Associations of Chronic Diabetes Complications and Cardiovascular Risk with the Risk of Obstructive Sleep Apnea in Patients with Type 2 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
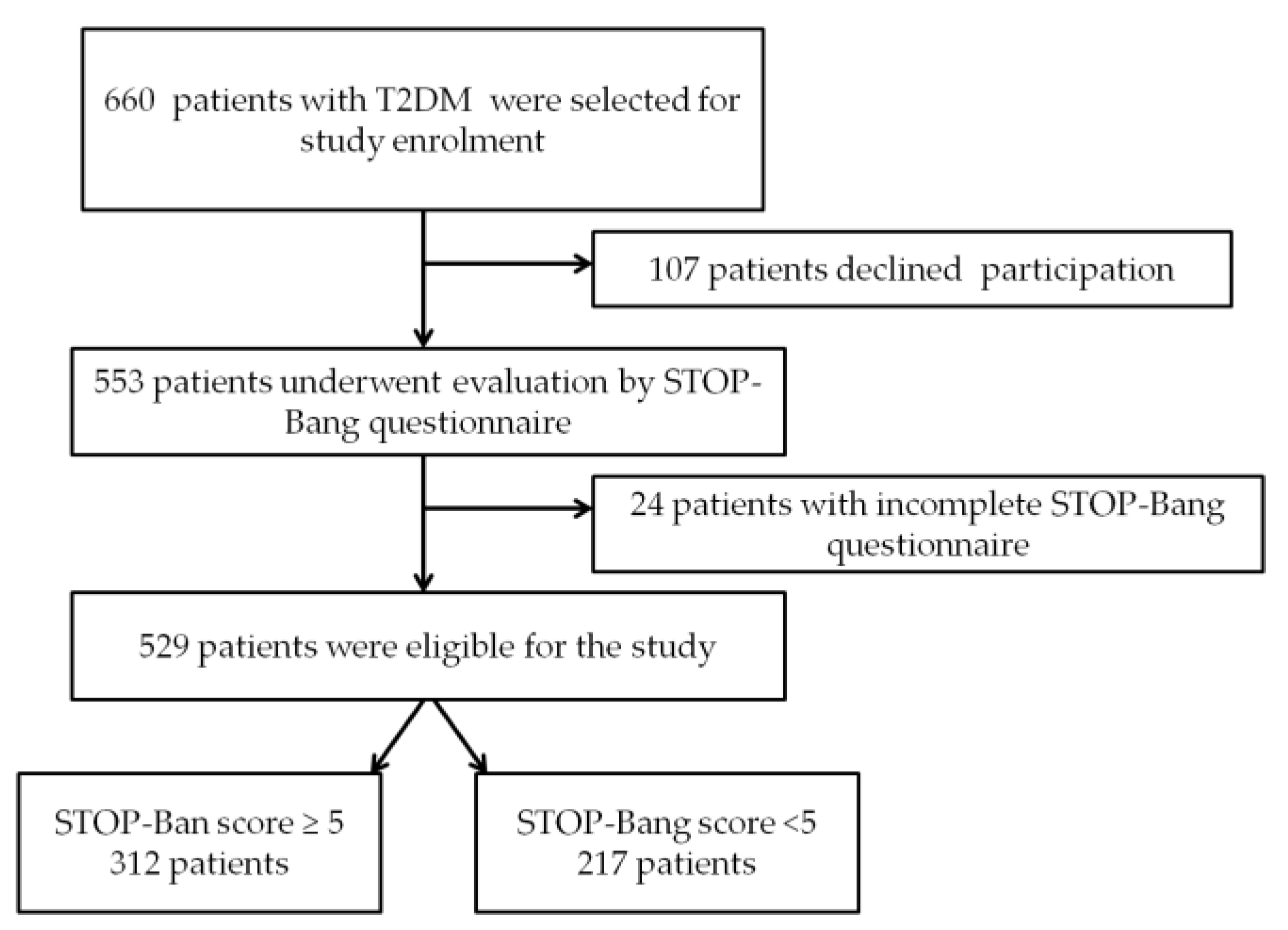
2.1. Participants
- -
- Group 1—217 patients with STOP-Bang score <5;
- -
- Group 2—312 patients with STOP-Bang score ≥5 (patients with moderate-to-severe OSA risk [18]).
2.2. Laboratory Exams
2.3. Evaluation of Obstructive Sleep Apnea Syndrome risk
2.4. Evaluation of Cardiovascular Risk
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lee, J.J.; Sundar, K.M. Evaluation and Management of Adults with Obstructive Sleep Apnea Syndrome. Lung 2021, 199, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.M.; Syed, U.; Arshad, A. Predictive Value of Clinical and Questionnaire Based Screening Tools of Obstructive Sleep Apnea in Patients with Type 2 Diabetes Mellitus. Cureus 2021, 13, e18009. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.C.; Mak, J.C.; Ip, M.S. Obesity, obstructive sleep apnoea and metabolic syndrome. Respirology 2012, 17, 223–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapur, V.; Strohl, K.P.; Redline, S.; Iber, C.; O’Connor, G.; Nieto, J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath. 2002, 6, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Patel, S.R. Obstructive Sleep Apnea. Ann. Intern. Med. 2019, 171, ITC81–ITC96. [Google Scholar] [CrossRef]
- Huang, T.; Lin, B.M.; Stampfer, M.J.; Tworoger, S.S.; Hu, F.B.; Redline, S. A Population-Based Study of the Bidirectional Association between Obstructive Sleep Apnea and Type 2 Diabetes in Three Prospective U.S. Cohorts. Diabetes Care 2018, 41, 2111–2119. [Google Scholar] [CrossRef] [Green Version]
- Trinh, M.D.; Plihalova, A.; Gojda, J.; Westlake, K.; Spicka, J.; Lattova, Z.; Pretl, M.; Polak, J. Obstructive sleep apnoea increases lipolysis and deteriorates glucose homeostasis in patients with type 2 diabetes mellitus. Sci. Rep. 2021, 11, 3567. [Google Scholar] [CrossRef]
- Su, X.; Li, J.H.; Gao, Y.; Chen, K.; Gao, Y.; Guo, J.J.; Shi, M.; Zou, X.; Xu, W.; Zhao, L.B.; et al. Impact of obstructive sleep apnea complicated with type 2 diabetes on long-term cardiovascular risks and all-cause mortality in elderly patients. BMC Geriatr. 2021, 21, 508. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [Green Version]
- Netzer, N.C.; Stoohs, R.A.; Netzer, C.M.; Clark, K.; Strohl, K.P. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann. Intern. Med. 1999, 131, 485–491. [Google Scholar] [CrossRef]
- Chung, F.; Abdullah, H.R.; Liao, P. STOP-Bang Questionnaire: A Practical Approach to Screen for Obstructive Sleep Apnea. Chest 2016, 149, 631–638. [Google Scholar] [CrossRef] [Green Version]
- Chiu, H.Y.; Chen, P.Y.; Chuang, L.P.; Chen, N.H.; Tu, Y.K.; Hsieh, Y.J.; Wang, Y.C.; Guilleminault, C. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: A bivariate meta-analysis. Sleep Med. Rev. 2017, 36, 57–70. [Google Scholar] [CrossRef]
- Chung, F.; Yang, Y.; Liao, P. Predictive performance of the STOP-Bang score for identifying obstructive sleep apnea in obese patients. Obes. Surg. 2013, 23, 2050–2057. [Google Scholar] [CrossRef]
- Teng, Y.; Wang, S.; Wang, N.; Muhuyati. STOP-Bang questionnaire screening for obstructive sleep apnea among Chinese patients with type 2 diabetes mellitus. Arch. Med. Sci. 2018, 14, 971–978. [Google Scholar] [CrossRef]
- Westlake, K.; Plihalova, A.; Pretl, M.; Lattova, Z.; Polak, J. Screening for obstructive sleep apnea syndrome in patients with type 2 diabetes mellitus: A prospective study on sensitivity of Berlin and STOP-Bang questionnaires. Sleep Med. 2016, 26, 71–76. [Google Scholar] [CrossRef]
- Hwang, M.; Zhang, K.; Nagappa, M.; Saripella, A.; Englesakis, M.; Chung, F. Validation of the STOP-Bang questionnaire as a screening tool for obstructive sleep apnoea in patients with cardiovascular risk factors: A systematic review and meta-analysis. BMJ Open Respir. Res. 2021, 8, e000848. [Google Scholar] [CrossRef]
- Valdivia, G.; Schmidt, A.; Schmidt, B.; Rivera, F.; Oñate, A.; Navarrete, C.; Campos, J.; Labarca, G. Association between cardiovascular mortality and STOP-Bang questionnaire scores in a cohort of hospitalized patients: A prospective study. J. Bras. Pneumol. 2021, 47, e20210039. [Google Scholar] [CrossRef]
- Archontogeorgis, K.; Voulgaris, A.; Nena, E.; Strempela, M.; Karailidou, P.; Tzouvelekis, A.; Mouemin, T.; Xanthoudaki, M.; Steiropoulos, S.; Froudarakis, M.E.; et al. Cardiovascular Risk Assessment in a Cohort of Newly Diagnosed Patients with Obstructive Sleep Apnea Syndrome. Cardiol. Res. Pract. 2018, 2018, 6572785. [Google Scholar] [CrossRef]
- Pafili, K.; Steiropoulos, P.; Papanas, N. The relationship between obstructive sleep apnoea and coronary heart disease. Curr. Opin. Cardiol. 2015, 30, 439–446. [Google Scholar] [CrossRef]
- Bertoluci, M.C.; Rocha, V.Z. Cardiovascular risk assessment in patients with diabetes. Diabetol. Metab. Syndr. 2017, 9, 25. [Google Scholar] [CrossRef] [Green Version]
- Bradley, T.D.; Floras, J.S. Obstructive sleep apnoea and its cardiovascular consequences. Lancet 2009, 373, 82–93. [Google Scholar] [CrossRef]
- Das, S.R.; Everett, B.M.; Birtcher, K.K.; Brown, J.M.; Januzzi, J.L., Jr.; Kalyani, R.R.; Kosiborod, M.; Magwire, M.; Morris, P.B.; Neumiller, J.J.; et al. 2020 Expert Consensus Decision Pathway on Novel Therapies for Cardiovascular Risk Reduction in Patients with Type 2 Diabetes: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2020, 76, 1117–1145. [Google Scholar] [CrossRef]
- Rosu, M.M.; Popa, S.G.; Mota, E.; Popa, A.; Manolache, M.; Guja, C.; Bala, C.; Mota, C.; Mota, M. Cardiovascular Risk Assessment in the Adult (Aged 40–79 Years) Romanian Population. Acta Endocrinol. 2018, 14, 227–234. [Google Scholar] [CrossRef]
- Kavaric, N.; Klisic, A.; Ninic, A. Cardiovascular Risk Estimated by UKPDS Risk Engine Algorithm in Diabetes. Open Med. 2018, 13, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.J.; Kothari, V.; Adler, A.I.; Stratton, I.M.; United Kingdom Prospective Diabetes Study (UKPDS) Group. The UKPDS risk engine: A model for the risk of coronary heart disease in Type II diabetes (UKPDS 56). Clin. Sci. 2001, 101, 671–679, Erratum in Clin. Sci. 2002, 102, 679. [Google Scholar] [CrossRef]
- Available online: https://www.calculator.net/sample-size-calculator.html (accessed on 20 January 2018).
- World Health Organization. Waist Circumference and Waist–Hip Ratio: Report of a WHO Expert Consultation; WHO: Geneva, Switzerland, 2008.
- American Diabetes Association Professional Practice Committee; Draznin, B.; Aroda, V.R.; Bakris, G.; Benson, G.; Brown, F.M.; Freeman, R.; Green, J.; Huang, E.; Isaacs, D.; et al. 12. Retinopathy, Neuropathy, and Foot Care: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S185–S194. [Google Scholar] [CrossRef] [PubMed]
- Lameire, N.H.; Levin, A.; Kellum, J.A.; Cheung, M.; Jadoul, M.; Winkelmayer, W.C.; Stevens, P.E.; Conference Participants. Harmonizing acute and chronic kidney disease definition and classification: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2021, 100, 516–526. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation. CKD-EPI Creatinine Equation 2021. Available online: https://www.kidney.org/content/ckd-epi-creatinine-equation-2021 (accessed on 30 March 2022).
- Oshita, H.; Ito, N.; Senoo, M.; Funaishi, K.; Mitama, Y.; Okusaki, K. The STOP-Bang Test Is Useful for Predicting the Severity of Obstructive Sleep Apnea. JMA J. 2020, 3, 347–352. [Google Scholar] [CrossRef]
- Simmons, R.K.; Coleman, R.L.; Price, H.C.; Holman, R.R.; Khaw, K.T.; Wareham, N.J.; Griffin, S.J. Performance of the UK Prospective Diabetes Study Risk Engine and the Framingham Risk Equations in Estimating Cardiovascular Disease in the EPIC-Norfolk Cohort. Diabetes Care 2009, 32, 708–713. [Google Scholar] [CrossRef] [Green Version]
- Floras, J.S. Sleep apnea and cardiovascular risk. J. Cardiol. 2014, 63, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Hermans, M.P.; Ahn, S.A.; Mahadeb, Y.P.; Rousseau, M.F. Sleep apnoea syndrome and 10-year cardiovascular risk in females with type 2 diabetes: Relationship with insulin secretion and insulin resistance. Diabetes Metab. Res. Rev. 2013, 29, 227–234. [Google Scholar] [CrossRef]
- Labarca, G.; Valdivia, G.; Oñate, A.; Navarrete, C.; Araya, J.; Fernandez-Bussy, I.; Dreyse, J.; Jorquera, J. Prevalence of STOP BANG questionnaire and association with major cardiovascular events in hospitalized population: Is it enough with currently used cardiovascular risk measurements? Sleep Med. 2019, 61, 82–87. [Google Scholar] [CrossRef]
- Azman, M.; Sani, A.; Kamaruddin, N.A. Insulin resistance using HOMA model in obstructive sleep apnea: A cross sectional study. Ann. Saudi Med. 2014, 34, 476–481. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.; Liu, Y.; Xu, H.; Meng, L.; Zou, J.; Qian, Y.; Chen, B.; Yi, H.; Guan, J.; Yin, S. Association of apnea-hypopnea index during rapid eye movement sleep with insulin resistance in patients with suspected obstructive sleep apnea: A cross-sectional study. Ann. Transl. Med. 2021, 9, 243. [Google Scholar] [CrossRef]
- Michalek-Zrabkowska, M.; Macek, P.; Martynowicz, H.; Gac, P.; Mazur, G.; Grzeda, M.; Poreba, R. Obstructive Sleep Apnea as a Risk Factor of Insulin Resistance in Nondiabetic Adults. Life 2021, 11, 50. [Google Scholar] [CrossRef]
- Otake, K.; Sasanabe, R.; Hasegawa, R.; Banno, K.; Hori, R.; Okura, Y.; Yamanouchi, K.; Shiomi, T. Glucose intolerance in Japanese patients with obstructive sleep apnea. Intern. Med. 2009, 48, 1863–1868. [Google Scholar] [CrossRef] [Green Version]
- Vladu, I.M.; Forțofoiu, M.; Clenciu, D.; Forțofoiu, M.C.; Pădureanu, R.; Radu, L.; Cojan, Ș.T.Ț.; Rădulescu, P.M.; Pădureanu, V. Insulin resistance quantified by the value of HOMA-IR and cardiovascular risk in patients with type 2 diabetes. Exp. Ther. Med. 2022, 23, 73. [Google Scholar] [CrossRef]
- Hui, M.; Li, Y.; Ye, J.; Zhuang, Z.; Wang, W. Obstructive sleep apnea-hypopnea syndrome (OSAHS) comorbid with diabetes rather than OSAHS alone serves an independent risk factor for chronic kidney disease (CKD). Ann. Palliat. Med. 2020, 9, 858–869. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, P.; Zhao, F.; Han, X.; Ji, L. Association of Diabetic Microvascular Complications and Parameters of Obstructive Sleep Apnea in Patients with Type 2 Diabetes. Diabetes Technol. Ther. 2016, 18, 415–420. [Google Scholar] [CrossRef]
- Antza, C.; Ottridge, R.; Patel, S.; Slinn, G.; Tearne, S.; Nicholls, M.; Cooper, B.; Ali, A.; Tahrani, A.A. The impact of sleep disorders on microvascular complications in patients with type 2 diabetes (SLEEP T2D): The protocol of a cohort study and feasibility randomised control trial. Pilot Feasibility Stud. 2021, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J. Diabetes Res. 2020, 2020, 7489795. [Google Scholar] [CrossRef] [PubMed]
- Jelani, Q.U.; Mena-Hurtado, C.; Gosch, K.; Mohammed, M.; Labrosciano, C.; Regan, C.; Scierka, L.E.; Spertus, J.A.; Nagpal, S.; Smolderen, K.G. Association of sleep apnea with outcomes in peripheral artery disease: Insights from the PORTRAIT study. PLoS ONE 2021, 16, e0256933. [Google Scholar] [CrossRef] [PubMed]
- Chung, F.; Yegneswaran, B.; Liao, P.; Chung, S.A.; Vairavanathan, S.; Islam, S.; Khajehdehi, A.; Shapiro, C.M. STOP questionnaire: A tool to screen patients for obstructive sleep apnea. Anesthesiology 2008, 108, 812–821. [Google Scholar] [CrossRef] [Green Version]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Williams, B.A. The clinical epidemiology of fatigue in newly diagnosed heart failure. BMC Cardiovasc. Disord. 2017, 17, 122. [Google Scholar] [CrossRef] [Green Version]
- Drager, L.F.; McEvoy, R.D.; Barbe, F.; Lorenzi-Filho, G.; Redline, S.; on behalf of the INCOSACT Initiative (International Collaboration of Sleep Apnea Cardiovascular Trialists). Sleep Apnea and Cardiovascular Disease: Lessons from Recent Trials and Need for Team Science. Circulation 2017, 136, 1840–1850. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [Green Version]
- Ioannidou, D.; Kalamaras, G.; Kotoulas, S.C.; Pataka, A. Smoking and Obstructive Sleep Apnea: Is There an Association between These Cardiometabolic Risk Factors?—Gender Analysis. Medicina 2021, 57, 1137. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Zhou, J.; Wei, Z.; Li, X.; Xu, J.; Zhang, F.; Wang, W. Smoking and sleep apnea duration mediated the sex difference in daytime sleepiness in OSA patients. Sleep Breath. 2021, 25, 289–297. [Google Scholar] [CrossRef]
- Bielicki, P.; Trojnar, A.; Sobieraj, P.; Wąsik, M. Smoking status in relation to obstructive sleep apnea severity (OSA) and cardiovascular comorbidity in patients with newly diagnosed OSA. Adv. Respir. Med. 2019, 87, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Popa, S.G.; Moţa, M.; Mihălţan, F.D.; Popa, A.; Munteanu, I.; Moţa, E.; Serafinceanu, C.; Guja, C.; Hâncu, N.; Catrinoiu, D.; et al. Associations of smoking with cardiometabolic profile and renal function in a Romanian population-based sample from the PREDATORR cross-sectional study. Eur. J. Gen. Pract. 2017, 23, 164–170, Erratum in Eur. J. Gen. Pract. 2017, 23, i–ii. [Google Scholar] [CrossRef]
- Pan, A.; Wang, Y.; Talaei, M.; Hu, F.B. Relation of Smoking with Total Mortality and Cardiovascular Events among Patients with Diabetes Mellitus: A Meta-Analysis and Systematic Review. Circulation 2015, 132, 1795–1804. [Google Scholar] [CrossRef] [Green Version]
- Zhu, P.; Pan, X.F.; Sheng, L.; Chen, H.; Pan, A. Cigarette Smoking, Diabetes, and Diabetes Complications: Call for Urgent Action. Curr. Diabetes Rep. 2017, 17, 78. [Google Scholar] [CrossRef]
- Muraki, I.; Wada, H.; Tanigawa, T. Sleep apnea and type 2 diabetes. J. Diabetes Investig. 2018, 9, 991–997. [Google Scholar] [CrossRef]
- Fu, L.; Zhou, Y.; Sun, J.; Zhu, Z.; Xing, Z.; Zhou, S.; Wang, Y.; Tai, S. Atherogenic index of plasma is associated with major adverse cardiovascular events in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2021, 20, 201. [Google Scholar] [CrossRef]



| Characteristic | Total | STOP-Bang < 5 | STOP-Bang ≥ 5 | p |
|---|---|---|---|---|
| Fatal CHD 10-year risk (median (IQR)) * | 14.00 (19.0) | 12.20 (16.7) | 15.50 (20.9) | 0.001 |
| Nonfatal CHD 10-year risk (median (IQR)) * | 20.70 (22.6) | 19.00 (18.7) | 22.70 (24.8) | 0.001 |
| Fatal stroke 10-year risk (median (IQR)) * | 1.30 (2.2) | 1.20 (2.0) | 1.30 (2.2) | 0.187 |
| Nonfatal stroke 10-year risk (median (IQR)) * | 8.00 (13.0) | 7.70 (12.7) | 8.40 (13.2) | 0.261 |
| 10-Year Risk | OR † [95% CI] | p † | OR * [95% CI] | p * |
|---|---|---|---|---|
| Moderate-increased fatal CHD 10 years risk | 1.275 [1.129; 1.440] | <0.001 | 1.576 [1.108; 2.241] | 0.011 |
| Moderate-increased nonfatal CHD 10 years risk | 1.311 [1.151; 1.493] | <0.001 | 1.648 [1.141; 2.380] | 0.008 |
| Moderate-increased fatal stroke 10 years risk | 1.891 [0.987; 3.622] | 0.055 | 0.734 [0.332; 1.622] | 0.443 |
| Moderate-increased nonfatal stroke 10 years risk | 1.141 [1.002; 1.300] | 0.046 | 1.531 [0.843; 2.781] | 0.161 |
| Characteristic | Total | STOP-Bang < 5 | STOP-Bang ≥ 5 | p |
|---|---|---|---|---|
| T2DM duration, years (median (IQR)) * | 7.00 (11) | 6.00 (10) | 8.00 (12) | <0.001 |
| HbA1c, % (median (IQR)) * | 8.80 (3.60) | 8.30 (3.59) | 9.20 (3.42) | <0.001 |
| HOMA-IR (median (IQR)) * | 2.08 (0.52) | 1.90 (0.34) | 2.31 (1.06) | <0.001 |
| Chronic kidney disease, no (%) | 168 (31.8%) | 56 (10.6%) | 112 (21.2%) | 0.014 |
| Diabetic retinopathy, no (%) | 202 (38.2%) | 65 (30%) | 137 (43.9%) | 0.005 |
| Diabetic peripheral neuropathy, no (%) | 448 (84.7%) | 184 (84.8%) | 264 (84.6%) | 0.956 |
| ASCVD, no (%) | 325 (61.4%) | 116 (53.5%) | 209 (67.0%) | 0.002 |
| Analyzed Parameter | OR [95% CI] | p |
|---|---|---|
| Waist circumference | 0.871 [0.824; 0.920] | <0.001 |
| Male gender | 3.124 [1.183; 8.250] | 0.021 |
| Established ASCVD | 0.191 [0.068; 0.534] | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Protasiewicz Timofticiuc, D.C.; Vladu, I.M.; Ștefan, A.-G.; Clenciu, D.; Mitrea, A.; Pădureanu, V.; Efrem, I.C.; Diaconu, I.-D.; Turcu, A.; Țenea-Cojan, T.Ș.; et al. Associations of Chronic Diabetes Complications and Cardiovascular Risk with the Risk of Obstructive Sleep Apnea in Patients with Type 2 Diabetes. J. Clin. Med. 2022, 11, 4403. https://doi.org/10.3390/jcm11154403
Protasiewicz Timofticiuc DC, Vladu IM, Ștefan A-G, Clenciu D, Mitrea A, Pădureanu V, Efrem IC, Diaconu I-D, Turcu A, Țenea-Cojan TȘ, et al. Associations of Chronic Diabetes Complications and Cardiovascular Risk with the Risk of Obstructive Sleep Apnea in Patients with Type 2 Diabetes. Journal of Clinical Medicine. 2022; 11(15):4403. https://doi.org/10.3390/jcm11154403
Chicago/Turabian StyleProtasiewicz Timofticiuc, Diana Cristina, Ionela Mihaela Vladu, Adela-Gabriela Ștefan, Diana Clenciu, Adina Mitrea, Vlad Pădureanu, Ion Cristian Efrem, Ileana-Diana Diaconu, Adina Turcu, Tiberiu Ștefăniță Țenea-Cojan, and et al. 2022. "Associations of Chronic Diabetes Complications and Cardiovascular Risk with the Risk of Obstructive Sleep Apnea in Patients with Type 2 Diabetes" Journal of Clinical Medicine 11, no. 15: 4403. https://doi.org/10.3390/jcm11154403
APA StyleProtasiewicz Timofticiuc, D. C., Vladu, I. M., Ștefan, A.-G., Clenciu, D., Mitrea, A., Pădureanu, V., Efrem, I. C., Diaconu, I.-D., Turcu, A., Țenea-Cojan, T. Ș., Hâncu, A. M., Forțofoiu, M., Mirea Munteanu, O., & Moța, M. (2022). Associations of Chronic Diabetes Complications and Cardiovascular Risk with the Risk of Obstructive Sleep Apnea in Patients with Type 2 Diabetes. Journal of Clinical Medicine, 11(15), 4403. https://doi.org/10.3390/jcm11154403









