10,092 | 90.9 | 0.78 (0.77–0.79) | 0.81 (0.76–0.85) | 1.3 | −0.6 (−0.9–−0.3) 0.7 (0.6–0.8) | <0.001 |
| G2 | 0 | 2304 | 77 | 0 | 0 | 0 | 2381 | |||||||
| G3a | 0 | 0 | 110 | 14 | 0 | 0 | 124 | |||||||
| G3b | 0 | 0 | 0 | 27 | 2 | 0 | 29 | |||||||
| G4 | 0 | 0 | 0 | 0 | 7 | 1 | 8 | |||||||
| G5 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | |||||||
| CKD-EPICysC | G1 | 8588 | 613 | 0 | 0 | 0 | 0 | 9201 | 89.9 | 0.78 (0.77–0.79) | 0.71 (0.67–0.75) | 3.1 | 1.3 (0.8–1.6) 1.7 (1.4–2.0) | <0.001 |
| G2 | 443 | 2584 | 10 | 0 | 0 | 0 | 3037 | |||||||
| G3a | 0 | 167 | 140 | 0 | 0 | 0 | 307 | |||||||
| G3b | 0 | 1 | 37 | 39 | 0 | 0 | 77 | |||||||
| G4 | 0 | 0 | 0 | 2 | 9 | 1 | 12 | |||||||
| G5 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | |||||||
| Total | 9031 | 3365 | 187 | 41 | 9 | 2 | 12,635 | |||||||
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Korean Disease Control and Prevention Agency; National Center for Health Statistics. Korean Sational Health and Nutrition Examination Survey. Chronic Disease Health Survey. Prevalence of Chronic KIDNEY Disease. Available online: https://health.kdca.go.kr/healthinfo/biz/pblcVis/details.do?ctgrSn=38 (accessed on 10 March 2022).
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Levey, A.S.; Coresh, J.; Tighiouart, H.; Greene, T.; Inker, L.A. Measured and estimated glomerular filtration rate: Current status and future directions. Nat. Rev. Nephrol. 2020, 16, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Guideline Updates Team (UK); National Institute for Health and Care Excellence. Evidence Reviews for the Diagnostic Accuracy of eGFR Calculations in Adults, Children, and Young People from Black, Asian and Other Minority Ethnic Groups with Chronic Kidney Disease; NICE guideline NG203, NICE Evidence Reviews Collection; National Institute for Health and Care Excellence (NICE): London, UK; ISBN 978-1-4731-4233-6.
- Pottel, H.; Björk, J.; Bökenkamp, A.; Berg, U.; Åsling-Monemi, K.; Selistre, L.; Dubourg, L.; Hansson, M.; Littmann, K.; Jones, I.; et al. Estimating glomerular filtration rate at the transition from pediatric to adult care. Kidney Int. 2019, 95, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Pottel, H.; Björk, J.; Courbebaisse, M.; Couzi, L.; Ebert, N.; Eriksen, B.O.; Dalton, R.N.; Dubourg, L.; Gaillard, F.; Garrousteet, C.; et al. Development and validation of a modified full age spectrum creatinine-based equation to estimate glomerular filtration rate: A cross-sectional analysis of pooled data. Ann. Intern. Med. 2020, 174, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Björk, J.; Nyman, U.; Larsson, A.; Delanaye, P.; Pottel, H. Estimation of the glomerular filtration rate in children and young adults by means of the CKD-EPI equation with age-adjusted creatinine values. Kidney Int. 2021, 99, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Giavarina, D.; Husain-Syed, F.; Ronco, C. Clinical implications of the new equation to estimate glomerular filtration rate. Nephron 2021, 145, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Stämmler, F.; Grassi, M.; Meeusen, J.W.; Lieske, J.C.; Dasari, S.; Dubourg, L.; Lemoine, S.; Ehrich, J.; Schiffer, E. Estimating glomerular filtration rate from serum myo-inositol, valine, creatinine and cystatin C. Diagnostics 2021, 11, 2291. [Google Scholar] [CrossRef] [PubMed]
- Gama, R.M.; Clery, A.; Griffiths, K.; Heraghty, N.; Peters, A.M.; Palmer, K.; Kibble, H.; Vincent, R.P.; Sharpe, C.C.; Cairns, H.; et al. Estimated glomerular filtration rate equations in people of self-reported black ethnicity in the United Kingdom: Inappropriate adjustment for ethnicity may lead to reduced access to care. PLoS ONE 2021, 16, e0255869. [Google Scholar] [CrossRef]
- Olié, V.; Cheddani, L.; Stengel, B.; Gabet, A.; Grave, C.; Blacher, J.; Halimi, J.M. Prevalence of chronic kidney disease in France, Esteban study 2014–2016. Nephrol. Ther. 2021, 17, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Delanaye, P.; Björk, J.; Courbebaisse, M.; Couzi, L.; Ebert, N.; Eriksen, B.O.; Dalton, R.N.; Dubourg, L.; Gaillard, F.; Garrouste, C.; et al. Performance of creatinine-based equations to estimate glomerular filtration rate with a methodology adapted to the context of drug dosage adjustment. Br. J. Clin. Pharmacol. 2021, 88, 2118–2127. [Google Scholar] [CrossRef]
- Ehrich, J.; Dubourg, L.; Hansson, S.; Pape, L.; Steinle, T.; Fruth, J.; Höckner, S.; Schiffer, E. Serum myo-inositol, dimethyl sulfone, and valine in combination with creatinine allow accurate assessment of renal insufficiency-a proof of concept. Diagnostics 2021, 11, 234. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G.; Holdenrieder, S.; Neumann, F.J.; Lahu, S.; Cassese, S.; Joner, M.; Xhepa, E.; Kufner, S.; Wiebe, J.; Laugwitzet, K.L.; et al. Prognostic value of glomerular function estimated by Cockcroft-Gault creatinine clearance, MDRD-4, CKD-EPI and European Kidney Function Consortium equations in patients with acute coronary syndromes. Clin. Chim. Acta 2021, 523, 106–113. [Google Scholar] [CrossRef]
- Xia, F.; Hao, W.; Liang, J.; Wu, Y.; Yu, F.; Hu, W.; Zhao, Z.; Liu, W. Applicability of creatinine-based equations for estimating glomerular filtration rate in elderly Chinese patients. BMC Geriatr. 2021, 21, 481. [Google Scholar] [CrossRef]
- Wang, Y.; Levey, A.S.; Inker, L.A.; Jessani, S.; Bux, R.; Samad, Z.; Khan, A.R.; Karger, A.B.; Allen, J.C.; Jafar, T.H. Performance and determinants of serum creatinine and cystatin C-based GFR estimating equations in South Asians. Kidney Int. Rep. 2021, 6, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Delgado, C.; Baweja, M.; Crews, D.C.; Eneanya, N.D.; Gadegbeku, C.A.; Inker, L.A.; Mendu, M.L.; Miller, W.G.; Moxey-Mims, M.M.; Roberts, G.V.; et al. A unifying approach for GFR estimation: Recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. Am. J. Kidney Dis. 2022, 79, 268–288. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, J.W.; Kasozi, R.N.; Larson, T.S.; Lieske, J.C. Clinical impact of the refit CKD-EPI 2021 creatinine-based eGFR equation. Clin. Chem. 2022, 68, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Duggal, V.; Thomas, I.C.; Montez-Rath, M.E.; Chertow, G.M.; Tamura, M.K. National estimates of CKD prevalence and potential impact of estimating glomerular filtration rate without race. J. Am. Soc. Nephrol. 2021, 32, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Ojo, A. Eliminating racial inequities in kidney health: Much more than revising estimating equations. Ann. Intern. Med. 2022, 175, 446–447. [Google Scholar] [CrossRef] [PubMed]
- Bundy, J.D.; Mills, K.T.; Anderson, A.H.; Yang, W.; Chen, J.; He, J.; CRIC Study Investigators. Prediction of end-stage kidney disease using estimated glomerular filtration rate with and without race: A prospective cohort study. Ann. Intern. Med. 2022, 175, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Jeong, T.D.; Lee, W.; Chun, S.; Lee, S.K.; Ryu, J.S.; Min, W.K.; Park, J.S. Comparison of the MDRD study and CKD-EPI equations for the estimation of the glomerular filtration rate in the Korean general population: The fifth Korea National Health and Nutrition Examination Survey (KNHANES V-1), 2010. Kidney Blood Press. Res. 2013, 37, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Rhee, H.; Seong, E.Y.; Lee, D.W.; Lee, S.B.; Kwak, I.S. Comparison of glomerular filtration rates calculated by different serum cystatin C-based equations in patients with chronic kidney disease. Kidney Res. Clin. Pract. 2014, 33, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.S.; Lee, Y.K.; Choi, H.Y.; Choi, S.O.; Shin, S.K.; Ha, S.K.; Lee, K.W.; Kim, Y.W.; Kim, Y.L.; Yasuda, Y.; et al. Is the new GFR equation using inulin clearance a more accurate method for Asian patients? Clin. Nephrol. 2015, 84, 331–338. [Google Scholar] [CrossRef]
- Ji, M.; Lee, Y.H.; Hur, M.; Kim, H.; Cho, H.I.; Yang, H.S.; Navarin, S.; Di Somma, S.; GREAT Network. Comparing results of five glomerular filtration rate-estimating equations in the Korean general population: MDRD study, revised Lund-Malmö, and three CKD-EPI equations. Ann. Lab. Med. 2016, 36, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.J.; Cha, R.H.; Lee, S.H.; Yu, K.S.; Kim, S.E.; Kim, H.; Kim, Y.S. Validation of the Korean coefficient for the modification of diet in renal disease study equation. Korean J. Intern. Med. 2016, 31, 344–356. [Google Scholar] [CrossRef]
- Jeong, T.D.; Lee, W.; Yun, Y.M.; Chun, S.; Song, J.; Min, W.K. Development and validation of the Korean version of CKD-EPI equation to estimate glomerular filtration rate. Clin. Biochem. 2016, 49, 713–719. [Google Scholar] [CrossRef]
- Jeong, T.D.; Cho, E.J.; Lee, W.; Chun, S.; Hong, K.S.; Min, W.K. Accuracy assessment of five equations used for estimating the glomerular filtration rate in Korean adults. Ann Lab Med. 2017, 37, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Jeong, T.D.; Cho, E.J.; Lee, W.; Chun, S.; Hong, K.S.; Min, W.K. Efficient reporting of the estimated glomerular filtration rate without height in pediatric patients with cancer. Clin. Chem. Lab. Med. 2017, 55, 1891–1897. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jeong, T.D. Estimated glomerular filtration rates show minor but significant differences between the single and subgroup creatinine-based Chronic Kidney Disease Epidemiology Collaboration Equations. Ann. Lab. Med. 2019, 39, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Measurement Procedure Comparison and Bias Estimation Using Patient Samples, 3rd ed.; CLSI Guideline EP09c; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; ISBN 978-1-68440-006-5. [Google Scholar]
- Mukaka, M.M. A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. (Zagreb) 2012, 22, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Chan, I.S.; Zhang, Z. Test-based exact confidence intervals for the difference of two binomial proportions. Biometrics 1999, 55, 1202–1209. [Google Scholar] [CrossRef]
- Mulder, P.G.H. An exact method for calculating a confidence interval of a poisson parameter. Am. J. Epidemiol. 1983, 117, 377. [Google Scholar] [CrossRef]
- Stevens, L.A.; Claybon, M.A.; Schmid, C.H.; Chen, J.; Horio, M.; Imai, E.; Nelson, R.G.; Van Deventer, M.; Wang, H.Y.; Zuo, L.; et al. Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011, 79, 555–562. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, O.M.; Sang, Y.; Grams, M.E.; Ballew, S.H.; Surapaneni, A.; Matsushita, K.; Go, A.S.; Shlipak, M.G.; Inker, L.A.; Eneanya, N.D.; et al. Association of estimated GFR calculated using race-free equations with kidney failure and mortality by black vs. non-black race. JAMA 2022, 327, 2306–2316. [Google Scholar] [CrossRef]
- Shafi, T.; Zhu, X.; Lirette, S.T.; Rule, A.D.; Mosley, T.; Butler, K.R.; Hall, M.E.; Vaitla, P.; Wynn, J.J.; Tio, M.C.; et al. Quantifying individual-level inaccuracy in glomerular filtration rate estimation: A cross-sectional study. Ann. Intern. Med. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Delgado, C.; Baweja, M.; Burrows, N.R.; Crews, D.C.; Eneanya, N.D.; Gadegbeku, C.A.; Inker, L.A.; Mendu, M.L.; Miller, W.G.; Moxey-Mims, M.M.; et al. Reassessing the inclusion of race in diagnosing kidney diseases: An interim report from the NKF-ASN task force. J. Am. Soc. Nephrol. 2021, 32, 1305–1317. [Google Scholar] [CrossRef]
- Levey, A.S.; Titan, S.M.; Powe, N.R.; Coresh, J.; Inker, L.A. Kidney disease, race, and GFR estimation. Clin. J. Am. Soc. Nephrol. 2020, 15, 1203–1212. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Yang, W.; Parikh, R.V.; Anderson, A.H.; Chen, T.K.; Cohen, D.L.; He, J.; Mohanty, M.J.; Lash, J.P.; Mills, K.T.; et al. Race, genetic ancestry, and estimating kidney function in CKD. N. Engl. J. Med. 2021, 385, 1750–1760. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.X.; Sun, L.; Nicholas, S.B.; Lu, Y.; Sinha K, S.; Hua, R. Comparison of bias and accuracy using cystatin C and creatinine in CKD-EPI equations for GFR estimation. Eur. J. Intern. Med. 2020, 80, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Titan, S.; Inker, L.A. Measurement and estimation of GFR for use in clinical practice: Core curriculum 2021. Am. J. Kidney Dis. 2021, 78, 736–749. [Google Scholar]
- Earley, A.; Miskulin, D.; Lamb, E.J.; Levey, A.S.; Uhlig, K. Estimating equations for glomerular filtration rate in the era of creatinine standardization: A systematic review. Ann. Intern. Med. 2012, 156, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Ebert, N.; Delanaye, P.; Shlipak, M.; Jakob, O.; Martus, P.; Bartel, J.; Gaedeke, J.; van der Giet, M.; Schuchardt, M.; Cavalier, E.; et al. Cystatin C standardization decreases assay variation and improves assessment of glomerular filtration rate. Clin. Chim. Acta 2016, 456, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Rowe, C.; Sitch, A.J.; Barratt, J.; Brettell, E.A.; Cockwell, P.; Dalton, N.R.; Deeks, J.J.; Eaglestone, G.; Pellatt-Higgins, T.; Kalra, P.A.; et al. Biological variation of measured and estimated glomerular filtration rate in patients with chronic kidney disease. Kidney Int. 2019, 96, 429–435. [Google Scholar] [CrossRef] [Green Version]
- Jonker, N.; Aslan, B.; Boned, B.; Marqués-García, F.; Ricós, C.; Alvarez, V.; Bartlett, W.; Braga, F.; Carobene, A.; Coskun, A.; et al. Critical appraisal and meta-analysis of biological variation estimates for kidney related analytes. Clin. Chem. Lab. Med. 2020, 60, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Coşkun, A.; Sandberg, S.; Unsal, I.; Cavusoglu, C.; Serteser, M.; Kilercik, M.; Aarsand, A.K. Personalized reference intervals in laboratory medicine: A new model based on within-subject biological variation. Clin. Chem. 2021, 67, 374–384. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Hur, M.; Lee, S.; Lee, G.-H.; Moon, H.-W.; Yun, Y.-M. European Kidney Function Consortium Equation vs. Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Refit Equations for Estimating Glomerular Filtration Rate: Comparison with CKD-EPI Equations in the Korean Population. J. Clin. Med. 2022, 11, 4323. https://doi.org/10.3390/jcm11154323
Kim H, Hur M, Lee S, Lee G-H, Moon H-W, Yun Y-M. European Kidney Function Consortium Equation vs. Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Refit Equations for Estimating Glomerular Filtration Rate: Comparison with CKD-EPI Equations in the Korean Population. Journal of Clinical Medicine. 2022; 11(15):4323. https://doi.org/10.3390/jcm11154323
Chicago/Turabian StyleKim, Hanah, Mina Hur, Seungho Lee, Gun-Hyuk Lee, Hee-Won Moon, and Yeo-Min Yun. 2022. "European Kidney Function Consortium Equation vs. Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Refit Equations for Estimating Glomerular Filtration Rate: Comparison with CKD-EPI Equations in the Korean Population" Journal of Clinical Medicine 11, no. 15: 4323. https://doi.org/10.3390/jcm11154323
APA StyleKim, H., Hur, M., Lee, S., Lee, G.-H., Moon, H.-W., & Yun, Y.-M. (2022). European Kidney Function Consortium Equation vs. Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Refit Equations for Estimating Glomerular Filtration Rate: Comparison with CKD-EPI Equations in the Korean Population. Journal of Clinical Medicine, 11(15), 4323. https://doi.org/10.3390/jcm11154323








